Abstract
The overall objective of these guidelines is to provide evidence-based recommendations for the diagnosis and management of immunoglobulin G4 (IgG4)-related digestive disease in adults and children. IgG4-related digestive disease can be diagnosed only with a comprehensive work-up that includes histology, organ morphology at imaging, serology, search for other organ involvement, and response to glucocorticoid treatment. Indications for treatment are symptomatic patients with obstructive jaundice, abdominal pain, posterior pancreatic pain, and involvement of extra-pancreatic digestive organs, including IgG4-related cholangitis. Treatment with glucocorticoids should be weight-based and initiated at a dose of 0.6–0.8 mg/kg body weight/day orally (typical starting dose 30-40 mg/day prednisone equivalent) for 1 month to induce remission and then be tapered within two additional months. Response to initial treatment should be assessed at week 2–4 with clinical, biochemical and morphological markers. Maintenance treatment with glucocorticoids should be considered in multi-organ disease or history of relapse. If there is no change in disease activity and burden within 3 months, the diagnosis should be reconsidered. If the disease relapsed during the 3 months of treatment, immunosuppressive drugs should be added.
Keywords: IgG4-related, digestive, disease, glucocorticoids, other organ involvement, biomarkers, autoimmune pancreatitis type 1, immune-related cholangitis, cancer, diabetes mellitus
Introduction and methodology
Aim of the guidelines
The overall objective of these guidelines is to provide evidence-based recommendations for the diagnosis and management of IgG4-related digestive disease in adults and children. Target users of the guidelines are clinicians involved in the care of patients with IgG4-related digestive disease.
Literature review
A comprehensive literature search for relevant articles was performed using the PubMed, Embase, and Cochrane databases was conducted.
The following keywords were used in various combinations: “pancreas” OR “pancreatic” OR “pancreatitis” AND “autoimmune” OR “IgG4” OR “rheumatoid”; “cholangitis” OR cholagiopathy”; “other organ involvement” OR “systemic disease”. Furthermore, additional keywords were used by the working parties to accommodate their specific topics, e.g. “therapy”, “treatment”, children”, “pediatric”, “kidney”, etc. The search was limited to human subjects with language restriction to English studies until 1 September 2019. The snowball strategy, including a manual search of the references listed by studies retrieved from the online databases and from previously published reviews, was also performed to identify potential additional studies. The following inclusion criteria were used: (a) randomized or observational cohort studies, including systematic reviews, on patients with IgG4-related digestive disease, which focused on specific study questions; (b) studies published in the English language; and (c) studies available in full text.
In view of the relatively small number of studies on IgG4-related digestive disease, which is rare in everyday clinical practice, even non-randomized studies with less than 20 patients were used.
Recommendations, grades of evidence and outcome reporting
The recommendations format comprised the question, the statement, its level of evidence, strength of recommendation, and the percentage agreement of the global consensus group with the final version. Statements are followed by qualifying comments, written by each working party (WP) (a list of abbreviations is part of the supplement) and reviewed by the entire scientific board (executive committee). Relevant comments and suggestions made by the global consensus group (expert readers) were also taken into consideration.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was applied (Table S1). All participants and reviewers used a GRADE system tutorial (link on UpToDate: http://www.uptodate.com/home/grading-tutorial).
The final outcomes of the systematic reviews were discussed among the members of each WP.
The review groups provided the following for each clinical question:
Recommendation: the GRADE strength of recommendation (1=strong, 2=weak) and the quality of evidence (A=high, B=moderate, C=low).
The first meeting of the group had taken place during the United European Gastroenterology Week (UEGW) in Vienna, Austria (October 2018). The working groups were finalized and a responsible leader for each group was named together with a time manager. The Conflict of Interest forms were distributed to all participants and signed scanned copies were sent to United European Gastroenterology (UEG) central in Vienna according to UEG rules. After the meeting in Vienna, we determined WPs, members of the WP (Table S2) and proposals for questions. After all WPs completed the first draft of the guidelines, the questions and statements were distributed among the entire expert group. The questions and answers, including related comments, were uploaded to the Delphi platform and voted upon. All questions with less than 80% agreement were discussed at a meeting during the European Pancreatic Club meeting in Bergen, Norway (June 2019) with Test and Evaluation Directorate (TED) voting. The comments to all questions, and particularly those with less than 80% agreement during the TED voting, were returned to the WP. A second round of the Delphi voting was performed during autumn 2019 and the final round of discussion, including TED voting, was performed during the UEG week in Barcelona, Spain (October 2019). Following the consensus reached after the UEG week (2019) and a final round of adjustments, a first draft of the manuscript was prepared in December 2019 and was sent out to external readers and finalized according to the comments received. In addition to this written version, an interactive smartphone app was developed (free download).
The working group received endorsements and funding from UEG with Swedish Society of Gastroenterology (SGF) as the National Society leading the development of these guidelines.
Overview
Biomarkers in IgG4-related gastrointestinal diseases
IgG4-related disease of pancreas
IgG4-related diseases of liver and bile ducts
IgG4-related gastrointestinal diseases of esophagus, stomach, and bowel
Clinical manifestations and management of systemic IgG4-related diseases
IgG4-related digestive diseases in children
IgG4-related gastrointestinal diseases and diabetes mellitus
IgG4-related gastrointestinal diseases and cancer
Systemic treatment of IgG4-related digestive diseases
WP 1: Biomarkers in IgG4-related gastrointestinal disease
Q1.1 Are there serum biomarkers that can be measured to establish the diagnosis of IgG4-related gastrointestinal disease?
Statement 1.1: IgG4 serum level alone lacks sensitivity and specificity, but can be helpful to establish the diagnosis, and therefore should be measured if IgG4-related gastrointestinal disease is suspected. (GRADE 2C; strong agreement)
Comments: To diagnose IgG4-related disease, current recommendations propose a comprehensive work-up, including histology, organ morphology at imaging, serology, search for other organ involvement, and response to glucocorticoid treatment.1,2 Several groups have reported a lack of sensitivity and specificity of the IgG4 serum level to establish the diagnosis of IgG4-related disease or to distinguish from primary sclerosing cholangitis or cholangiocarcinoma.3–6 IgG4 serum levels seem to have diagnostic value when the level is higher than four times the upper level of normal, which is the case in only a minority of patients.7 One large cohort study in the UK demonstrated that only 22.4% of patients with IgG4 levels above the normal range of 1.4 mg/ml fulfilled the criteria to diagnose an IgG4-related disease. When the cut-off level was set at 2.8 mg/ml, specificity increased to 96%, whereas sensitivity was lost, and the positive predictive value was less than 50%.8 Thus, new biomarkers are urgently required, and preliminary studies have reported promising results. Next-generation sequencing identified that class-switched IgG4-positive clones were predominantly present in the IgG B-cell receptor repertoire and were able to differentiate active IgG4-related from other hepato-pancreato-biliary disease.9,10 Finally, a quantitative polymerase chain reaction (qPCR) assay was designed to analyse the IgG4/IgG RNA ratio, which demonstrates promising potential for the efficient differentiation of IgG4-cholangitis from malignancy and other inflammatory processes. Further prospective trials are needed to better understand the validity of this assay for IgG4-related disease of the hepato-pancreato-biliary system, as well as for systemic forms, although there are already recommendations to include this test into the diagnostic work-up of patients with suspected IgG4-related disease.11 Finally, circulating plasmablasts and CC-chemokine ligand 18 (CCL18) levels appear valuable in diagnosing IgG4-related diseases, and monitoring the disease course.12 In addition, confirmation of preliminary results in larger cohorts is warranted for both of these markers.
Q1.2. Does the measurement of IgG4 help to monitor the disease course?
Statement 1.2: The measurement of IgG4 serum levels to monitor the disease course may be helpful in some patients. (GRADE 2C, strong agreement)
Comments: As with its poor quality in establishing the diagnosis of IgG4-related disease serum, IgG4 levels cannot contribute to accurately monitoring disease course, nor does it sufficiently correlate with the development of complications or even with relapse.13,14 In certain cases, treatment induces normalization of elevated IgG4 levels or a dramatic decrease, whereas other patients exhibit normal IgG4 levels at initiation, as shown for patients with second-line therapy with immunomodulators.15 In another cohort, clinical response with glucocorticoid treatment was achieved and maintained, while serum IgG4 levels did not normalize in 63% of patients.16 However, relapse rates were significantly higher in patients with elevated IgG4 levels compared with patients with normalization of IgG4 (34/115, 30% versus 7/69, 10%; p=0.003).16 Thus, serum IgG4 concentrations do not appear to be reliable biomarkers of disease activity, except in a minority of patients. In one study, circulating immune complexes were reportedly useful predictors of relapses, but these results have not been confirmed yet.17 Interestingly, the IgG4/IgG RNA ratio correlated with response to glucocorticoid treatment in 20 patients with IgG4-related disease after 4 and 8 weeks, and as such, represents a promising novel biomarker to monitor the disease.9 These results, however, need to be confirmed in larger cohorts before a general recommendation on their validity can be made. Apart from biomarkers alone, activity scores such as the M-ANNHEIM-AiP-Activity-Score, including biomarkers suitable for IgG4-related diseases, may help in the future to predict and assess the effectiveness of therapies.18
Q1.3. Is the measurement of the serum carbohydrate antigen (CA) 19-9 level recommended to differentiate autoimmune pancreatitis (AIP) from pancreatic adenocarcinoma?
Statement 1.3: Elevated serum CA 19-9 levels are influenced by factors, such as cholangitis, and when used alone CA 19-9 displays limited accuracy in differentiating AIP from pancreatic adenocarcinoma, but given that it is an easy-to-perform and cheap test with acceptable sensitivity and specificity, its use integrated with other second-level diagnostics (e.g. biopsy, computed tomography (CT) scan) is encouraged. (GRADE 2C; strong agreement)
Comments: For these guidelines, AIP is referring to AIP type 1, i.e. IgG4-related autoimmune pancreatitis, unless specified otherwise. AIP type 1 is a rare disease. In contrast, pancreatic adenocarcinoma (PDAC) is a relatively frequent tumour, being the 12th most frequent cancer worldwide.19 A pancreatic mass caused by AIP type 1 and PDAC is difficult to distinguish with imaging techniques, thus biological markers may aid in differential diagnoses. CA 19-9, a cheap, simple test, is the most commonly used and best validated serum tumour marker for PDAC; it has the best accuracy in patients with advanced PDAC.20 However, the value of CA 19-9 measurement is limited if jaundice is present. Studies addressing the diagnostic accuracy of CA 19-9 to distinguish AIP from pancreatic cancer are summarized in Table 1.21–23 Using different cut-offs for CA 19-9, sensitivity and specificity ranged from 56 to 84% and 73 to 96%, respectively. Sensitivity and specificity improved by using different combinations with other parameters (CA 19-9 plus IgG420,21 or CA 19-9, eosinophil percentage, globulin and haemoglobin23), but no validation cohorts have confirmed those strategies with specific cut-offs. CA 19-9 should be considered in the diagnostic work-up, not as a single and definitive marker of the presence or absence of pancreatic adenocarcinoma, but as a test to be interpreted together with other clinical, laboratory, and imaging characteristics of the patient.
Table 1.
(Statement 1.3): Value of CA 19-9 to differentiate AIP from pancreatic cancer.
| Study | Sample | CA 19-9 cut-off(U/ml) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|
| Chang et al., 201421 | AIP: 188PDAC: 130CP: 86 | 37 | 84 | 73 | 68 | 86 |
| 85 | 74 | 91 | 87 | 83 | ||
| Van Heerde et al., 201422 | AIP: 33PDAC: 53Other diseases: 145 | 74 | 73 | 74 | – | – |
| Yan et al., 201723 | AIP: 25PDAC: 100 | 306.75 | 56 | 96 | – | – |
AIP: autoimmune pancreatitis. PDAC: pancreatic adenocarcinoma; CP: chronic pancreatitis (other aetiologies than autoimmune).
WP 2: IgG4-related disease of the pancreas
Q2.1: What are the pathological characteristics of AIP type 1?
