Abstract
Background
Autoimmune pancreatitis (AIP) is a rare, and relatively new, form of chronic pancreatitis. The management of AIP can vary considerably among different centres in daily clinical practice.
Objectives
The aim of this study is to present a picture of epidemiological, clinical characteristics, outcomes, and the real-life practice in terms of management in several academic and non-academic centres in Italy.
Methods
Data on the clinical presentation, diagnostic work-up, treatments, frequency of relapses, and long-term outcomes were retrospectively collected in a cohort of AIP patients diagnosed at 14 centres in Italy.
Results
One hundred and six patients were classified as type 1 AIP, 48 as type 2 AIP, and 19 as not otherwise specified. Epidemiological, clinical, radiological, and serological characteristics, and relapses were similar to those previously reported for different types of AIP. Endoscopic cytohistology was available in 46.2% of cases, and diagnostic for AIP in only 35.2%. Steroid trial to aid diagnosis was administered in 43.3% cases, and effective in 93.3%. Steroid therapy was used in 70.5% of cases, and effective in 92.6% of patients. Maintenance therapy with low dose of steroid (MST) was prescribed in 25.4% of cases at a mean dose of 5 (±1.4) mg/die, and median time of MST was 60 days. Immunosuppressive drugs were rarely used (10.9%), and rituximab in 1.7%. Faecal elastase-1 was evaluated in only 31.2% of patients, and was pathological in 59.2%.
Conclusions
In this cohort of AIP patients, diagnosis and classification for subtype was frequently possible, confirming the different characteristics of AIP1 and AIP2 previously reported. Nevertheless, we observed a low use of histology and steroid trial for a diagnosis of AIP. Steroid treatment was the most used therapy in our cohort. Immunosuppressants and rituximab were rarely used. The evaluation of exocrine pancreatic insufficiency is underemployed considering its high prevalence.
Keywords: Autoimmune pancreatitis, endoscopic ultrasound, fine needle aspiration/biopsy, steroid trial, pancreatic insufficiency
Introduction
Autoimmune pancreatitis (AIP) is an unusual form of chronic pancreatitis of presumed autoimmune aetiology. Two histological subtypes of AIP have been recognized: type 1 (AIP1) and type 2 (AIP2),1–7 with distinct histological and clinical characteristics.5–7
Diagnosing AIP is difficult, requiring a combination of different data. Several diagnostic criteria have been developed over the past few years.8–11 In 2012, the International Association of Pancreatology proposed the International Consensus Diagnostic Criteria (ICDC),1 which are the most commonly employed criteria worldwide. ICDC combine pancreatic parenchyma and ductal changes at abdominal imaging, serum IgG4 level, other organ involvement (OOI), histology, and response to steroid treatment to reach a diagnosis. Differently from other criteria, ICDC can diagnose AIP1 and AIP2 independently. In addition, the ICDC defined the criteria for AIP not otherwise specified (AIP-NOS) for those cases not clearly diagnosed as either AIP1 or AIP2.
Over the last decade, data from several case series and small cohorts of AIP patients have been reported.6,11–17 However, most of those data regard AIP patients diagnosed and managed in tertiary referral centres, and detailed data on the clinical, and radiological features of patients, and on adherence to guidelines are scant.
We have hypothesized that the management of patients with AIP, and the adherence to the most commonly used guidelines, may differ significantly in the routine clinical practice of various Italian centres. We therefore decided to carry out a national survey in both academic and non-academic centres throughout Italy on the management of AIP.
Methods
This observational multicentre retrospective survey was done with the aim of presenting a picture of real-life daily practice, in referral and non-referral centres, in the care of patients with AIP, in Italy. Definitions of terms used in the study are reported in Supplementary Table 1.
Patients with a definitive diagnosis of AIP between January, 2000 and December, 2017 were included. Among the 22 centres initially interested in participating, eight dropped out due to a low volume of AIP cases. In the end, 14 Italian centres participated in the study, for a total of 173 patients. Ninety-four percent of patients (163/173) were diagnosed after 2010. Each centre was free to manage patients according to the guidelines it preferred. Diagnosis and histological adequacy for AIP were based on the local investigators’ judgment at each centre. Treatments and follow-up modality were chosen locally, as well.
Data, at time of diagnosis and during the follow-up, on demographics, clinical presentation, laboratory, radiological, endoscopic, and histological findings, diagnostic criteria, and type of AIP, treatments, pattern and timing of relapses, duration of follow-up, and long-term outcomes were recorded.
The study was promoted and coordinated by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO), and was endorsed by the Italian Association for the Study of the Pancreas (AISP). IRCCS-ISMETT (Mediterranean Institute for Transplantation and Highly Specialized Therapies), was the coordinating centre of the study.
The study was approved by ISMETT’s Ethics Committee on 20 January 2017 (IRRB/27/16), and by the local committees of all the participating institutions, and was in accordance with the Declaration of Helsinki. All patients gave written consent. An electronic case report form was created, and investigators at all the participating centres filled it out anonymously with patient data. Particular attention was paid to the compilation of each data sheet in the database in order to provide, consonant with the retrospective nature of the study, the largest amount of data required for each patient. The patient files in the database were checked at the coordinating centre by two of the authors (L.B., M.T.) and any missing data or inaccuracies were reported to the individual co-authors to supplement or modify the missing or inaccurate information. Furthermore, to make sure that there were no obvious misdiagnoses, all the information contained in the patient’s card was revised.
The study was drafted in accordance with the STROBE statements.18
Statistics
Categorical variables are reported as percentage and were compared using the chi-square or Fisher’s exact test when appropriate. Continuous variables are reported as mean and standard deviation (SD) or median and interquartile range (IQR), and were compared using the Student t-test or the paired t-test when appropriate. Recurrence-free probability was assessed with the Kaplan–Meier estimator. Data handling and analyses were done with SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). A p-value of <0.05 was considered statistically significant.
Results
Patient characteristics
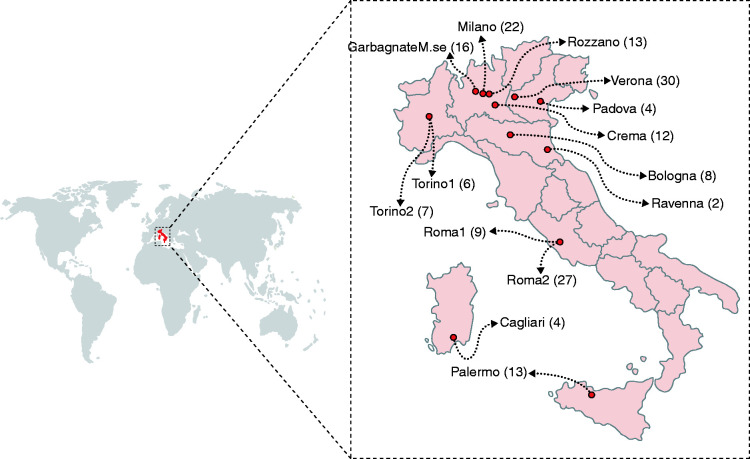
Data from 173 patients from 14 Italian centres (seven academic and seven non-academic) (Figure 1) were collected: 106 (61%) patients were classified as AIP1, 48 (28%) as AIP2, and 19 (11%) as AIP-NOS. Mean follow-up time was 1257±1169 days (median: 962 days; IQR 424–1704). Baseline characteristics at diagnosis and clinical presentation are summarized in Table 1. The percentage of males was 66.9% for patients with AIP1, and 54.2% in AIP2 (p = NS). Median age at diagnosis was significantly different in AIP1 and AIP2 (62.5, IQR 51–70, and 48 years, IQR 28.5–65, respectively; p < 0.001). Cardiovascular comorbidities were observed in 32.4% of patients, diabetes in 10.4%, pulmonary comorbidities in 9.2%, autoimmune diseases in 8.7%, and gastrointestinal comorbidities in 16.2%. No significant differences were found regarding comorbidities, except for gastrointestinal ones (7.5% in AIP1 vs. 35.4% in AIP2; p < 0.001), due to the higher probability of developing an inflammatory bowel disease (IBD), specifically ulcerative colitis (UC) in patients with AIP2. In fact, IBD was present in 2/106 (1.9%, both UC) patients with AIP1, and in 13/48 (27.1%, 11 UC and two undetermined IBD) patients with AIP2 (p < 0.001).
Figure 1.
Distribution of recruited AIP patients in the study by each centre.
Table 1.
Baseline characteristics of AIP patients at diagnosis and clinical presentation.
| Overall | Type 1 AIP§ (n = 106) | Type 2 AIP§ (n = 48) | p-value | NOS-AIP§ (n = 19) | |
|---|---|---|---|---|---|
| Male (%) | 108 (62.4) | 71 (66.9) | 26 (54.2) | 0.13 | 11 |
| Age at diagnosis (median) | 61 | 62.5 | 48 | <0.001 | 62 |
| BMI* at diagnosis (median) | 23.5 | 23.8 | 23.5 | 0.9 | 28.8 |
| Comorbidities | |||||
| Cardiovascular | 56 (32.4) | 40 (37.7) | 13 (27.1) | 0.2 | 3 (15.8) |
| Diabetes | 18 (10.4) | 13 (12.3) | 3 (6.2) | 0.25 | 2 (10.5) |
| Cancers | 9 (5.2) | 6 (5.7) | 1 (2.1) | 0.3 | 2 (10.5) |
| Pulmonary | 16 (9.2) | 12 (11.3) | 3 (6.2) | 0.32 | 1 (5.3) |
| Rheumatological | 14 (8.1) | 7 (6.6) | 5 (10.4) | 0.41 | 2 (10.5) |
| Gastrointestinal | 28 (16.2) | 8 (7.5) | 17 (35.4) | <0.001 | 3 (15.8) |
| Autoimmune | 15 (8.7) | 11 (10.4) | 3 (6.2) | 0.4 | 1 (5.3) |
| Prostatic | 12 (6.9) | 8 (7.5) | 3 (6.2) | 0.4 | 1 (5.3) |
| Other organ involvement in type 1 AIP§ | |||||
| Overall | 61 (57.5) | ||||
| Biliary ducts | 42 (39.6) | ||||
| Salivary glands | 8 (7.5) | ||||
| Kidneys | 12 (11.3) | ||||
| Lymph nodes | 10 (9.4) | ||||
| Retroperitoneal fibrosis | 2 (1.9) | ||||
| Lungs | 8 (7.5) | ||||
| Other | 7 (6.6) | ||||
| IBD# | 21 (12.1) | 2 (1.9) | 13 (27.1) | <0.001 | 2 (10.5) |
| Symptoms | |||||
| Dark urine | 30 (17.3) | 22 (20.7) | 7 (14.6) | 0.37 | 1 (5.3) |
| Pale faeces | 28 (16.2) | 21 (19.8) | 6 (12.5) | 0.27 | 1 (5.3) |
| Jaundice | 70 (40.5) | 52 (49.0) | 12 (25.0) | 0.005 | 6 (31.6) |
| Acute pancreatitis | 37 (21.4) | 21 (19.8) | 12 (25.0) | 0.47 | 4 (21) |
| Epigastric pain | 51 (29.5) | 26 (24.5) | 15 (31.25) | 0.38 | 10 (5.3) |
| Radiating epigastric pain | 27 (15.6) | 12 (11.3) | 12 (25.0) | 0.03 | 3 (15.8) |
| Lumbar pain | 13 (7.5) | 7 (6.6) | 4 (8.3) | 0.7 | 2 (10.5) |
| Nausea | 14 (8.1) | 10 (9.4) | 3 (6.25) | 0.51 | 1 (5.3) |
| Astenia | 25 (14.4) | 16 (15.1) | 8 (16.7) | 0.8 | 1 (5.3) |
| Anorexia | 21 (12.1) | 16 (15.1) | 5 (10.4) | 0.43 | 0 |
| Weight loss | 40 (23.1) | 26 (24.5) | 10 (20.8) | 0.62 | 4 (21) |
| Analgesics | 71 (41) | 33 (31.1) | 26 (54.2) | 0.006 | 12 (63.2) |
§AIP: autoimmune pancreatitis; °NOS: not otherwise specified; *BMI: body mass index; #IBD: inflammatory bowel disease.