Statement 2.1: There are four key histological features of AIP type 1: (a) lymphoplasmacytic infiltration affecting the tissue either diffusely or in a patchy manner, (b) storiform fibrosis, (c) obliterative phlebitis, and (d) increased numbers of IgG4+ plasma cells. (GRADE 1B; strong agreement)
Comments: There are four key histological features of AIP type 1.24 Lymphoplasmacytic infiltration is heavy within the lobules and focuses on medium-sized ducts. Eosinophils are often, but not always, present.25,26 The characteristic cartwheel appearance of storiform fibrosis may be present only focally, while cellular fibrosis with marked chronic inflammatory cell infiltration is usually extensively present. Cellular inflammation may become less apparent in long-standing cases or following treatment.27 Obliterative phlebitis presents as partial or complete venous obliteration or as an inflammatory nodule next to a patent artery. Elastica-van Gieson or Verhoeff staining may be helpful. Immunohistochemical staining for IgG4 is crucial for reaching a diagnosis of AIP type 1. For the diagnosis of AIP, the number of IgG4+ plasma cells should exceed 50 cells/high-power field (HPF) in surgical specimens and 10 cells/HPF in biopsy samples (average of counts in three hot spots [400×]). In addition, the IgG4/IgG ratio should be more than 40%. Although an increased IgG4 plasma cell count is an important finding, it is not diagnostic of AIP type 1 if found in isolation.28,29 Especially for the evaluation of needle biopsies, systems to categorize the likelihood of AIP (highly suggestive, probable, inconclusive) based on various combinations of features have been proposed,1,24,30,31 but require clinical validation. A biopsy showing little or no evidence of AIP cannot be used in isolation to exclude this diagnosis, unless a positive alternative diagnosis can be made.30 Biopsies from tumefactive lesions with lymphoplasmacytic infiltration should be stained for IgG and IgG4, and elevated counts should trigger clinical evaluation for AIP, regardless of the presence or absence of storiform fibrosis or obliterative phlebitis.32
While AIP type 2 shares several features with AIP type 1 (see Table 2), the presence of few or no IgG4+ plasma cells in combination with the presence of granulocytic epithelial lesions (GELs) is considered confident histological evidence. GELs are characterized by the infiltration of neutrophilic granulocytes in the duct epithelial lining, causing degenerative epithelial changes, often including epithelial detachment. The presence of an acinar infiltrate, (including neutrophils), in the absence of GELs or an elevated IgG4+ plasma cell count (≤10/HPF) is regarded as a probable diagnosis of AIP type 2.1
Table 2.
(Statement 2.1): Diagnostic microscopic features of type 1 and type 2 autoimmune pancreatitis (adapted from Zhang et al.226).
| AIP Type 1 | AIP Type 2 | |
|---|---|---|
| Periductal lymphoplasmacytic infiltrate | Present | Present |
| Inflammation of lobules | Present | Patchy,less marked, commonly admixed with neutrophils |
| Storiform fibrosis | Prominent | Occasional |
| Obliterative phlebitis | Yes | Rare |
| Lymphoid follicles | Prominent | Occasional |
| IgG4+ plasma cell infiltration | Marked | Scant or absent |
| GEL | Absent | Present |
| Inflammation of peripancreatic fat | Possible | Rare |
AIP: autoimmune pancreatitis; GEL: granulocytic epithelial lesion
Q2.2: What are the imaging features of AIP?
Statement 2.2: The classical imaging features of AIP are parenchymal enlargement, ‘sausage-like’ shape, peripancreatic edematous rim, and main pancreatic duct narrowing without upstream dilatation. These features may be diffuse or focal but can also be highly variable. (GRADE 2C; strong agreement)
Comments: The imaging features below are depicted using clinically available CT, magnetic resonance imaging (MRI), ultrasound (US), and positron emission tomography-computed tomography (PET-CT) systems.8,33–41
Parenchymal changes suggestive of AIP are:
Diffuse or (multi-) focal enlargement with loss of the normal multilobulated pattern (‘sausage-like’ shape); with diffuse involvement, more frequent in type 1 and focal involvement in AIP type 2 (Figure 1).
Altered imaging characteristics, such as lower signal intensity (SI)/echogenicity on unenhanced T1-w MRI/(E)US, respectively, moderately higher SI on T2-w MRI, impeded diffusion on MRI, and increased 18F-fluorodeoxyglucose (FDG)-uptake on PET-CT compared with normal parenchyma. Post injection of (iodine-, gadolinium-, or microbubble-based) contrast media, there is dotted/patchy enhancement in the late arterial/pancreatic phase that progressively increases towards the later vascular phases.
Rectangular shape of the tail (‘cut-tail sign’).
Thin peripancreatic edematous rim or progressively enhancing true capsule.
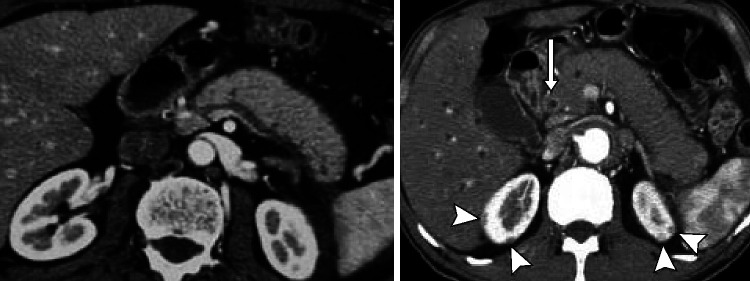
Figure 1.
CT pictures of IgG4-RD in the abdomen. Sausage-like pattern of the pancreatic gland with loss of lobulation (left). Contrast enhancement of the distal bile duct (CBD, arrow) indicating IgG4-related cholangitis (IRC). Note the typical kidney lesions (arrowhead) pathognomonic to IgG4-RD underscoring the diagnosis.225
Ductal changes suggestive of AIP are:
Long-segment (i.e. ≥1/3 of the length) or multifocal main pancreatic duct (MPD) involvement (narrowing or vanishing) without upstream dilatation or other signs of obstructive pancreatitis.
Skip lesions, i.e. ≥2 involved MPD-segments separated by a normal MPD-segment.
‘Duct-penetrating’ (i.e. visible MPD- and/or common bile duct (CBD)-lumen) and ‘icicle’ (i.e. a progressive decrease of MPD-diameter) signs within an enlarged parenchymal area.
Q2.3: What is the role of endoscopy in diagnoses of AIP type 1?
Statement 2.3: Endoscopic ultrasound (EUS) provides pancreatic imaging findings suggestive of AIP and is used for obtaining tissue samples for the histological diagnosis of the disease. (GRADE 2B; strong agreement)
Comments: EUS plays a major role in the diagnosis of AIP and IgG4-related cholangitis (IRC).1,42–45
AIP must be differentiated from pancreatic carcinoma.1,42–46 Pancreatography findings, such as a long narrowing of the main pancreatic duct (>1/3 the length of the MPD), lack of upstream dilatation, skipped narrowed lesions, and side branches arising from the narrowed portion, suggest AIP rather than pancreatic carcinoma.1,42–45 IgG4-immunostaining of biopsy specimens obtained from the major duodenal papilla supports the diagnosis of AIP.1,43,45 IRC must be differentiated from cholangiocarcinoma and primary sclerosing cholangitis (PSC).43–45 Specific cholangiography findings support the diagnosis of IRC (see below).43–45 IgG4-immunostaining of trans-papillary biopsy specimens of bile duct strictures may help exclude cholangiocarcinoma and support the diagnosis of IRC.43–45 EUS may demonstrate diffuse hypoechoic pancreatic enlargement and other features of pancreatic disease in patients with AIP.43–45 EUS-guided tissue acquisition is used for obtaining adequate tissue samples for the histological diagnosis of AIP and to exclude pancreatic carcinoma.1,43–45 EUS-guided tissue acquisition with a core biopsy, with a 19-gauge needle, is recommended, but even use of a 22-gauge needle for a sample allowing histological evaluation can be obtained.43–45 EUS and intraductal ultrasonography may show wall thickening of the CBD in patients with IRC (see below).43–45
Q2.4: What is the role of surgery in AIP type 1?
Statement 2.4: Surgery is generally not indicated for AIP type 1. Surgery might be considered in patients when suspicion of pancreatic cancer cannot be excluded after complete diagnostic work-up. (GRADE 2B; strong agreement)
Comments: Diagnosis of AIP is not always straightforward and, in some cases, it is not easy to differentiate it from pancreatic cancer. Furthermore, an incidence of concomitant pancreatic tumours (benign and malign) in patients with AIP has been reported in up to 7% of cases.47 The International Study Group of Pancreatic Surgery consensus statement reported that 5–13% of patients undergoing surgical resections because of suspected malignancy had benign findings on pathology, with AIP accounting for 30–43% of these findings.48 In a retrospective study, including pathological analysis of 274 patients who underwent pancreaticoduodenectomy because of presumed malignancy, the prevalence of benign disease was 8.4%, and overall prevalence of AIP was 2.6%.49 European patients treated with pancreatic resection with a postoperative diagnosis of AIP were included in a multicentre study. There were 63 patients with AIP type 1 who underwent operations due to suspected pancreatic cancer, intractable pain, or jaundice (or a combination of these symptoms or signs). Relapse of disease after surgery was 41.2%.50 In 74 patients from a North American series undergoing pancreatectomy with the final diagnosis of AIP, the long-term relapse rate of 17% was much lower.51 The long-term surgical outcomes of 13 patients with pathologically diagnosed type 1 AIP with immunohistochemical staining for IgG4 were retrospectively compared with those of 34 patients with conventional chronic pancreatitis to evaluate the residual pancreatic function in Japanese patients.52 Relapse of AIP, in terms of the clinical manifestations and diagnostic imaging, was not found in any of these patients during the postoperative course. A study from the USA reported that eight out of 29 (28%) patients with AIP developed recurrence after resection: seven with jaundice and one with recurrent pancreatitis (median time to recurrence, 11 months; median follow-up, 38 months).53 A possible cause of these discrepancies in the reported recurrence rate of AIP after surgery may be the difficulty in diagnosing a recurrence of AIP in the remnant pancreas.52 However, careful long-term follow-up is needed for patients undergoing pancreatectomy for AIP type 1, as the disease may return or remnant pancreatic function can deteriorate as severely as that of patients who undergo pancreatectomy for conventional chronic pancreatitis.52
Q2.5: What is the expected outcome and optimal follow-up of patients with AIP type 1?
Statement 2.5.1: AIP is a special and treatable form of chronic pancreatitis with a good response to initial glucocorticoid therapy, but high rates of disease relapses. Other organ involvement (OOI), defined as the presence of extra-pancreatic disease, is common. (GRADE 1A; strong agreement)
Statement 2.5.2: Long-term sequelae, such as exocrine and endocrine insufficiency, often occur in patients with AIP type 1. (GRADE 1B; strong agreement)
Statement 2.5.3: Screening for a deficiency of fat-soluble vitamins (A, D, E, and K), zinc, calcium, and magnesium should be considered in line with UEG evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU).54 (GRADE 2A; strong agreement)
Statement 2.5.4: Life-long follow-up of patients with AIP type 1 is advisable. (GRADE 2C; strong agreement)
Comments: AIP is a special form of chronic pancreatitis with a good response to glucocorticoid therapy, but high rate of disease relapses.13,55,56 Relapse occurrence in European studies varies from 7% to 55%.57–62 OOI, defined as the presence of extra-pancreatic disease, is reported in 47–84% of patients in different European studies.57,59–62 The likelihood of pancreatic exocrine insufficiency with all consequences is further increased in patients with chronic pancreatitis, diabetes mellitus, and after pancreatic resections.54 Deficiency of fat-soluble vitamins, namely magnesium, zinc, calcium, iron, haemoglobin, albumin, and prealbumin, have been associated with pancreatic insufficiency.54,63–66 Patients with all aetiologies of chronic pancreatitis, including those of autoimmune cause, are also at high risk of developing osteoporosis and osteopenia, especially in patients with glucocorticoid treatment.54,67,68 Pancreatic duct stones were reported in 5–40% of patients with AIP.69,70 Known (exocrine and endocrine insufficiency, pancreatic duct stones) long-term sequelae require ongoing surveillance to further understand their full clinical significance and, at this point, yearly life-long follow-up of patients with AIP type 1 is advisable.
WP 3: IgG4-related diseases of liver and bile ducts
Q3.1. What is the definition and proposed nomenclature of IgG4-related hepatobiliary disease?