In patients with AIP1, the most common OOI was biliary duct (39.6%), followed by kidneys (11.3%), lymph nodes (9.4%), salivary glands (7.5%), and lungs (7.5%).
Jaundice was the most common presentation symptom (40.5%), with a statistically significant difference between AIP1 and AIP2 (49% vs. 25%; p = 0.005). Other frequent presentation symptoms were acute pancreatitis (overall 21.4%; 19.8% in AIP1, and 25.0% in AIP2; p = NS), epigastric pain (overall 29.5%), and weight loss (overall 23.1%).
Imaging findings and histology
The employed imaging techniques with their relative findings are summarized in Figure 2 and in Supplementary Table 2. Most of the patients underwent computed tomography (CT) scan (83.8%), followed by endoscopic ultrasound (EUS) (68.2%), ultrasonography (66.5%), and magnetic resonance imaging (MRI) (62.4%). In more than 85% of patients there was diffuse or focal pancreatic enlargement (both on CT and MRI imaging), with a higher prevalence of the latter. The pathognomonic capsule-like rim sign was seen in only about 13% of patients, with no differences between CT and MRI. The rate of main pancreatic duct stenosis found on EUS, MRI, and endoscopic retrograde cholangiopancreatography (ERCP) was similar (9.3%, 15.7%, and 7.7%, respectively; p = NS).
Figure 2.
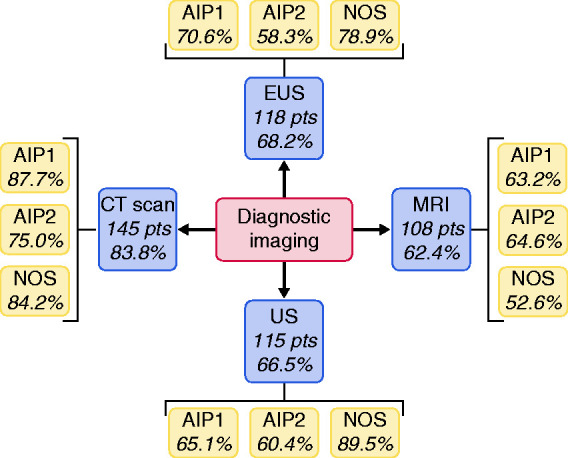
Distribution of diagnostic imaging techniques performed.
Histological specimens were available in 105/173 patients (60.7%). Nineteen patients (11%) had surgical histology (18 on the pancreas, and one on the common bile duct), while 86 (49.7%) patients had cytohistology obtained endoscopically. Among patients with a cytohistological sample obtained during endoscopic procedures, 80/86 (93%) underwent pancreatic EUS-fine needle aspiration/biopsy (FNA/B), 14/86 (16.3%) common bile duct wall biopsy (during ERCP), 8/86 (9.3%) ampulla of Vater biopsy, and 2/86 (2.3%) EUS-FNA/B of lymph nodes.
When stratified by AIP subtypes, EUS-FNA/B pancreatic cytohistology was obtained more frequently in AIP1 (57/106 patients, 53.8%) compared with AIP2 (13/48 patients, 27.1%; p < 0.01). This was judged diagnostic for AIP in 43/57 (75.4%) AIP1, and 10/13 (76.9%) AIP2 (p = 0.91). In AIP-NOS patients, pancreatic EUS-FNA/B was done in 10/19 (52.6%) cases.
EUS-FNA/B of the pancreas was done mostly in the focal form of AIP (85% of cases).
Endoscopic (with EUS, ERCP or gastroscopy) histology was judged diagnostic for AIP in 61/86 (70.9%), with no statistically significant differences between AIP1 and AIP2 (p = NS). However, in the overall cohort, an endoscopic histology diagnostic for AIP was obtained in only 61/173 (35.2%) of patients.
Laboratory findings and steroid trial
IgG measurement was available in 128/173 (74%) patients. Median total IgG value was 955 (IQR 837–1326) mg/dl, with no statistically significant differences between AIP1 and AIP2 (1107.5, IQR 850–1300 mg/dl, and 1150, IQR 767–1329 mg/dl, respectively). IgG4 levels, measured at diagnosis before any treatment, were recorded in 140 (80.9%) patients, 84 with AIP1, 40 with AIP2, and 16 with AIP-NOS. IgG4 overall median value was 177.5 (IQR 70–402) mg/dl. The difference in median IgG4 value between AIP1 and AIP2 was statistically significant (234, IQR 133–567 mg/dl, and 87, IQR 25–161 mg/dl, respectively; p = 0.03). In 78/140 (55.7%) patients, IgG4 values were above upper normal range (UNR): 62/84 (73.8%) in AIP1 patients, and 11/40 (27.5%) in AIP2 patients (p = <0.001). In 40/84 (47.6%) AIP1 patients, IgG4 values were two times above UNR. Also, 5/40 (12.5%) AIP2 patients had IgG4 values >2 times UNR (Supplementary Table 3).
A steroid trial was administered in 75/173 (43.3%) patients, and considered diagnostic for AIP in 70/75 (93.3%) cases. In all cases, the steroid trial was carried out for lack of sufficient diagnostic criteria for AIP.
Treatment and relapses
In the entire cohort, 52 patients (30.1%) underwent ERCP. In 94.2% (n = 49) the indication was the presence of biliary duct stenosis, and in all these cases a biliary stent was placed (plastic in 72.9%, and metallic in 27.1% of patients). Of the remaining three patients, ERCP was done with diagnostic intent in order to better characterize a main pancreatic duct stenosis.
Twenty-four of the 173 patients (13.9%) did not receive any treatment, and among these only one with AIP1 relapsed. Regarding the other 149 patients, 122/173 (70.5%) were treated with steroids as first-line therapy (in 11 patients steroids were associated with azathioprine or methotrexate), 15/173 (8.7%) with surgery upfront, 4/173 (2.3%) with steroid therapy followed by surgical resection, and in 8/173 (4.6%) with only azathioprine. The main reason for surgery, both alone and with associated steroid treatment (n=19), was the suspicion of pancreatic cancer (in 94.4% of cases). In the steroid group, the mean treatment dose received by each patient was 46.7 ± 17.1 mg/day, with a primary success rate of 92.6% (113/122).
Among patients treated only with steroids, a dosage tapering of prednisone was done in 121/122 (98.4%), while a maintenance steroid therapy (MST) was administered in 31/122 (25.4%) patients after the first episode of AIP (22/72 AIP1, and 7/34 AIP2 patients; p = 0.30). Median dosage of prednisone for MST was 5 (±1.4) mg/die, and median time of MST was 60 (IQR 30–180) days. Rituximab was used in 2/37 (5.4%) patients after the first episode of relapse, and in 1/10 (10%) after the second relapse.
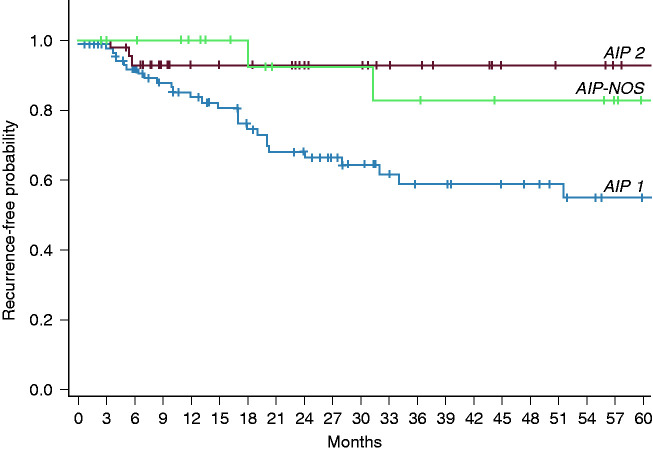
Relapses (Table 2 and Figure 3) were observed in 37/173 (21.4%), with a statistically significant difference among patients with AIP1 and AIP2 (30.2% vs. 6.25%, respectively; p = 0.001). In only 1/37 (2.7%) patient the relapse was observed during MST with prednisone 5 mg/die.
Table 2.
Pharmacological and surgical treatments.
| Treated |
Primary efficacy |
Relapses |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Total number of patients | ||||||
| Overall | 173 | 123 | 71.1 | 37 | 21.4 | |
| Type 1 AIP§ | 106 | 61.3 | 72 | 67.9 | 32 | 30.2 |
| Type 2 AIP§ | 48 | 27.7 | 37 | 77 | 3 | 6.25 |
| NOS-AIP§ | 19 | 11.0 | 14 | 7.6 | 2 | 10.5 |
| No treatment | ||||||
| Overall (on 173) | 24 | 13.9 | 0 | 0 | 1 | 4.2 |
| Type 1 AIP§ | 13 | 12.3 | 0 | 0 | 1 | 7.7 |
| Type 2 AIP§ | 8 | 16.7 | 0 | 0 | 0 | 0 |
| NOS-AIP§ | 3 | 15.7 | 0 | 0 | 0 | 0 |
| Surgery | ||||||
| Overall (on 173) | 15 | 8.6 | 0 | 0 | 1 | 7.1 |
| Type 1 AIP§ | 13 | 12.2 | 0 | 0 | 1 | 8.3 |
| Type 2 AIP§ | 2 | 4.2 | 0 | 0 | 0 | 0 |
| NOS-AIP§ | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery + Steroid | ||||||
| Overall (on 173) | 4 | 2.3 | 3 | 75 | 1 | 25 |
| Type 1 AIP§ | 3 | 2.8 | 2 | 66.7 | 1 | 33.3 |
| Type 2 AIP§ | 1 | 2 | 1 | 100 | 0 | 0 |
| NOS-AIP§ | 0 | 0 | 0 | 0 | 0 | 0 |
| Steroid | ||||||
| Overall* (on 173) | 122 | 70.5 | 113 | 91.9 | 32 | 26.0 |
| Type 1 AIP§ | 72 | 67.9 | 66 | 90.4 | 27 | 36.9 |
| Type 2 AIP§ | 34 | 70.8 | 33 | 97 | 3 | 8.8 |
| NOS-AIP§ | 16 | 84.2 | 14 | 87.5 | 2 | 12.5 |
| Azathioprine alone | ||||||
| Overall (on 173) | 8 | 4.6 | 7 | 87.5 | 2 | 25 |
| Type 1 AIP§ | 5 | 4.7 | 4 | 80 | 2 | 40 |
| Type 2 AIP§ | 3 | 6.25 | 3 | 100 | 0 | 0 |
| NOS-AIP§ | 0 | 0 | 0 | 0 | 0 | 0 |
§AIP: Autoimmune pancreatitis; °NOS: Not Otherwise Specified.
Figure 3.
Kaplan–Meier curves for relapses in AIP1, AIP2, and AIP-NOS.