Statement 3.1: The most common manifestation of IgG4-related hepatobiliary disease is IgG4-related cholangitis. (GRADE 2C; strong agreement)
Comments: Immunoglobulin G4-related hepatobiliary disease is the hepatobiliary manifestation of IgG4-related systemic disease. IgG4-related hepatobiliary disease mostly manifests as glucocorticoid-responsive cholangitis of the extrahepatic and perihilar bile ducts, but the intrahepatic bile ducts can also be involved. IgG4-related hepatobiliary disease is often associated with other organ manifestations of IgG4-related disease, in particular autoimmune pancreatitis type 1.71 Glucocorticoid-responsive cholangitis is the most common manifestation of hepatobiliary disease, but inflammatory pseudotumours of the liver and biliary cirrhosis can also develop as a late-stage manifestation of this condition. There is considerable doubt that an IgG4-related hepatitis exists as a primary manifestation of IgG4-related disease, partly because IgG4+ plasma cell tissue infiltrations can be found in the setting of various pathological conditions independent of IgG4-related disease.72 The HISORt criteria proposed by Ghazale et al.73 as diagnostic criteria for IgG4-related cholangitis include histopathological and imaging features, high serum IgG4 levels, other organ involvement (e.g. pancreas, salivary/lacrimal/thyroid glands, lungs, mediastinal and abdominal lymph nodes, retroperitoneum, aorta, kidneys, ureters, prostate or testes), and response to glucocorticoid therapy. IgG-related cholecystitis also occurs. There is an ongoing debate over the nomenclature of IgG4-related hepatobiliary disease. Among others, IgG4-related cholangitis has been named IgG4-associated cholangitis,71,73,74 IgG4-related sclerosing cholangitis,75,76 autoimmune cholangitis (in the past defined as anti-mitochondrial antibodies (AMA)-negative primary biliary cirrhosis,74 thus confusing), or IgG4 cholangiopathy. Worldwide use of the same term appears highly desirable. The disease is completely reversible under early glucocorticoid treatment, favouring the terms IgG4-related cholangitis (IRC), IgG4-associated cholangitis (IAC), or IgG4 cholangiopathy (IC), whereas more advanced and irreversible stages are reflected by the term IgG4-related sclerosing cholangitis (IgG4-SC). The term IgG4-SC creates associations in affected patients with PSC and, in particular, with serum IgG4-positive PSC, a progressive disease with dismal prognosis. In analogy with the name change from cirrhosis to cholangitis in primary biliary cholangitis, formerly primary biliary cirrhosis,77 we advocate, for the sake of clarity for our patients, future use of the more benign term, IgG4-related cholangitis (IRC), a compromise between IAC and IgG4-SC. Accordingly, IgG4-related hepatopathy (including IgG4-related hepatic pseudotumours) and IgG4-related cholecystitis are the nomenclature suggested for liver and gallbladder involvement. It remains unclear if IgG4-related hepatopathy truly is a manifestation distinct from biliary tract involvement or rather a consequence of IgG4-related cholangitis.
Q3.2. What are the clinical, biochemical, pathological, and radiological characteristics leading to the diagnosis of IgG4-related cholangitis?
Statement 3.2: Jaundice, a cholestatic serum enzyme profile, includes elevated serum IgG4 concentrations, histological features (including lymphoplasmacellular infiltrates with >10 IgG4+ plasma cells/HPF, storiform fibrosis, and/or obliterative phlebitis), and extrahepatic, hilar, and/or intrahepatic bile duct strictures, which are characteristic features of IgG4-related cholangitis. (GRADE 2C; strong agreement)
Comments: Clinical signs and symptoms of IRC include jaundice (mostly painless), pruritus, weight loss, and abdominal discomfort. IgG4-related disease is often associated with diabetes mellitus.73,78 Decompensated biliary cirrhosis or cholangiocarcinoma are very rare at time of diagnosis.73,78 Biochemical characteristics of IRC are elevation of serum markers of cholestasis, including alkaline phosphatase, gamma-glutamyl transferase, and conjugated bilirubin. The ‘tumour marker’ CA19-9 can be excessively high in IRC and responds rapidly to glucocorticoid treatment. Serum IgG4 is elevated in about 75% of patients and reliable for the diagnosis of IgG4-related disease when above 4× the upper limit of normal.3,6 Moderate elevation of serum IgG4 is also described in about 10% of patients with PSC, cholangiocarcinoma, pancreatitis, and pancreatic carcinoma.3,6,9 The blood IgG4/IgG RNA ratio, as determined using qPCR, showed excellent accuracy for the diagnosis of IRC (in comparison to PSC and cholangio-/pancreatic carcinoma) in one study9 but was rebutted in a large observational study with more than 200 patients.79 Clearly, it requires prospective re-evaluation in well-defined cohorts for further characterization. Histopathological criteria of IRC include lymphoplasmacellular infiltrates including >10 IgG4+ plasma cells per HPF (defined as microscopic visible area under 400-fold magnification), storiform fibrosis, and obliterative phlebitis.24 Minor criteria include eosinophilia and partial obliterative phlebitis.24
The diagnosis of IRC may be difficult without histological sampling and, as it happens under ongoing steroid therapy, since then IgG4 may be already within normal range.80,81 The measurement of IgG subclass 2 (IgG2) may help in confirming the diagnosis of IRC, as demonstrated in a recent study.82
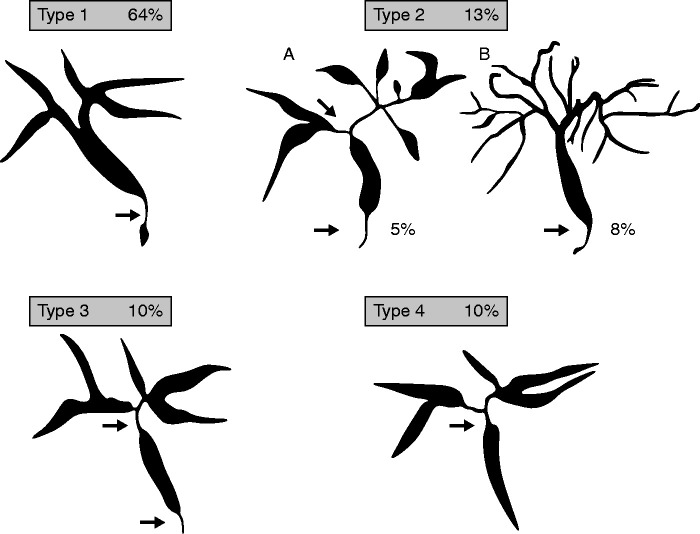
Cholangiographic characteristics and a classification are shown in Figure 2.83 Lower strictures of the CBD without strictures of upstream bile ducts represent the most common finding (type 1). Intrahepatic segmental (type 2a) and diffuse (type 2b) strictures, in addition to a lower CBD stricture, when taken together represent the second most common finding. The combination of hilar and lower CBD strictures (type 3) and hilar strictures only (type 4) are additional variants.83
Figure 2.
Classification of IgG4-related cholangitis83 (related to statement 3.2).
Q3.3. Is glucocorticoid treatment response indispensable for the diagnosis of IgG4-related hepatobiliary disease?
Statement 3.3: Treatment response is regarded as a major diagnostic criterion but is not indispensable for the diagnosis of IgG4-related cholangitis. (GRADE 2C; strong agreement)
Comments: To affirm that treatment response to glucocorticoids represents a conditio sine qua non for the diagnosis, it is necessary to retrieve the evidence that almost 100% of patients are responders or that treatment failure unequivocally excludes a definite diagnosis of IgG4-related hepatobiliary disease. Based on available literature, the question can be answered only as far as IRC is concerned. No randomized controlled study on the short-term treatment of IRC has been performed and no randomized controlled trial has been performed testing glucocorticoid-response as a criterion for the diagnosis of IRC. Two prospective studies84,85, six retrospective, observational cohort studies (>20 patients each),73,78,86–89 and one systematic review90 evaluating the response to glucocorticoids have been considered for analysis; different studies have been excluded, as these were non-randomized and with less than 20 patients enrolled. These studies73,78,84–90 showed differences in inclusion criteria and bile duct involvement, definition of response (clinical, biochemical, radiologic), type of glucocorticoids, doses and length of treatment, modalities of tapering, and additional treatments (surgery, stenting). Nonetheless, these studies demonstrated a rate of response from 62 to 100% with a relapse of approximately 30% during glucocorticoid tapering or after withdrawal of glucocorticoids. Despite advances in the initial treatment and tapering/maintenance of glucocorticoids, current available studies show how, based on biliary tree imaging, treatment failure exists in a minority of cases associated with a more fibrotic phenotype, multiple bile duct strictures, and multi-organ involvement. We believe that further research is unlikely to change our confidence in the estimate of benefit and risk.
Q3.4. What are the evidence-based manifestations of IgG4-related hepatobiliary disease in addition to IgG4-related cholangitis?
Statement 3.4: IgG4-related cholangitis and IgG4-related hepatic pseudotumours are hepatic manifestations within the spectrum of IgG4-related disease. Other histopathological features of liver tissue might also be interpreted as reactive changes due to IgG4-related cholangitis or autoimmune pancreatitis. (GRADE 2C; strong agreement)
Comments: In patients with AIP, five manifestations of liver involvement were defined: (a) portal inflammation with or without interface activity, (b) lobular hepatitis, (c) portal sclerosis, (d) lobular (perivenular) cholestasis, and (e) (large) bile duct obstructive pattern.91 However, it may be very difficult to distinguish primary hepatic involvement of IgG4-related disease, including IRC, from reactive obstructive changes in the liver secondary to AIP. The lobular hepatitis pattern is considered by some as a separate entity, resembling classical autoimmune hepatitis (AIH), with an increasing number of IgG4-positive plasma cells. One European and two Japanese cohorts described an IgG4-associated AIH.92–94 However, diagnostic criteria varied between the studies and the number of cases was limited, so the clinical relevance of IgG4-associated AIH remains unclear in the context of IgG4-related disease and deserves further study. After liver transplantation, the occurrence of a ‘post-transplant de novo AIH’ has been described, sometimes called ‘plasma cell hepatitis’, with an important contribution of IgG4-positive plasma cells. The relationship of this entity to IgG4-related disease, if any, is highly questionable. Characteristic features, such as storiform fibrosis or obliterative phlebitis, are lacking.95 Finally, the occurrence of inflammatory (lymphoplasmacytic) IgG4-positive pseudotumours is reported in the liver in the context of IRC.96–99 In conclusion, different manifestations of hepatic involvement of IgG4-related disease are reported mostly in small, retrospective cohort studies or case reports in which definitions of disease entities varied between authors. Distinction between reactive changes due to AIP and/or IRC and primary hepatic manifestations of IgG4-relate disease is often difficult.
WP 4: IgG4-related gastrointestinal disease of esophagus, stomach, and bowel
Q4.1. How often do IgG4-related diseases occur in the esophagus, stomach, and bowel?
Statement 4.1: Involvement of esophagus, stomach, and bowel in IgG4-related disease is rare or non-existing. (GRADE 2C; strong agreement)
Comments: Only single clinical observations100–102 and small series (groups)102-105of patients are presented. Topal et al.105 detected IgG4-positive plasma cell staining in the colon biopsies of 21 out of 119 (17.6%) patients with inflammatory bowel disease without AIP. Of these 21 patients, five displayed elevated serum IgG4 levels (>140 mg/dl). Of the total, 4.2% (5/119) had both IgG4-immunstaining and elevated IgG4 serum levels. Obiorah et al.103 evaluated chronic esophagitis specimens with lymphoplasmacytic infiltrate obtained over 6 years using a chart review. IgG4 immunohistochemical staining of these specimens confirmed the diagnoses of IgG4-related esophagitis in eight out of 18 patients. Notohara et al.106 reported seven clinical cases, found through a multicentre-survey (these cases were found incidentally, detected radiologically or pathologically). Sporadic cases were reported also in small bowel (one case),100 pouchitis (two cases),102 ileocecal region (one case),101 and rectum (one case).107
Q4.2. What are the typical clinical features and diagnostic criteria of IgG4-related disease in the esophagus, stomach, and bowel?