Outcomes
Outcomes of patients with AIP are reported in Table 3. In our cohort there were 54 patients with a diagnosis of diabetes. In only 11% of cases (n=19) this was present before the onset of AIP, and in 35 patients it was caused/unmasked by the pancreatitis. In particular, 19 patients (11%) developed diabetes simultaneously with the onset of AIP, and 16 patients (9.2%) developed it during follow-up. Among patients with ‘acute’ diabetes, an improvement after therapy in 58% of cases was observed; 69% of patients who developed diabetes during the follow-up had undergone steroid therapy previously.
Table 3.
Outcomes of patients with AIP.
| Overall |
% | Type 1 AIP§ |
% | Type 2 AIP§ |
% | p-value | NOS-AIP§ |
% | |
|---|---|---|---|---|---|---|---|---|---|
| (n = 173) | (n = 106) | (n = 48) | (n = 19) | ||||||
| Diabetes | |||||||||
| Occurred before the beginning of AIP | 19 | 11 | 14 | 13.2 | 3 | 6.3 | 0.2 | 2 | 10.5 |
| Occurred simultaneously with AIP | 19 | 11 | 14 | 13.2 | 5 | 10.4 | 0.63 | 0 | 0 |
| Occurred during follow-up | 16 | 9.2 | 12 | 11.3 | 4 | 8.3 | 0.57 | 0 | 0 |
| Atrophy at imaging | 45 | 26 | 27 | 25.5 | 15 | 31.3 | 0.46 | 3 | 15.8 |
| Clinical evidence of steatorrhoea | 24 | 13.9 | 13 | 12.3 | 11 | 22.9 | 0.09 | 0 | 0 |
| Faecal elastase value (on 54 patients) | |||||||||
| >100 and <200 mcg/g | 9 (on 54) | 16.7 | 2 (on 106) | 1.9 | 6 (on 48) | 12.5 | 0.09 | 1 (on 19) | 5.3 |
| <100 mcg/g | 23 (on 54) | 42.6 | 17 (on 106) | 16 | 5 (on 48) | 10.4 | 0.36 | 1 (on 19) | 5.3 |
| Pancreatic calcifications | 17 | 9.8 | 9 | 8.5 | 5 | 10.4 | 0.7 | 3 | 15.8 |
§AIP: Autoimmune pancreatitis; °NOS: Not Otherwise Specified.
Regarding exocrine insufficiency, faecal elastase-1 was measured in only 31.2% of patients (n=54), and among these the value was under 200 mcg/g in 59.2% of cases. No differences between type 1 and type 2 AIP were found regarding low (<200 and >100 mcg/g; p = 0.09) and very low (<100 mcg/g; p = 0.36) levels of faecal elastase-1.
Pancreatic atrophy on imaging was observed in 45/173 (26%) of patients, while calcifications were found in 17 (9.8%) patients.
Only 1/173 (0.6%) patient was diagnosed with pancreatic adenocarcinoma during follow-up, within 1 year of diagnosis.
Discussion
This study reports results of a multicentre retrospective real-life survey on clinical features and management of patients with AIP diagnosed in Italy. The epidemiology, clinical manifestations, laboratory/radiological findings, and treatment outcomes, including the relapse rate, were found to be similar to those previously published.1,2,4,7 Elderly age (sixth or seventh decade), male gender, increased level of IgG4, involvement of the biliary tract, kidney and salivary glands, jaundice as presentation symptom, and frequent relapses were associated with AIP1. Younger age (fourth decade), abdominal pain and acute pancreatitis or persistent abdominal pain as presenting symptoms, associated IBD, rare IgG4 elevation, and a lower relapse rate were more frequently found in AIP2. However, the study also highlighted several critical points that deserve further discussion.
A first point regards the possibility of obtaining a histological sample and its reliability in daily clinical practice. In our cohort, EUS-FNA/B was the tissue-acquisition technique most used, and was done in 80/173 (46.2%) of cases, but only in 27.1% of patients initially classified as AIP2, where histology is a cornerstone for the diagnosis.1,7 Furthermore, the rate of all endoscopic sampling fulfilling the histological diagnostic criteria of AIP was observed in only 35.2% in the study population. Histological diagnosis and sub-classification of AIP remains a debated issue.19–21 Histology is a cardinal ICDC criterion for the diagnosis of AIP1 and AIP2, and its sub-classification. However, obtaining adequate histology in a preoperative setting is challenging, and AIP1 and AIP2 are often indistinguishable without an adequate histology. In this eventuality, some authors have proposed avoiding sub-classification,11,19 or including in a single category probable AIP1, probable AIP2, and AIP-NOS.20
The lack of agreement on a reliable tissue-acquisition technique for a diagnosis of AIP is a problem that has often been discussed.22–27 Most authors agree that EUS-FNA/B is the preferred technique for excluding pancreatic cancer in the focal form of AIP. However, there is much less agreement regarding the possibility of obtaining specimens diagnostic for AIP. In our cohort, pancreatic EUS-FNA/B was considered diagnostic for AIP in a minority of patients. Likely, indeed, the main goal of EUS-FNA/B was to exclude pancreatic cancer, being performed far more frequently (85%) in patients with focal pancreatic enlargement. Furthermore, a discrepancy concerning the routine use of EUS-FNA/B among centres was observed, and is likely related to the availability of pathologists with expertise in pancreatic disease in each centre.
The fact that the majority of patients were included in the study with a diagnosis after 2010 reflects a greater awareness acquired in the last 10 years of this new nosological entity, to which the ICDC guidelines have certainly contributed. However, our data confirm those previously observed on ICDC diagnostic criteria:6,19,20 while very accurate for a diagnosis and for sub-classification of AIP types, these criteria remain burdensome, and difficult to apply in real-life daily practice, particularly when the absence of histology makes the sub-classification extremely difficult. In any event, epidemiological and clinical data, and natural history of our cohort are generally in agreement with the diagnosis and sub-classification proposed by the treating physicians. Therefore, we could speculate that EUS-FNA/B is essential in excluding pancreatic cancer, especially in the focal form of AIP, but may not be necessary for a diagnosis of AIP in all patients, though this might result in misclassification of the disease regarding the specific subtype, as previously reported.19,28
Recent advances in EUS needle technology, with needles specifically designed for EUS-FNB,29,30 could improve the histology procurement, and should be evaluated in future studies. Furthermore, we need specific training of pathologists to establish the diagnosis of AIP effectively.