Statement 4.2.1: Typical clinical features and diagnostic criteria of IgG4-related disease of the esophagus, stomach, and bowel have only rarely been reported – and the reported cases are often incomplete regarding diagnostic criteria. (GRADE 2C; strong agreement)
Comments 4.2.1: Based on the small observational reports related to esophagus and stomach, histological criteria are present in some cases, the same holds true for IgG4-positive cells above the threshold of 50/HPF and IgG4/IgG ratios above 40% as well as elevation of serum IgG4 above 1.5 times the upper limit of normal.1,2 For the bowel, only single case reports are available, and the histological criteria are rarely reported. Also, serum IgG4 is rarely reported/increased for the bowel.105,108 Most cases are mainly based on increased IgG4-positive cells,100,105,107–109 often without reported IgG4/IgG ratios.107,108 Thus, there remains major uncertainty regarding organ involvement of the esophagus, stomach, and intestine in IgG4-related disease.
Q4.3: What should be the treatment approach for IgG4-related disease of the esophagus, stomach, or bowel?
Statement 4.3.1. Pharmacological therapy of IgG4-related disease of the gut is based on the same principles as IgG4-related disease of other organs. (GRADE 2C; strong agreement)
Statement 4.3.2. In a patient with gastrointestinal mass lesion and equivocal/nondiagnostic histology for IgG4-related disease with negative malignant cells, empirical treatment with glucocorticoid for 1 month may be a suitable option. (GRADE 2C; strong agreement)
Comments: Most of the cases reported in the literature describe patients who were operated on for small bowel masses without preoperative testing for IgG4 serum level, and the only indicator for the presence of IgG4-related disease was postoperative histology. Subsequently, patients received no glucocorticoid or other immune-suppressive medications and had short follow-up, if any.100,110–114 IgG4- related disease of the gut, in many cases, was diagnosed postoperatively for presumed tumour, and patients did not receive any specific treatment with less than 6 months of follow-up. Most patients were treated with surgical or endoscopic resection, whereas a minority received glucocorticoids and/or immunosuppressants. In most cases, where glucocorticoid therapy was initiated, patients exhibited good treatment response, though it was incomplete in long-standing lesions, which could be explained by the prominent fibrotic component of the lesion. IgG4-related lesions of the stomach may respond to antisecretory drugs.115 In few cases, patients received maintenance treatment with immunosuppressive agents (mycophenolate, cyclosporine, azathioprine), which appeared effective.116–118 Careful monitoring of patients is also required, as in the natural history of IgG4-related disease, further lesions may appear as late as years after initial manifestation is diagnosed and could be located in different organs.119
WP 5: Clinical manifestations and management of systemic IgG4-related diseases
Q5.1 What is the spectrum of organ involvement and clinical presentations in IgG4-related disease?
Statement 5.1.1: Clinical manifestations of IgG4-related disease are extremely variable depending on the type and number of organ/tissues involved. (GRADE 1A; strong agreement)
Statement 5.1.2: IgG4-related disease is a systemic condition typically involving two or more organs. (GRADE 1B; strong agreement)
Statement 5.1.3: The most frequently involved organs are: the pancreato-hepatobiliary tract, salivary and lacrimal glands, the retroperitoneum, kidneys, lungs, and aorta. (GRADE 1A; strong agreement)
Comments: IgG4-related disease is a systemic condition with ≥2 organs involved in as much as 75% of patients in large case series.120–128 Different organ involvement can occur at the same time or metachronously (for example, at relapse). The patient’s history should be screened cautiously, as previous medical issues often reveal unrecognized manifestations of this condition. IgG4-related disease classically affects middle-aged individuals, but paediatric cases have been described (see WP 6). IgG4-related disease clinical manifestations are related to either the tumoural mass, the stricture of tissues and/or organs by the tumour, or signs of organ dysfunction. Sustained fever and constitutional symptoms are not inherently associated with IgG4-related disease, but can occur as complications of the disease, e.g. ascending cholangitis occurring in the setting of damaged bile ducts.122 IgG4-related disease typically presents in the form of a tumour-like lesion leading to compression of adjacent organs, strictures, and, eventually, organ dysfunction. Neurological symptoms, for instance, may occur in cases of meningeal involvement, and abdominal, flank, and/or lower back pain may occur in cases of retroperitoneal fibrosis.129 Tumour masses can be identified by clinical examination or imaging studies.129 According to the largest international cohorts of patients with IgG4-related disease, the most frequently involved organs are: the pancreato-hepatobiliary tract (∼45%), major salivary glands (∼37%), the lacrimal gland (∼26%), the retroperitoneum (∼15%), the kidneys (∼15%), the lungs (∼14%), and the aorta (∼10%).122 IgG4-related disease is also frequently associated with enlarged lymph nodes, but the pathological relevance of isolated lymph node involvement in the absence of other characteristic IgG4-related disease manifestations remains to be fully elucidated. Other localizations of IgG4-related disease include: arteries (other than aorta), orbits, meninges, prostate, testicles, skin, nasal sinuses, mesentery, mediastinum, pericardium, pleura, peripheral nerves, bones, and muscles.120–122,124–128,130 Based partly on organ involvement, four clinical phenotypes of IgG4-related disease have been proposed: (a) pancreato-hepato-biliary disease; (b) retroperitoneal fibrosis and/or aortitis; (c) head and neck-limited disease; and (d) classic Mikulicz syndrome with systemic involvement. There is considerable overlap in several of these proposed phenotypes, however, and the biological basis (and ultimate validity) of the proposed phenotypes is not clear. IgG4-related disease phenotypes differ in terms of demographical and serological features. The ‘head and neck’ phenotype, for instance, is more frequently observed in female patients, although the overall prevalence of IgG4-related disease is higher in male individuals. ‘Mikulicz syndrome with systemic involvement’ is the IgG4-related disease phenotype associated with higher levels of serum IgG4.122
Q5.2 What is the optimal diagnostic work-up and follow-up strategy for IgG4-related disease?
Statement 5.2.1: The most accurate diagnostic assessment of IgG4-related disease is based on a full clinical history, physical examination, laboratory investigations, pathology, and imaging studies. Life-long follow-up of patients with IgG4-related disease is advisable. (GRADE 1B; strong agreement)
Statement 5.2.2: Whenever possible the diagnosis of IgG4-related disease should be confirmed by pathological examination from a guided biopsy. (GRADE 1A; strong agreement)
Statement 5.2.3: Patients with systemic manifestations of IgG4-related disease should be followed over time with specific serological exams and imaging studies depending on the spectrum of organ involvement, as well as with the IgG4-RD Responder Index. (GRADE 1C; strong agreement)
Comments: Appropriate diagnostic work-up and follow-up strategies for systemic IgG4-related disease requires integrated information from clinical history, physical examination, laboratory investigations, pathology findings, and imaging studies.131 Available biomarkers, in fact, are not sufficiently accurate for either diagnostic purpose or for longitudinal assessment.131 Serum IgG4 measurement, for instance, the most widely used biomarker of IgG4-related disease, has a specificity of 60% and a positive predictive value of only 34%, being elevated in several other inflammatory disorders and normal in up to 50% of patients with IgG4-related disease (see also WP1; Q1). Circulating plasma blasts, T-follicular helper cells, and CCL18 serum levels are associated with disease activity, but their utility as disease biomarkers has not been validated in prospective studies.132–137 Similarly, the IgG4/IgG RNA ratio on peripheral blood (see also WP1; Q1) reliably distinguishes pancreato-biliary IgG4-related disease from PSC or cholangiocarcinoma, but its utility in the evaluation of patients with extra-gastrointestinal manifestations of IgG4-related disease has never been confirmed. Definitive diagnosis of IgG4-related disease, therefore, still relies on pathological examination of biopsy specimens, and requires fulfilment of the organ-specific criteria outlined in the ‘Consensus statement on the pathology of IgG4-RD’. Conversely, comprehensive evaluation of blood test, imaging, and functional studies remains the cornerstone for an appropriate follow-up strategy. Laboratory exams should include complete blood cell count, liver and renal function tests, serum protein electrophoresis, and measurement of IgG subclasses and complement. Imaging studies – including CT, MRI, and US – should be decided according to the spectrum of organs involved.131 Finally, functional assessment through 18F-FDG PET/CT can be used to distinguish active disease from chronic fibrotic damage and to reveal asymptomatic localizations of IgG4-related disease.132,136–139 The IgG4-RD Responder Index (IgG4-RD RI, Table S3) currently represents the only validated score to monitor IgG4-related disease activity and to combine all aforementioned clinical, serological, and radiological information.140 In particular, the IgG4-RD RI collects information regarding disease activity (through a 0–3 organ/site score), symptoms, need for urgent care, and organ damage. An IgG4-RD RI score of ≥3 was recently used to identify patients with active disease.140
Q5.3 How do we assess disease activity and differentiate chronic damage from active lesions in IgG4-related disease?
Statement 5.3.1: There is no reliable biological marker to assess disease activity on its own. (GRADE 1A; strong agreement)
Statement 5.3.2: IgG4-RD Responder Index can assess changes in multi-organ disease activity and is now being used in multicentre clinical trials. (GRADE 1C; strong agreement)
Statement 5.3.3: The most accurate assessment of IgG4-related disease activity relies on the combination of findings from physical examination, laboratory exams, histology, and imaging studies. (GRADE 1B; strong agreement)
Comments: Reliable assessment of disease activity and end-stage disease-related fibrosis poses significant challenges to clinicians due to the multi-organ nature of this condition. A combination of factors is typically assessed to define IgG4-related disease activity, but none of these alone is sufficiently specific and sensitive from patient to patient to reliably capture the overall disease status. Different biomarkers have been proposed to reflect IgG4-related disease activity, including serum IgG4 levels, circulating plasmablasts, the IgG4/IgG RNA-ratio, CCL18, complement, organ-specific enzymes, and renal function in peripheral blood.9,12,121,123,124,141–147 Plasmablasts are elevated in a proportion of patients presenting with normal serum IgG4 levels at diagnosis and normalize with disease remission in most cases.12,133,144,148,149 Imaging is complementary and represents a reliable tool for assessing systemic involvement of IgG4-related disease, response to immunosuppressive therapy, and disease relapse. US (for salivary glands), contrast enhanced CT scan, and MRI play central roles in differentiating active disease from organ-specific damage related to fibrosis.150–154 In general, active tissue inflammation and end-stage fibrosis display characteristic radiologic features.152 18F-FDG uptake reflects the pathological expansion of circulating plasmablasts, rather than processes related to fibroblast activation, and is useful to differentiate IgG4-related disease activity from end-stage fibrosis.132,137,138,153,154 IgG4-RD RI represents a promising tool for evaluating IgG4-related disease activity in a systematic manner, by integrating clinical, laboratory, and imaging information.140 Experience with sequential assessment of the IgG4-RD RI in randomized clinical trials, however, remains limited.
WP 6: IgG4-related digestive diseases in children
Q6.1: What is the prevalence of IgG4-related digestive disease in children?
Statement 6.1: There are currently insufficient data regarding prevalence of IgG4-related digestive disease in children. IgG4-related digestive disease is extremely rare in childhood. The most common IgG4-related digestive disease in the paediatric population is AIP type I, which is rare, but the accurate prevalence remains unknown. (GRADE 2C; strong agreement)
Comments: IgG4-related digestive diseases are increasingly recognized; however, the underlying aetiology remains unclear. The average age of patients with IgG4-related disease is reported to be older than 50 years,155 and data in the paediatric population is limited. Improved awareness may increase detection of IgG4-related disease in children. Our systematic literature search on paediatric patients with IgG4-related digestive disease indicated that there are fewer than 100 published cases to date; with regard to pancreatitis, these are AIP type 2.156–161
Q6.2: What is the difference in diagnosis of IgG4-related digestive diseases in childhood compared with adults?