A second point that deserves discussion regards the use of the steroid trial. Despite the fact that this trial is counted in ICDC as one of the diagnostic criteria for diagnosis of AIP,1 in our cohort steroid trial was used in a relatively low percentage of patients (43.3%) to reach a diagnosis. The high rate of diagnostic steroid trial in our population (93%) underlines its diagnostic usefulness. Therefore, we believe that, after excluding malignancy, in cases with a suspicion of AIP and diagnostic doubt, a steroid trial, if not otherwise contraindicated, is useful to achieve a sufficient number of diagnostic criteria, in accordance with the ICDC guidelines, regardless of whether the patients merit a prolonged steroid therapy.
Regarding the employed therapeutic approach, surgery was performed in 11% of patients, in the majority for suspicion of pancreatic cancer, and most of the patients received steroids as initial treatment and as maintenance therapy, with a very high response rate, as previously documented.31 Immunosuppressants and rituximab were used especially after relapses, even if rarely; only a small number of patients received azathioprine as first-line treatment, because other comorbidities contraindicated the use of steroids. Both immunosuppressive drugs and rituximab deserve to be further explored in randomized studies, in particular rituximab, which in a recent retrospective study on relapsing autoimmune pancreatitis looked very promising, having been shown to be more efficient than immunosuppressant drugs, and with better tolerance.32
Regarding the natural history of AIP, our results confirm its association with diabetes both at presentation of the disease and during follow-up.33–36 In some instances diabetes might improve after treatment for AIP, while in other cases the development of diabetes is irreversible.37 We observed the development of diabetes during follow-up in about 10% of patients in our study, likely related to the atrophy of the pancreas, observed in about 25% of cases.
As regards pancreatic exocrine insufficiency, only a minority of patients were investigated by means of faecal elastase-1 measurement, particularly during follow-up. Indeed, faecal elastase-1 value was measured in only 30% of patients, but a pathologic result was observed in about 60% (in 43% of which <100 mcg/g). These data should be carefully evaluated: these high values may be related to the fact that only one-third of patients were tested for faecal elastase-1, suggesting that in the majority of cases only symptomatic patients were measured. A dedicated prospective study would be desirable to establish the unbiased probability of exocrine pancreatic insufficiency in AIP patients. However, although faecal elastase-1 assay has several limitations in diagnosing pancreatic exocrine insufficiency,38 it is currently considered the most available and accurate non-invasive test. Furthermore, it is inexpensive, non-invasive, and largely available, so we believe its use in this setting should be encouraged.
The risk of developing pancreatic cancer in our cohort seemed to be extremely low (1/173; 0.6%), as previously reported.39 Moreover, the single case of pancreatic cancer was diagnosed within a year of AIP-NOS diagnosis; thus, it was most likely present at the beginning of the clinical history, and initially misdiagnosed.40
The retrospective nature of this study is its most relevant limitation, which entailed partial availability of information with inherent biases, and the fact that comparisons could only be done in an indirect manner. Also, the study represents a picture of common real-life practice with AIP among different centres with heterogeneous behaviours and clinical approaches that were not standardized. Another limitation is the long period of recruitment. Nevertheless, 94% of enrolled patients had a diagnosis of AIP after 2010, making our cohort more uniform in terms of knowledge of the disease, and available guidelines and treatments. Finally, the relatively small size of the study cohort makes categorization in subpopulations difficult. However, considering the rarity of this pathology, the participation of 14 centres with a total of 173 patients allows this study to be considered one of the most robust to date. The distribution of cases covers the entire national territory, and the participation of community, and not only referral hospitals, makes this survey a real picture of the general gastroenterology community in Italy.
In conclusion, in real-life daily clinical practice in AIP patients, pancreatic EUS-FNA/B for specific diagnosis of AIP seems underutilized, and its diagnostic yield low in this setting of patients. We need better tissue-acquisition modalities, but also specific training for pathologists to effectively establish the diagnosis of AIP. However, in most instances the real need for histology, apart from excluding malignancy, should be further investigated.
Second, steroid trial is not employed frequently in real-life clinical practice. Its use should be implemented to allow a more frequent adherence to the ICDC criteria, which, however, remain burdensome and difficult to apply in daily clinical practice.
Immunosuppressants and rituximab are rarely used, and their role has to be assessed in future studies.
Finally, the rate of exocrine pancreatic insufficiency development is not routinely investigated during the follow-up of AIP, but is rather common in AIP patients, and so measurement of faecal elastase test should be recommended.
The present data from a real-life setting highlight some weaknesses in the routine diagnostic work-up and management of AIP patients that, if confirmed in prospective studies, could be improved in the future.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620924302 for Multicentric Italian survey on daily practice for autoimmune pancreatitis: Clinical data, diagnosis, treatment, and evolution toward pancreatic insufficiency by Luca Barresi, Matteo Tacelli, Stefano Francesco Crinò Fabia Attili, Maria Chiara Petrone, Germana De Nucci, Silvia Carrara, Guido Manfredi, Gabriele Capurso, Claudio Giovanni De Angelis, Lucia Crocellà, Alberto Fantin, Maria Francesca Dore, Alessandra Tina Garribba, Ilaria Tarantino, Nicolò De Pretis, Danilo Pagliari, Gemma Rossi, Gianpiero Manes, Paoletta Preatoni, Ilenia Barbuscio, Fabio Tuzzolino, Mario Traina, Luca Frulloni, Guido Costamagna, Paolo Giorgio Arcidiacono, Elisabetta Buscarini, Raffaele Pezzilli and Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO), Italian Association for the Study of the Pancreas (AISP) in United European Gastroenterology Journal
Acknowledgements
This study was promoted and coordinated by the Italian Association of Hospital Gastroenterologists (AIGO), and was endorsed by AISP (Italian Association for the Study of the Pancreas).