Statement 6.2.1: There are currently insufficient data regarding differences in diagnosis of IgG4-related digestive disease in children. The diagnosis of IgG4-related digestive disease in children should be based on adult criteria, in the absence of paediatric consensus on diagnostic criteria. (GRADE 2C; strong agreement)
Comments: One characteristic of IgG4-related digestive disease is unexplained enlargement of one or more organs in both children and adults. Compared with adult patients, in whom malignancy must be excluded, cancer is a rare diagnosis in children, in whom infectious and other inflammatory disorders are more common. In adults elevated IgG4 serum levels and diagnosis of AIP type I are more common than in children, where most published cases are AIP type II.158–160 In the diagnostic work-up, obtaining proper biopsies is challenging due to the difficulty of EUS or endoscopic retrograde cholangiopancreatography (ERCP) in children. One of the reasons the IgG4 cut-off level is proposed is to distinguish IgG4-related diseases from other conditions, like malignancy (including cholangiocarcinoma and pancreatic cancer), that do not affect children in the same extent as adults.162 The application of adult cut-off values for IgG4 may be inappropriate for paediatric patients, but there are no data available. Some 70% of the histologically confirmed cases displayed elevated IgG4 serum levels compared with 48 paediatric AIP cases, where elevated serum IgG4 levels were observed in 9/48 (22%) of spatients.158
Comments: Current classification of paediatric autoimmune liver diseases comprise AIH and autoimmune sclerosing cholangitis, which is a form of sclerosing cholangitis with strong autoimmune features overlapping with AIH, originally described at paediatric age and affecting children more frequently than adults.163 The extent of IgG4-related component involvement in these conditions remains unclear. Evaluation for IgG4-related histopathological features should be considered in children with diagnosed autoimmune liver disease and concomitant AIP or other IgG4 disorders. Growing awareness and prospective studies are mandatory to establish proper definitions, improve understanding of pathogenesis and natural course, and assess response to treatment in paediatric IgG4-related liver disorders.
Q6.3: What are the differences in approaches to treatment of IgG4-related digestive disease in children as opposed to adults?
Statement 6.3.1: There are currently insufficient data regarding different treatments of IgG4-related digestive diseases in children. (GRADE 2C; strong agreement)
Statement 6.3.2: There are currently insufficient data on the differences in treatment of IgG4-related liver disorders in children compared with adults. (GRADE 2C; strong agreement)
Comments: Because of the lack of data, it is not possible to provide evidenced recommendations for treatment of IgG4-related liver diseases. Nevertheless, in IRC it seems prudent to follow current paediatric guidelines, where glucocorticoids, immunomodulators, and ursodeoxycholic acid remain the mainstay of treatment.164
Indications for ERCP with balloon dilatation are limited and may be considered individually in cases of dominant or symptomatic biliary strictures.164,165
Q6.4: What are the clinical manifestations of AIP type I in children?
Statement 6.4.1: The classic form of AIP (type 1) is rarely diagnosed in childhood. The diagnosis of AIP, in the absence of paediatric consensus on diagnostic criteria, should be carried out at a specialized paediatric pancreatic centre based on adult criteria. There is currently insufficient data about transition from AIP to chronic pancreatitis in the paediatric population. (GRADE 2C; strong agreement)
Statement 6.4.2: Children with AIP type 1 may present acutely with jaundice, pancreatic mass, pain, vomiting, and weight loss. Patients usually respond well to glucocorticoid therapy with a lower likelihood of recurrence. Some paediatric patients may exhibit resolution of symptoms without any treatment. (GRADE 2C; strong agreement)
Statement 6.4.3: Transabdominal ultrasonography is recommended as the ‘first step’ in a diagnostic work-up. In the suspicion of AIP, presence of pancreas enlargement, or pancreatic mass lesions with jaundice, magnetic resonance cholangiopancreatography (MRCP) and/or EUS is recommended. (GRADE 2C; strong agreement)
Statement 6.4.4: Pancreatic biopsy is not necessary to start immunosuppressive treatment. (GRADE 2C; strong agreement)
Statement 6.4.5: Glucocorticoids are the first line of treatment for remission induction, unless there are contraindications for their use. Children with AIP type 1 inflammation with low disease activity at the beginning of treatment do not require any maintenance treatment. (GRADE 2C; strong agreement)
Comments: Published data regarding AIP incidence in children are limited and AIP type 2 is predominant (not associated with IgG4). Due to sporadic case reports of AIP and rarely elevated IgG4 levels in children with a final diagnosis of AIP type 1, the diagnosis may be more difficult than in adults.158–160 Paediatric AIP is often characterized by sudden onset with variable symptoms, most commonly including painless jaundice and general weakness. Abdominal pain, if any, is mild, whereas episodes of acute pancreatitis are rarely the first manifestation of the disease. Imaging studies often reveal diffuse enlargement or focal changes in pancreas. AIP can also be asymptomatic, with abnormalities present only in laboratory tests and diagnostic imaging. Some paediatric patients may experience resolution of symptoms without any therapy. However, there are no long-term data comparing complications or recurrence rates with and without treatment.166 Description of IgG4 tissue infiltration is rare in published paediatric AIP cases.158–160,166,167 EUS-guided biopsy or brushings obtained from ERCP should be considered in cases of pancreatitis associated with pancreatic mass;168 however, is not necessary for initiation of treatment, mostly because of the very low incidence of tumours in this population.169 There are currently no data regarding the number of IgG4+ plasma cells per HPF that should be present in different gastrointestinal tissues in children. Among 48 AIP paediatric patients, collected from literature review and International Study Group of Pediatric Pancreatitis: In search for a cure (INSPPIRE) database, lymphoplasmacytic inflammation, pancreatic fibrosis, and ductal granulocyte infiltration were the main histological findings in 18/25 patients (72%).158
INSPPIRE recommends oral prednisolone as a first-line treatment at a dose of 1–1.5 mg/kg/day to maximum 40–60 mg given in one or two divided doses for 2–4 weeks. Thereafter, the dose should be tapered. In case of relapse, a new course of prednisolone is recommended.166
WP 7: IgG4-related digestive disease and diabetes mellitus
Q7.1 What is the prevalence of diabetes mellitus in IgG4-related disease of the pancreas?
Statement 7.1: Diabetes mellitus is very common in IgG4-related disease of the pancreas (AIP type 1). Its prevalence ranges between 21% and 77%. (GRADE 2C; strong agreement)
Comments: Diabetes mellitus associated with autoimmune pancreatitis is a ‘pancreatogenic’ form of diabetes mellitus. There are very few studies and small case series addressing the prevalence of diabetes in IgG4-RD of the pancreas. They all report a rather high prevalence of diabetes mellitus in patients with type 1 AIP, ranging from 21% in a Swedish study population61 up to 77% in a Japanese study population.170 Most patients seem to develop diabetes mellitus simultaneously with the onset of type 1 AIP (53%). However, there is a subset of patients exhibiting diabetes mellitus before AIP type 1 onset (33%) and a subset of patients developing or exacerbating diabetes mellitus as a complication of glucocorticoid treatment.171–173 Unlike the course of classical chronic pancreatitis, its prevalence appears to not rise with the duration of the disease, possibly as glucocorticoid treatment might ameliorate the disease course. Notably, in a study investigating glucose tolerance and diabetes mellitus in patients with IgG4-related disease, with AIP ruled out, 52% of cases suffered from diabetes mellitus and a further 17% displayed impaired glucose tolerance, with insulin secretion being preserved in most patients and a high prevalence of glucagon hyper-reactivity.174
Q7.2 Are there any features of IgG4-related disease of the pancreas associated with the risk and/or severity of diabetes mellitus?
Statement 7.2: Among patients with IgG4-RD of the pancreas (AIP type 1), radiologically defined pancreatic atrophy, pancreatic exocrine insufficiency, age, and smoking are all associated with a significantly higher risk of diabetes mellitus, while there are no specific data on features associated with the severity of diabetes mellitus. (GRADE 2C; strong agreement)
Comments: There are few studies investigating features associated with the risk of diabetes mellitus in patients with IgG4-related disease. Most of these studies investigate patients with AIP without distinction between the 2 subtypes. Frulloni et al. investigated the exocrine and endocrine pancreatic function in 21 patients with AIP, and reported that all five patients (24% of total) with diabetes mellitus exhibited very low levels of faecal elastase-1 concentrations of <19 µg/g.175 Patients with diabetes mellitus were also older than patients without diabetes mellitus.175 Maire and colleagues reported data on 96 patients with type 1 (73%) or type 2 (27%) AIP. Smokers (>10 pack/years) presented with diabetes mellitus more frequently (50% vs. 27%, p <0.04) compared with no/low-amount smokers.176 The rate of diabetes mellitus is higher in patients with AIP type 1 as compared with AIP type 2 in most published series. Rates of diabetes of 68% vs. 14%,106 23% vs. 11%,61 36% vs. 40%,62 and 40% vs. 22%13 have been reported in AIP type 1 vs. AIP type 2, globally suggesting that pancreatic endocrine dysfunction is more common in IgG4-RD. Pancreatic atrophy has also been associated with an increased risk of diabetes mellitus by Masuda and colleagues, who reported a 75% incidence of diabetes mellitus in cases with pancreatic atrophy, as assessed by CT scan, compared with 10% in cases without pancreatic atrophy.177 There are no specific studies regarding the association between the severity of diabetes and features of IgG4-RD. It has been reported that insulin requirement, which can be a proxy of severity of diabetes mellitus, is more common in type 1 AIP compared with type 2 AIP.13
Q7.3. What is the impact of glucocorticoid/immunosuppressive treatment on the risk and/or severity of diabetes mellitus in IgG4-related disease of the pancreas?
Statement 7.3: Glucocorticoid treatment of IgG4-related disease of the pancreas (AIP type 1) induces beneficial effects on the clinical course of diabetes mellitus in approximately 50–60% of all cases. Patients with simultaneous-onset diabetes mellitus show greater glucocorticoid responsiveness than patients with pre-existing diabetes mellitus. (GRADE 2C; strong agreement)
Comments: There are only a few studies with small numbers of patients addressing the impact of glucocorticoid treatment on type 1 AIP. Nearly all studies, however, describe an improvement of diabetes mellitus in more than half of the patients. Nishimori and colleagues conducted a nationwide survey in Japan and found an improvement in diabetes mellitus, concerning parameters of glucose control, in 55% of patients with simultaneous-onset diabetes. However, only 36% of patients with pre-existing diabetes mellitus improved and 20% showed an exacerbation or new-onset diabetes mellitus following glucocorticoid treatment.178 Miyamoto and colleagues evaluated 69 patients for short- and long-term outcome of diabetes mellitus in type 1 AIP after glucocorticoid therapy in a retrospective study. They describe similar results, with improvement rates of 55–66% for simultaneous-onset diabetes mellitus, as well as for pre-existing diabetes mellitus. Worsening is only reported in 9–15% of cases with at least 3 months of treatment. This group also evaluated the effect of long-term glucocorticoid treatment on diabetes mellitus in patients with at least 3 years of treatment. The rate of improvement rises up to 66% with none of the patients reporting worsening after 3 years.179 In a smaller series, the diabetes rate increased from 24% to 48% during glucocorticoid treatment, but eventually dropped to 19% at the end of tapering, also confirming a positive effect of long-term glucocorticoid treatment.175
WP 8: IgG4-related digestive disease and cancer
Q8.1 What is the risk of cancer development in the context of IgG4-related disease?
Statement 8.1: IgG4-related disease, and particularly AIP, may be associated with an increased risk of developing malignant disease compared with the general population. Life-long surveillance in patients with IgG4-related disease is advised. (GRADE 2C; strong agreement)
Comments: Several studies (Table 3) have investigated the risk of various malignancies in patients with IgG4-related disease.126,180–184 In one study, the incidence of malignancies in patients with IgG4-related disease was similar to the general population.180 In the remaining studies, the risk of malignancy development was significantly increased in patients with IgG4-related disease compared with the general population.181–184 Other investigations focused on the risk of malignancy development in patients with AIP.185–188 Patients with AIP, diagnosed by Asian diagnostic criteria, revealed a high risk of developing various cancers.185 The highest risk of cancer occurred in the first year after AIP diagnosis, and the absence of an AIP relapse after successful cancer treatment suggested that AIP may represent a paraneoplastic syndrome in certain individuals with AIP.185 In line with this observation, an investigation of a mixed cohort of patients with various forms of AIP demonstrated that the risk of malignancy significantly increased compared with the general population.186 In contrast, another investigation observed a similar cancer risk in patients with AIP, before and after diagnosis, compared with control subjects.187 In an investigation of patients with type 1 AIP, no pancreatic cancer occurred in any patient.188 The investigation of all malignant diseases in a prospective cohort of patients with type 1 AIP revealed that malignancy occurred in 11% shortly before or after diagnosis of IgG4-RD, including three hepatic, biliary, or pancreatic cancers.84 The risk of any cancer at diagnosis or during follow-up was significantly increased compared with matched national statistics.84 In summary, various malignant diseases (e.g. lung cancer, colon cancer, pancreatic cancer, bladder cancer, lymphoma, leukaemia, and others) occurred in patients with IgG4-related disease. Several lines of evidence suggest that IgG4-related disease might be associated with an increased risk of development of malignant diseases. However, these data might be influenced by a bias due to a more careful surveillance of patients with IgG4-related disease or AIP. Thus, future prospective studies are required to analyse the incidence of cancer in patients with IgG4-related disease compared to age-, sex-, and risk factor-matched control subjects.189
Table 3.
(Statement 8.1): Prevalence rates of malignant diseases in patient cohorts with IgG4-related disease or in patient cohorts with AIP.
| Author | Patients with malignant disease and IgG4-related disease | Control cohort | Study conclusion: In IgG4-related disease, the risk of various malignant diseases … |
|---|---|---|---|
| Hirano et al.180 | 12/95 (13%) | Japan cancer registry | … is not increased. |
| Yamamoto et al.181 | 11/106 (10%) | Japan cancer registry | … is increased. |
| Asano et al.227 | 28/109 (26%) | Japan cancer registry | … is increased. |
| Wallace et al.183 | 20/125 (16%) | Matched controls, general population | … is increased. |
| Ahn et al.230 | 12/118 (10%) | General population | … is increased. |
|
Author |
Patients with malignant disease and AiP |
Control cohort |
Study conclusion: In AiP, the risk of various malignant diseases … |
| Shiokawa et al.185 | 15/108 (14%) | Matched controls, Japan cancer registry | … is increased. |
| Schneider et al.229 | 5/28 (18%) | German cancer registry | … is increased. |
| Hart et al.187 | 19/116 (16%) | Matched controls | … is not increased. |
| Lee et al.188 | 0/138 (0%) | No control group | … is not increased. |
| Inoue et al.126 | 13/235 (0%) | No control group | … is not increased. |
Q8.2. How can we distinguish IgG4-related disease and cancer clinically and radiologically?
Statement 8.2.1: There are no specific clinical features or blood tests that can differentiate between IgG4-related disease and cancer. (GRADE 2B; strong agreement)
Statement 8.2.2. Radiological differentiation between IgG4-related disease and cancer is challenging. A few imaging findings may aid in the differential diagnosis. (GRADE 2C; strong agreement)
Comments: Symptom profiles in IgG4-related disease and cancer can depend on the organs involved, but for pancreatic and biliary disease the presentations are broadly similar. These include weight loss, obstructive jaundice, abdominal pain, and diarrhoea. There are no specific clinical features that can differentiate between IgG4-related disease and cancer.73,84 Involvement of multiple organ systems lends support for IgG4-related disease. Constitutional symptoms, including fever and night sweats, are more suggestive of cancer (e.g. lymphoma) and are rarely present in IgG4-related disease. Serum IgG4 levels can be elevated and/or normal in both IgG4-related disease and cancer. Mild elevations (<2 times upper limit) cannot reliably differentiate the conditions.142 Marked elevations (≥4 times the upper limit) can differentiate between IgG4-related cholangitis and cholangiocarcinoma with high specificity,6 although cancers with IgG4+ infiltrates and elevated serum IgG4 are described. Tumour markers, such as CA 19-9, can be elevated in both conditions, especially in the context of an obstructed biliary system.
The most challenging radiological diagnosis is differentiating between focal AIP and pancreatic cancer, and isolated IgG4-SC (type IV) and extrahepatic cholangiocarcinoma, respectively. The combination of a few MR/CT features may be useful (Table 1).41,190–193 PET/CT may identify a diffuse uptake in the pancreas in focal AIP and show evidence of other organ involvement.41,190 The role of imaging in the differentiation between IgG4-related lymphadenopathy and IgG4-related retroperitoneal fibrosis (RPF) from malignancy (e.g. lymphoma or retroperitoneal malignancies) has been poorly investigated and is still unclear. A biopsy and clonality assessment are often required to clarify the diagnosis and may still be inconclusive.
Q8.3. How can we distinguish IgG4-related disease and cancer histologically?
Consensus Statement on the Pathology of IgG4-RD (histological Boston criteria):
Includes three key morphological features: dense lymphoplasmacytic infiltrate, fibrosis that is at least focally storiform, and obliterative phlebitis. Additional morphological features: phlebitis and increased eosinophils. The number of IgG4-positive plasma cells are measured as the mean IgG4+ plasma cell count per HPF within the three HPFs containing the greatest number of these cells when the distribution is patchy. If the IgG4-positive plasma cells are distributed diffusely, the mean IgG4+ plasma cell count is determined in three random HPFs.
Histologically highly suggestive of IgG4-related disease: Two or more key morphological features and immunohistochemistry demonstrating IgG4-positive plasma cells above 30–100 per HPF (organ-specific cut-off in resection specimen) or 10–200 per HPF (organ-specific cut-off in a biopsy specimen) and IgG4/IgG plasma cell ratio of over 40%.
Probable histological features of IgG4-related disease: One key morphological feature and immunohistochemistry demonstrating IgG4-positive plasma cells above 30–100 per HPF (organ-specific cut-off in resection specimen) or 10–200 per HPF (organ-specific cut-off in a biopsy specimen) and IgG4/IgG plasma cell ratio of over 40%.
Statement 8.3.1: In resection specimens, there are established histological criteria to distinguish cancer and IgG4-related disease (the so-called Boston criteria: three morphological features of lymphoplasmacytic infiltrate, storiform fibrosis, obliterative phlebitis, and in addition, immunohistochemistry demonstrating IgG4-positive plasma cells above 50–100 per HPF (organ-specific cut-off) and IgG4/IgG ratios of over 40%). (GRADE 2B; strong agreement)
Statement 8.3.2. In biopsy specimens, distinguishing IgG4-related disease and cancer is more challenging. Risk of sampling error should be considered in a negative biopsy. Non-specific inflammation, with increased IgG4-positive cells can occur in both cancer and IgG4-related disease. (GRADE 2C; strong agreement)
Statement 8.3.3. An increased frequency of synchronous or metachronous lymphomas and IgG4-related disease has been reported. Immunohistochemical, flow cytometric, and molecular studies may help to differentiate them. (GRADE 2C; strong agreement)
Comments: In resection specimens from the pancreas, liver, or extrahepatic bile ducts, the histological differential diagnosis of IgG4-related disease from cancer is generally easy. However, in histological biopsy specimens this differentiation is often challenging and depends on the representativeness and the size of the biopsy material available. If two or three ‘Boston criteria’ for the histological diagnosis of IgG4-related disease are present with increased IgG4-positive plasma cells and increased ratio of IgG4- to IgG-positive cells, a histological diagnosis of IgG4-related disease is highly suggestive.24,30 However, synchronous presence of IgG4-related disease and cancer is reported, requiring careful clinical and radiological correlation.84,182,184 EUS-guided fine needle aspiration (FNA) from adenocarcinomas of the pancreas or hepatic bile ducts have a sensitivity and specificity of 75–90% and almost 100% for the diagnosis of malignancy, but are not sufficient for a diagnosis of IgG4-related disease when only cytological material is obtained, as the architectural changes characteristic of this disease cannot be appreciated. However, sometimes pancreatic FNAs may contain small tissue (micro)-fragments, and EUS-guided FNA with 22-gauge (22G) needles has been used for the diagnosis of AIP with varying results, mainly in South-East Asia.44,194–196 EUS-guided fine needle biopsy is preferred for tissue differential diagnosis between focal benign and malignant lesions in the pancreas,197–199 where certain histological changes characteristic of AIP are appreciated, although these can be patchy in distribution (e.g. lymphoplasmacytic infiltration, focal storiform fibrosis, obliterative phlebitis), and where IgG and IgG4 immunostaining for plasma cells can be performed. Diagnostic difficulties arise when only one of the Boston criteria or unspecific inflammation is present, with or without increased IgG4-positive cells. Peri-tumoural inflammation may show lymphoplasmacytic inflammation and increased IgG4-positive cells. Intraductal papillary mucinous neoplasms have been associated with IgG4-related disease.44 Distinguishing IgG4-related disease from lymphoma is challenging. Immunohistochemistry for kappa and lambda Ig light chains, polymerase chain reaction (PCR) for immunoglobulin heavy chain (IGH) gene rearrangement, flow cytometric, and molecular studies may be necessary. A few studies indicate that patients with IgG4-related disease have an increased frequency of synchronous or metachronous lymphomas.184,200 In the end, a small number of pancreatic resections due to suspicious of malignancy needs to be accepted in light of the dire differential diagnosis.
Q8.4. Is IgG4-related disease a paraneoplastic condition?
Statement 8.4.1: Malignancy in patients with IgG4-related disease, and particularly AIP, is most often identified in organs distinct from those affected by the disease. (GRADE 2B; strong agreement)
Statement 8.4.2: IgG4 antibodies can be found in both the context of IgG4-related disease and in several patients with cancer. Further studies are needed to establish the relationship between IgG4 antibodies, IgG4-related disease, and cancer. (GRADE 2C; strong agreement)
Comments: It is well established that long-standing chronic inflammation plays a critical role in the development of cancer through the process of inflammation-associated carcinogenesis.201 However, the presence of AIP is more closely associated with extra-pancreatic cancer than pancreatic cancer itself.84,180,181,185–187 The majority of cancer cases detected in AIP occur at or within a year of diagnosis.183 Remission of AIP has also been achieved after the successful treatment of co-existing cancer,185 as in the case of other autoimmune paraneoplastic disorders. K-ras gene mutations have been detected in the gastrointestinal tract in patients with AIP.202 Prospective matched controlled studies of cancer incidence are required to evaluate this further.
Cancer and pancreatic tissues of AIP patients with cancer share key immune responses leading to the enhancement of IgG4 antibody production.185 IgG4 antibodies may be specifically generated in response to malignant disease and represent a mechanism of tumour-induced immune escape. In human melanoma patients,203 tumour-associated B cells are stimulated by a Th2 (IL-10 secreting) tumour microenvironment and polarized to produce IgG4. Strikingly, IgG4 antibodies can inhibit the anti-tumour effector functions of IgG1 antibodies, and IgG4 serum levels are associated with decreased patient survival.203 IgG4 responses have also been reported in other cancers, including extrahepatic cholangiocarcinoma, pancreatic cancer, and glioblastoma, and elevated serum IgG4 levels have been associated with poorer prognosis in malignant melanoma and biliary tract cancers.
WP 9: Systemic treatment of IgG4-related digestive disease
Q9.1 What are indications and modalities of initial systemic treatment of IgG4-related gastrointestinal diseases?
Statement 9.1.1: All symptomatic patients (e.g. suffering from pancreatic pain, obstructive jaundice) should be considered for treatment, sometimes urgently in cases of organ insufficiency. Treatment can also be proposed to asymptomatic patients in case of: (1) persistence of a pancreatic mass in imaging to rule out cancer, (2) persistence of liver test abnormalities (cholestasis) in case of associated IgG4-related cholangitis, and (3) in subclinical situations that could lead to severe or irreversible organ failure. (GRADE 1C)
Statement 9.1.2: There is no relevant data to support that a treatment should be proposed in patients with AIP without symptoms, just to limit the risk of exocrine or endocrine insufficiencies. (GRADE 1C)
Statement 9.1.3: Treatment with glucocorticoids should be initiated in a weight-based manner at a dose of 0.6–0.8 mg/kg body weight/day orally (typical starting dose 30–40 mg/day prednisone equivalent) for 1 month to induce remission. Response to initial treatment should be assessed at week 2–4 with clinical, biochemical, and morphological markers. Glucocorticoid therapy should gradually be tapered by 5 mg every two weeks (tapering duration 3–6 months). (GRADE 1C)
Comments: Indications for treatment of symptomatic patients: obstructive jaundice, abdominal pain, pancreatic pain, and involvement of extra-pancreatic digestive organs including IRC. In the literature, 10–25% of patients with IgG4-related disease present spontaneous resolution of symptoms without medical (glucocorticoids or immunosuppressive therapy), interventional endoscopic, or surgical treatment.13,14,204 A wait-and-see attitude therefore seems to be appropriate for a significant portion of patients.205 Data on the long-term functional outcome of gastrointestinal IgG4-related disease manifestations are limited, especially in cases of asymptomatic patients. Diabetes mellitus has been reported in 19–67% of these patients and exocrine insufficiency in 36–85%. The wide range of results might be due to small populations, short follow-up, and non-standardized criteria for endocrine and exocrine insufficiencies.34,206 Moreover one survival analysis did not assess any significant difference between patients with IgG4-related disease and the general population. Specific survival rates were 91% and 72% at 5 and 10 years, respectively.34 The aim of the treatment is to suppress inflammation, delay fibrotic progression, and prevent disease-related complications by maintaining the disease in a quiescent state.