Declaration of conflicting interests
None declared.
Funding
There is no financial funding or interest to report.
Ethics approval
The study was approved by ISMETT’s Ethics Committee on 20 January 2017 (IRRB/27/16), and by the local committees of all the participating institutions, and was in accordance with the Declaration of Helsinki.
Informed consent
All patients gave written consent.
Author contributions
L. Barresi, M. Tacelli and R. Pezzilli designed the study. M Tacelli, S. F. Crinò, F. Attili, M. C. Petrone, G. De Nucci, S. Carrara, G. Manfredi, G. Capurso, C. De Angelis, L. Crocellà, A. Fantin, M. F. Dore, A. T. Garribba, I. Tarantino, N. De Pretis, D. Pagliari, G. Rossi, G. Manes, P. Preatoni, I. Barbuscio, R. Pezzilli provided retrospective patient data. L. Barresi, M. Tacelli, R. Pezzilli, S. F. Crinò, Capurso. Tarantino, M. Traina, L. Frulloni, G. Costamagna, P. G. Arcidiacono, E. Buscarini, F. Tuzzolino interpreted the data. L. Barresi, M. Tacelli, R. Pezzilli, F. Tuzzolino carried out the statistical analysis. M. Tacelli, I. Barbuscio constructed the Tables and Figures. L. Barresi, M. Tacelli, R. Pezzilli, G. Capurso, S.F. Crinò drafted the article. Tarantino, M. Traina, L. Frulloni, G. Costamagna, P. G. Arcidiacono, E. Buscarini, F. Attili, M. C. Petrone, G. De Nucci, S. Carrara, G. Manfredi, C. De Angelis, L. Crocellà, A. Fantin, M. F. Dore, A. T. Garribba, N. De Pretis, D. Pagliari, G. Rossi, G. Manes, P. Preatoni, I. Barbuscio, F. Tuzzolino revised the manuscript. All authors have read and approved the final manuscript.
ORCID iDs
Luca Barresi https://orcid.org/0000-0002-3788-145X
Stefano Francesco Crinò https://orcid.org/0000-0003-4560-8741
Paolo Giorgio Arcidiacono https://orcid.org/0000-0001-6692-7720
Elisabetta Buscarini https://orcid.org/0000-0003-0863-0624
Raffaele Pezzilli https://orcid.org/0000-0001-9827-7451
Supplemental material
Supplemental material for this article is available online.
References
- 1.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011; 40: 352–358. [DOI] [PubMed] [Google Scholar]
- 2.Sugumar A, Klöppel G, Chari ST. Autoimmune pancreatitis: Pathologic subtypes and their implications for its diagnosis. Am J Gastroenterol 2009; 104: 2308–2310. [DOI] [PubMed] [Google Scholar]
- 3.Zamboni G, Lüttges J, Capelli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: A study on 53 resection specimens and 9 biopsy specimens. Virchows Arch 2004; 445: 552–563. [DOI] [PubMed] [Google Scholar]
- 4.Chari ST, Kloeppel G, Zhang L, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis. Pancreas 2010; 39: 549–554. [DOI] [PubMed] [Google Scholar]
- 5.Sah RP, Chari ST, Pannala R, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology 2010; 139: 140–148. [DOI] [PubMed] [Google Scholar]
- 6.Ikeura T, Manfredi R, Zamboni G, et al. Application of international consensus diagnostic criteria to an Italian series of autoimmune pancreatitis. United European Gastroenterol J 2013; 1: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamisawa T, Chari ST, Giday SA, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: An international multicenter survey. Pancreas 2011; 40: 809–814. [DOI] [PubMed] [Google Scholar]
- 8.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience. Clin Gastroenterol Hepatol 2006; 4: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 9.Otsuki M, Chung JB, Okazaki K, et al. Asian diagnostic criteria for autoimmune pancreatitis: Consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol 2008; 43: 403–408. [DOI] [PubMed] [Google Scholar]
- 10.Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol 2009; 7: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 11.Frulloni L, Scattolini C, Falconi M, et al. Autoimmune pancreatitis: Differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol 2009; 104: 2288–2294. [DOI] [PubMed] [Google Scholar]
- 12.Van Buuren H, Vleggaar F, Willemien Erkelens G, et al. Autoimmune pancreatocholangitis: A series of ten patients. Scand J Gastroenterol 2006; 243: 70–78. [DOI] [PubMed] [Google Scholar]
- 13.Church NI, Pereira SP, Deheragoda MG, et al. Autoimmune pancreatitis: Clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 2007; 102: 2417–2425. [DOI] [PubMed] [Google Scholar]
- 14.Czakó L, Gyökeres T, Topa L, et al. Autoimmune pancreatitis in Hungary: A multicenter nationwide study. Pancreatology 2011; 11: 261–267. [DOI] [PubMed] [Google Scholar]
- 15.Detlefsen S, Zamboni G, Frulloni L, et al. Clinical features and relapse rates after surgery in type 1 autoimmune pancreatitis differ from type 2: A study of 114 surgically treated European patients. Pancreatology 2012; 12: 276–283. [DOI] [PubMed] [Google Scholar]
- 16.Rasch S, Phillip V, Schmid RM, et al. Epidemiology, clinical presentation, diagnosis and treatment of autoimmune pancreatitis: A retrospective analysis of 53 patients. Pancreatology 2016; 16: 73–77. [DOI] [PubMed] [Google Scholar]
- 17.Nikolic S, Brehmer K, Panic N, et al. Cardiovascular and lung involvement in patients with autoimmune pancreatitis. J Clin Med 2020; 9: E409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 19.Schneider A, Michaely H, Rückert F, et al. Diagnosing autoimmune pancreatitis with the Unifying-Autoimmune-Pancreatitis-Criteria. Pancreatology 2017; 17: 381–394. [DOI] [PubMed] [Google Scholar]