Glucocorticoids remain the most effective initial treatment, although there are limited clinical trials on the effectiveness of glucocorticoid maintenance therapy.14,16 Glucocorticoids are the preferred first-line medication for active IgG4-related disease, with response rates around 97–100% and a significant decrease of serum IgG4 levels.14 Treatment with glucocorticoids should be initiated weight-based at a dose of 0.6–0.8 mg/kg body weight/day orally for 1 month to induce remission, but initial glucocorticoid dose can be adjusted. This adjustment must be based on body weight, in case of particularly aggressive disease (use initial dosages above 40 mg/d), or in case of elderly patients, with very mild clinical symptoms (use <20 mg/d). In patients with diabetes, it is important to optimize diabetic control and osteoporosis medication prior to initiation of glucocorticoids, if this is possible. A few data on remission rates using low glucocorticoid doses (e.g. equivalent prednisolone dosing of 10–20 mg/day) are available,14,16 but are too preliminary to be proposed. Response to glucocorticoids has become part of the diagnostic criteria.1 This suggests that biliary stenting is not mandatory in combination with glucocorticoids in cases of obstructive jaundice to limit the risk of cholangitis.1 Jaundice totally resolved in less than 15 days with glucocorticoids (without stenting) with a rapid normalization of serum liver tests. Biliary stenting may even propagate pancreatic stone formation in IRC and AIP.207 As shown by Yukutake et al., serum liver test abnormalities are normalized in 80% and 100% at 15 and 21 days, respectively.208–210 Despite the effectiveness of glucocorticoids, about one-third of patients experience disease relapse during glucocorticoid tapering, thus requiring re-induction therapy.14 This re-induction is generally managed with an increase in glucocorticoid dosing followed by a prolonged taper. Relapse may occur in the same organ being treated or, interestingly, in previously uninvolved organ systems.14,75 Recurrence occurs more often in patients without prior glucocorticoid therapy (about 40%) than in cases after previous glucocorticoid therapy (about 25%).14 In the Asian region, especially in Japan and China, a 12-month maintenance therapy is therefore recommended. To be weighed against this are potential side effects of long-term glucocorticoid maintenance therapy against the recurrence rate (about 5% with maintenance therapy versus 22% without maintenance therapy).211 No relevant data are available to recommend maintaining low-dose therapy for a few months. But several experts recommend maintaining glucocorticoids at ≤10 mg/day (equivalent to 2.5–10 mg/day prednisolone) for 12 months. Some Japanese centres continue low-dose (5 mg) prednisolone for as many as 3 years212 and beyond.213
Q9.2. What are the indications for immunosuppressant treatments for IgG4-related gastrointestinal disease?
Statement 9.2: Adding immunosuppressive agents should be considered in case of disease relapse as maintenance of remission strategy, and in patients with a high risk of disease relapse, particularly in the case of multi-organ involvement. If there is no change in disease activity or the disease relapsed during the 3 months of treatment (during glucocorticoid taper or discontinuation), then immunosuppressive drugs should be added. (GRADE 2C)
Comments: The use of immunosuppressants is still controversial as a combination therapy along with glucocorticoids as the initial treatment of IgG4-related disease.14 The remission-induction stage is defined as fulfilling each of the following criteria after 6 months of treatment: (1) ≥50% decline in IgG4 levels;204 (2) glucocorticoids tapered to maintenance dose ≤10 mg/day; and (3) no14 relapse during glucocorticoid tapering within 6 months. Taken together, IgG4-related disease is sensitive to glucocorticoid therapy, but the best way to maintain remission remains unclear. In addition, glucocorticoid monotherapy might not be sufficient to control IgG4-related disease, and some patients may relapse during glucocorticoid tapering. The relapse rate is high and ranges from 26% to more than 70%.13 A Japanese study showed that maintenance of low-dose glucocorticoids in the long term (2.5–7.5 mg/day for 3 years) could reduce the risk of relapse from 58% to 23%.211 However, the risk induced by long-term glucocorticoid therapy warrants reconsideration of this option. The risk factors for relapse are not well understood. However, several predictors of relapse may be high serum IgG4 levels, persistently high serum IgG4 levels after glucocorticoid treatment, and multiple organ involvement. Furthermore, to avoid long-term side effects of glucocorticoid therapy, conventional glucocorticoid-sparing agents, including azathioprine, 6-mercaptopurine, mycophenolate mofetil, cyclosporine A, tacrolimus, methotrexate, cyclophosphamide, or rituximab, must be considered. At least three treatment regimens have been used for relapse therapy: (a) high-dose glucocorticoids followed by maintenance treatment with low-dose glucocorticoids or a glucocorticoid-sparing agent; (b) high-dose glucocorticoids without maintenance treatment; or (c) rituximab induction with or without maintenance rituximab. As glucocorticoids remain highly successful for re-induction of remission (>95%), it is reasonable, if tolerated by the patient, to repeat a course of high-dose glucocorticoids.
Q9.3. Which immunosuppressant (for which patient) should be proposed as treatment of IgG4-related digestive disease and what are the secondary effects of the systemic treatment?
Statement 9.3.1: Rituximab should be considered if patients are resistant or intolerant to high-dose glucocorticoids to maintain remission or have failed to respond to immunosuppressive therapies. Dosing protocol (375 mg/m2 body surface area) is used weekly for 4 weeks, followed by infusions every 2–3 months or at two 1000 mg infusions 15 days apart every 6 months. (GRADE 2A)
Comments: There is a strong pathophysiological rationale for rituximab use in IgG4-related disease, allowing depletion of the CD20 + B-cell precursors of disease-specific clonal plasmablasts. Depletion of B cells by rituximab has been demonstrated to be highly effective at inducing and maintaining remission. Apart from glucocorticoids, rituximab is the only drug that has been shown to induce remission of IgG4-related disease. It has shown promise in treating patients with IgG4-related disease in a prospective open-label study of 30 patients, which revealed a 97% response rate with significant reduction in patients’ baseline IgG4-RD RI. A clinical response usually occurs within 4 weeks of rituximab therapy.15,214,215
In IgG4-related digestive disease, rituximab proved effective when administered both at 375 mg/m2 weekly for 4 weeks followed by maintenance infusions every 2–3 months (onco-haematological protocol) or at two 1000 mg infusions 15 days apart every 6 months (immunological/rheumatoid arthritis protocol).216 Maintenance therapy with rituximab, continued for up to 2 years, was associated with longer relapse-free survival. Adverse events, such as infusion reactions, hypogammaglobulinemia, and severe infections must be noted. Rituximab presents several advantages, such as a glucocorticoid-sparing effect, existing data on remission induction, and (possibly) better safety profile than glucocorticoids or immunosuppressants.
Statement 9.3.2: Immunosuppressive drugs. Immunosuppressants used include: thiopurines (azathioprine and 6-mercaptopurine), mycophenolate mofetil, methotrexate, or calcineurin inhibitors (tacrolimus and cyclosporine A). (GRADE 2A)15,217–219
Comments: Treatment with thiopurines (azathioprine and 6-mercaptopurine). Although data to support the use of one agent over another are limited, azathioprine use (2–2.5 mg/kg body weight) is suggested.15 According to a recent meta-analysis, azathioprine was used in 85% of cases, with the next common therapy being mycophenolate mofetil (MMF). Many patients have been reported to relapse on low doses of azathioprine (50 mg daily). Therefore, a target dose of 2–2.5 mg/kg body weight should be sought under close clinical and laboratory control. Reportedly, the disease has been controlled by increasing the dose, but upon evaluation of the case series most responses were achieved in combination with glucocorticoid therapy, making it difficult to assess their efficacy.90 In few cases, azathioprine and 6-mercaptopurine (6-MP) may cause myelosuppression, toxic hepatitis, or pancreatitis, which requires a switch to a different immunosuppressant. Many side effects (well-known in patients with inflammatory bowel disease) are reported. In the French prospective cohort of patients with inflammatory bowel disease, a risk of lymphoproliferative disease when taking thiopurines was evaluated at 5.28 (2.01–13.9), especially in men over 65, which is the profile of patients with IgG4-related disease.220
Therapy with mycophenolate mofetil (MMF). A recent Chinese randomized control trial showed that maintenance therapy with mycophenolate, in addition to glucocorticoids, reduced the risk of relapse (21% at 12 months) compared with glucocorticoids alone (40%), with no increase in adverse events.221 Therapy with MMF should commence with 1 g/day and can be increased to 1.5–2 g/day under close monitoring of complete blood count. Like azathioprine, many patients have been reported to relapse on low doses of MMF (1 g daily).
Therapy with methotrexate (MTX). Several series of cases reported the role of MTX in patients with relapsing IgG4-related disease .219 In 10 patients, oral or subcutaneous MTX was introduced on average 5 weeks (range 1–16) after initiation of oral glucocorticoids, at a mean daily prednisone dose of 20.8 mg (range 10–50). MTX administration began at a dose of 10 mg/week and increased to 20 mg/week. Twelve months after introduction of MTX, six patients were in disease remission and four maintained partial remission on a mean daily prednisone dose of 2 mg. In a second series, three patients were treated by MTX: one maintained remission at 34 months and two relapsed on MTX at 24 months.218,219
Other immunosuppressants. Calcineurin inhibitors (CNI), such as tacrolimus or cyclosporine A, can be used as a steroid-sparing regime in IgG4-related disease patients with contraindications or non-response to other therapies. A target level of 5–7 ng/ml for tacrolimus and one of 80–120 ng/ml for cyclosporine A is documented in the available case reports. There is little evidence for the efficacy of CNI as steroid-sparing agents in IgG4-related disease. One should take into consideration that long-term use of CNI might lead to hypertension or renal insufficiency in older patients.
Cyclophosphamide use has been adapted from the treatment of vasculitis, lupus, and rheumatoid diseases, and administered as an intravenous infusion or tablets 50–100 mg/day, and often for extra-pancreatobiliary disease manifestations. A Chinese controlled trial suggested that cyclophosphamide, in addition to glucocorticoids, lowered the risk of disease relapse (12% at 12 months) compared with glucocorticoids alone (39%), which was, however, associated with increased toxicity.222
Immunosuppressants display distinctly greater toxicity profiles, accompanied with frequent relapses when used as monotherapy. Too little is known regarding this subject to confidently suggest monotherapy with immunosuppressive agents.
As there has been no randomized controlled study on the treatment of IgG4-related disease, including various drugs, the best evidence-based treatment of this disorder is still unknown. The choice of using certain medication for treatment of IgG4-related disease varies in different countries, among specialties, and different organ involvement. Therefore, multicentre clinical trials with large numbers of patients are needed to define optimal treatment protocols in IgG4-related disease.