- 20.Song TJ, Kim MH, Kim MJ. et al. Clinical validation of the international consensus diagnostic criteria and algorithms for autoimmune pancreatitis: Combined IAP and KPBA meeting 2013 report. Pancreatology 2014; 14: 233–237. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramanian G, Sugumar A, Smyrk TC, et al. Demystifying seronegative autoimmune pancreatitis. Pancreatology 2012; 12: 289–294. [DOI] [PubMed] [Google Scholar]
- 22.Levy MJ, Reddy RP, Wiersema MJ, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc 2005; 61: 467–472. [DOI] [PubMed] [Google Scholar]
- 23.Buscarini E, De Lisi S, Arcidiacono PG, et al. Endoscopic ultrasonography findings in autoimmune pancreatitis. World J Gastroenterol 2011; 17: 2080–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwashita T, Yasuda I, Doi S, et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol 2012; 10: 316–322. [DOI] [PubMed] [Google Scholar]
- 25.Imai K, Matsubayashi H, Fukutomi A, et al. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis 2011; 43: 869–874. [DOI] [PubMed] [Google Scholar]
- 26.Kanno A, Ishida K, Hamada S, et al. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc 2012; 76: 594–602. [DOI] [PubMed] [Google Scholar]
- 27.Morishima T, Kawashima H, Ohno E, et al. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest Endosc 2016; 84: 241–248. [DOI] [PubMed] [Google Scholar]
- 28.Van Heerde MJ, Buijs J, Rauws EA, et al. A comparative study of diagnostic scoring systems for autoimmune pancreatitis. Pancreas 2014; 43: 559–564. [DOI] [PubMed] [Google Scholar]
- 29.Detlefsen S, Joergensen MT, Mortensen MB. Microscopic findings in EUS-guided fine needle (SharkCore) biopsies with type 1 and type 2 autoimmune pancreatitis. Pathol Int 2017; 67: 514–520. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya A, Cruise M, Chahal P. Endoscopic ultrasound guided 22 gauge core needle biopsy for the diagnosis of autoimmune pancreatitis. Pancreatology 2018; 18: 168–169. [DOI] [PubMed] [Google Scholar]
- 31.Tacelli M, Celsa C, Magro B, et al. Risk factors for rate of relapse and effects of steroid maintenance therapy in patients with autoimmune pancreatitis: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019; 17: 1061–1072. [DOI] [PubMed] [Google Scholar]
- 32.Soliman H, Vullierme MP, Maire F, et al. Risk factors and treatment of relapses in autoimmune pancreatitis: Rituximab is safe and effective. United European Gastroenterol J 2019; 7: 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamisawa T, Egawa N, Inokuma S, et al. Pancreatic endocrine and exocrine function and salivary gland function in autoimmune pancreatitis before and after steroid therapy. Pancreas 2003; 27: 235–238. [DOI] [PubMed] [Google Scholar]
- 34.Ito T, Kawabe K, Arita Y, et al. Evaluation of pancreatic endocrine and exocrine function in patients with autoimmune pancreatitis. Pancreas 2007; 34: 254–259. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto Y, Kamisawa T, Tabata T, et al. Short and long-term outcomes of diabetes mellitus in patients with autoimmune pancreatitis after steroid therapy. Gut Liver 2012; 6: 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vujasinovic M, Valente R, Maier P, et al. Diagnosis, treatment and long-term outcome of autoimmune pancreatitis in Sweden. Pancreatology 2018; 18: 900–904. [DOI] [PubMed] [Google Scholar]
- 37.Nishimori I, Tamakoshi A, Kawa S, et al. Influence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis. Pancreas 2006; 32: 244–248. [DOI] [PubMed] [Google Scholar]
- 38.Pezzilli R, Andriulli A, Bassi C, et al. Exocrine pancreatic insufficiency in adults: A shared position statement of the Italian association for the study of the pancreas. World J Gastroenterol 2013; 19: 7930–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider A, Hirth M, Münch M, et al. Risk of cancer in patients with autoimmune pancreatitis: A single-center experience from Germany. Digestion 2017; 95: 172–180. [DOI] [PubMed] [Google Scholar]
- 40.Pezzilli R, Vecchiarelli S, Di Marco MC, et al. Pancreatic ductal adenocarcinoma associated with autoimmune pancreatitis. Case Rep Gastroenterol 2011; 5: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620924302 for Multicentric Italian survey on daily practice for autoimmune pancreatitis: Clinical data, diagnosis, treatment, and evolution toward pancreatic insufficiency by Luca Barresi, Matteo Tacelli, Stefano Francesco Crinò Fabia Attili, Maria Chiara Petrone, Germana De Nucci, Silvia Carrara, Guido Manfredi, Gabriele Capurso, Claudio Giovanni De Angelis, Lucia Crocellà, Alberto Fantin, Maria Francesca Dore, Alessandra Tina Garribba, Ilaria Tarantino, Nicolò De Pretis, Danilo Pagliari, Gemma Rossi, Gianpiero Manes, Paoletta Preatoni, Ilenia Barbuscio, Fabio Tuzzolino, Mario Traina, Luca Frulloni, Guido Costamagna, Paolo Giorgio Arcidiacono, Elisabetta Buscarini, Raffaele Pezzilli and Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO), Italian Association for the Study of the Pancreas (AISP) in United European Gastroenterology Journal