Areas of uncertainty and future research
These UEG guidelines set out to provide a rational basis for diagnosing and treating IgG4-related digestive disease. We achieved this goal, however, inherent to a relatively rare disease, there are still several white spots on the map of the digestive tract in conjunction with this enigmatic disease. Consequently, for several areas, evidence is low or possibly non-existent, even if experts agree on a certain practice. Much of this is performed in analogy to other diseases, such as the consideration to treat IRC with ursodeoxycholic acid, as done in other cholangiopathies, such as PBC or PSC. Consequently, as is the case with many other guidelines, areas of future research were identified to provide evidence for future versions. The major topics are the following:
incidence of cancer in patients with IgG4-related digestive disease compared with age-, sex- and risk factor-matched control subjects, and any risk factors for its development should be explored in prospective studies.
prospective studies to evaluate the accuracy of imaging modalities (CT, MR, PET-CT) even with the aid of innovative post-processing methods (i.e. Radiomics, Texture Analysis) in the differentiation of focal autoimmune pancreatitis and isolated IRC from pancreatic cancer and cholangiocarcinoma, respectively.223
as there have been no randomized controlled studies on the treatment of IgG4-related digestive disease, the best evidence-based treatment of this disorder is still unknown. The use of certain medications for treatment of IgG4-related digestive disease varies in different countries, among different specialties, and different organ involvement; therefore, multicentre clinical trials with large numbers of patients are needed to define optimal treatment protocols.224
IgG4-related digestive disease in children is very rare, and multicentre paediatric studies are necessary for better understanding of the disease course in children, and to define the best treatment choice.
Working party and external expert reviewers
All members of the working party33 are listed as co-authors. The allocation to the WP is listed in the supplement (Table S2). The following colleagues served as external expert reviewers as part of the working party contributing substantially and are therefore also co-authors of these guidelines in accordance with GUIDE:
Marc G Besselink1, Marco J Bruno2, László Czakó3, Marco del Chiaro4, Oleksandra Filippova5, Akihisa Fukuda6, Sebastien Gaujoux7, Phil A Hart8, Peter Hegyi9, Eduard Jonas10, Alisan Kahraman11, Alexander Kleger12, Olexander Kuryata5, Johanna Laukkarinen13, Markus M Lerch14, Giovanni Marchegiani15, Hanns-Ulrich Marschall16, Celso Matos17, Yair Molad18, Dilek Oguz19, Aldis Pukitis20, Sohei Satoi21, John H Stone22, Joanne Verheij23, Niek de Vries24
1Department of Surgery, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands
2Department of Gastroenterology and Hepatology; Erasmus Medical Center, University Medical Center, Rotterdam, the Netherlands
3First Department of Medicine, University of Szeged, Szeged, Hungary
4Division of Surgical Oncology, Department of Surgery, University of Colorado Anschutz Medical Campus, Denver, USA, CO Division of Surgery, CLINTEC-Karolinska Institutet, Stockholm, Sweden, Division of Surgical Oncology, Department of Surgery, Johns Hopkins University, Baltimore, USA
5Internal Medicine 2 and Physiology, Dnipropetrovsk State Medical Academy, Dnipro, Ukraine
6Department of Gastroenterology & Hepatology; Kyoto University Graduate School of Medicine, Japan
7Department of Pancreatic and Endocrine Surgery, Cochin Hospital, Paris, APHP, France
Université de Paris, France
8Division of Gastroenterology, Hepatology, and Nutrition, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA
9Institute for Translational Medicine, Szentágothai Research Centre, Medical School, University of Pécs, Pécs, Hungary
10Surgical Gastroenterology Unit, Department of Surgery, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
11Department of Gastroenterology and Hepatology, University Clinic of Essen, Germany
12University Medical Center Ulm, Center for Internal Medicine, Department of Internal Medicine I, University of Ulm, Ulm, Germany
13Department of Gastroenterology and Alimentary Tract Surgery, Tampere University Hospital, and Faculty of Medicine and Health Technology, Tampere University, Finland
14Department of Medicine A, University Medicine Greifswald, Greifswald, Germany
15Department of Surgery, Pancreas Institute, University and Hospital Trust of Verona, Verona, Italy
16Department of Molecular and Clinical Medicine/Wallenberg Laboratory, University of Gothenburg, Gothenburg, Sweden
17Radiology Department, Champalimaud Centre for the Unknown, Lisbon, Portugal
18Institute of Rheumatology, Rabin Medical Center, Beilinson Hospital, and The Laboratory of Inflammation Research, Felsenstein Medical Research Center, Sackler Faculty of Medicine, Tel Aviv University, Petach Tikva, Israel
19Department of Gastroenterology, Kırıkkale University School of Medicine, Kırıkkale, Turkey
20Center of Gastroenterology, Hepatology and Nutrition, Pauls Stradins
Clinical University Hospital; University of Latvia, Riga, Latvia
21Division of Pancreatobiliary Surgery, Department of Surgery, Kansai Medical University, Japan
22Harvard Medical School The Edward A. Fox Chair in Medicine Massachusetts General Hospital, Boston, MA, USA
23Department of Pathology, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands
24Department of Clinical Immunology & Rheumatology, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
Online
Parts of these guidelines, are available online (introduce link). The essentials are available via an app, for both the Apple and Android iOS.
Disclaimer
These clinical practice guidelines (CPGs) are developed to assist clinicians with decisions about appropriate health care in patients with IgG4-related disease. They detail the assessment and management of many common (and some rare but important) conditions and have been developed for use in healthcare settings. As such, these CPGs are targeted at clinicians only. Patients or other community members using these CPGs should do so in conjunction with a health professional and should not rely on the information in these guidelines as professional medical advice.
These CPGs do not constitute a textbook and therefore deliberately provide little, if any, explanation or background to the conditions and treatment outlined. They are however designed to rapidly acquaint the reader with the clinical problem and provide practical advice regarding assessment and management.
These CPGs are developed by a multidisciplinary team of practising clinicians by consensus and based on the evidence available.
The recommendations contained in these CPGs do not indicate an exclusive course of action or standard of care. They do not replace the need for application of clinical judgment to each individual presentation, nor variations based on locality and facility type.
The inclusion of links to external websites does not constitute an endorsement of those websites nor the information or services offered.
The authors of these CPGs have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Users of these CPGs are strongly recommended to confirm that the information contained within them, especially drug doses, is correct by way of independent sources. The authors accept no responsibility for any inaccuracies, information perceived as misleading, or the success or failure of any treatment regimen detailed in the CPGs.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620934911 for European Guideline on IgG4-related digestive disease – UEG and SGF evidence-based recommendations by J-Matthias Löhr, Ulrich Beuers, Miroslav Vujasinovic, Domenico Alvaro, Jens Brøndum Frøkjær, Frank Buttgereit, Gabriele Capurso, Emma L Culver, Enrique de-Madaria, Emanuel Della-Torre, Sönke Detlefsen, Enrique Dominguez-Muñoz, Piotr Czubkowski, Nils Ewald, Luca Frulloni, Natalya Gubergrits, Deniz Guney Duman, Thilo Hackert, Julio Iglesias-Garcia, Nikolaos Kartalis, Andrea Laghi, Frank Lammert, Fredrik Lindgren, Alexey Okhlobystin, Grzegorz Oracz, Andrea Parniczky, Raffaella Maria Pozzi Mucelli, Vinciane Rebours, Jonas Rosendahl, Nicolas Schleinitz, Alexander Schneider, Eric FH van Bommel, Caroline Sophie Verbeke, Marie Pierre Vullierme, Heiko Witt and the UEG guideline working group in United European Gastroenterology Journal
Acknowledgements
We thank both the Swedish Society for Gastroenterology (SGF) and the Dutch Society for Gastroenterology for their readiness to serve as national societies of UEG supporting these guidelines. Furthermore, the support of the European Pancreatic Club and the United European Gastroenterology is acknowledged for providing space and time for the face-to-face meetings in the development of these guidelines.
Conflict of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AL reports speaker fees from Bracco, GE Healthcare, Merck, Bayer and Bristol-Myers-Squibb; DGD received fellowship grant from Gilead Sciences; DA received research support from Intercept Pharma and Vesta and speaker fees from Intercept Pharma and Aboca; ELC reports consultation fees from Xencor pharmaceuticals; MPV reports honoraria from Guerbet; JML reports research support from Mylan and lecture fees from Abbott and Mylan; MV reports research support from Mylan and lecture fees from Abbott and Mylan, NS reports honoraria and consultation fees from Baxalta, Shire, LFB, CSL Behring and Novartis; UB reports research support from Falk and Intercept, honoraria and consultation fees from Intercept and lecture fees from Abbvie, Falk Foundation and Intercept. The other authors have nothing to disclose regarding the work under consideration for publication.
Funding
We gratefully acknowledge the support from the National Societies Committee of the United European Gastroenterology (UEG) for the conduct of these guidelines independent from other sources. No other funding was received.
ORCID iDs
J-Matthias Löhr https://orcid.org/0000-0002-7647-198X
Jens Brøndum Frøkjær https://orcid.org/0000-0001-8722-0070
Enrique de-Madaria https://orcid.org/0000-0002-2412-9541
Luca Frulloni https://orcid.org/0000-0001-7417-2655
Andrea Laghi https://orcid.org/0000-0002-3091-7819
Fredrik Lindgren https://orcid.org/0000-0001-7984-7754
Eric FH van Bommel https://orcid.org/0000-0002-9803-1715
Supplemental material
Supplemental material for this article is available online.
References
- 1.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011; 40: 352–358. [DOI] [PubMed] [Google Scholar]
- 2.Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis 2020; 79: 77–87. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra K, Culver EL, de Buy Wenniger LM, et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 2014; 59: 1954–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubers LM, Beuers U. IgG4-related disease of the biliary tract and pancreas: Clinical and experimental advances. Curr Opin Gastroenterol 2017; 33: 310–314. [DOI] [PubMed] [Google Scholar]
- 5.Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol 2006; 101: 2070–2075. [DOI] [PubMed] [Google Scholar]
- 6.Oseini AM, Chaiteerakij R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 2011; 54: 940–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamisawa T, Takuma K, Tabata T, et al. Serum IgG4-negative autoimmune pancreatitis. J Gastroenterol 2011; 46: 108–116. [DOI] [PubMed] [Google Scholar]
- 8.Culver EL, Sadler R, Simpson D, et al. Elevated serum IgG4 levels in diagnosis, treatment response, organ involvement, and relapse in a prospective IgG4-related disease UK cohort. Am J Gastroenterol 2016; 111: 733–743. [DOI] [PubMed] [Google Scholar]
- 9.Doorenspleet ME, Hubers LM, Culver EL, et al. Immunoglobulin G4(+) B-cell receptor clones distinguish immunoglobulin G 4-related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology 2016; 64: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Maillette de Buy Wenniger LJ Doorenspleet ME, Klarenbeek PL, et al. Immunoglobulin G4+ clones identified by next-generation sequencing dominate the B cell receptor repertoire in immunoglobulin G4 associated cholangitis. Hepatology 2013; 57: 2390–2398. [DOI] [PubMed] [Google Scholar]
- 11.Tabibian JH, Lindor KD. Distinguishing immunoglobulin G4-related disease from its pancreatobiliary mimics: Are we there now? Hepatology 2016; 64: 340–343. [DOI] [PubMed] [Google Scholar]
- 12.Wallace ZS Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015; 74: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: A multicentre, international analysis. Gut 2013; 62: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut 2007; 56: 1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart PA, Topazian MD, Witzig TE, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: The Mayo Clinic experience. Gut 2013; 62: 1607–1615. [DOI] [PubMed] [Google Scholar]
- 16.Kamisawa T.Shimosegawa T, Okazaki Ket al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009; 58: 1504–1507. [DOI] [PubMed] [Google Scholar]
- 17.Muraki T.Hamano H, Ochi Yet al. Autoimmune pancreatitis and complement activation system. Pancreas 2006; 32: 16–21. [DOI] [PubMed] [Google Scholar]
- 18.Hirth M.Vujasinovic M, Munch M,et al. Monitoring and predicting disease activity in autoimmune pancreatitis with the M-ANNHEIM-AiP-Activity-Score. Pancreatology 2018; 18: 29–38. [DOI] [PubMed] [Google Scholar]
- 19.Globocan. (2018). https://www.iccp-portal.org/news/globocan-2018
- 20.Scara S, Bottoni P, Scatena R. CA 19-9: Biochemical and clinical aspects. Adv Exp Med Biol 2015; 867: 247–260. [DOI] [PubMed] [Google Scholar]
- 21.Chang MC.Liang PC, Jan Set al. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 levels. Pancreatology 2014; 14: 366–372. [DOI] [PubMed] [Google Scholar]
- 22.van Heerde MJ.Buijs J, Hansen BEet al. Serum level of Ca 19-9 increases ability of IgG4 test to distinguish patients with autoimmune pancreatitis from those with pancreatic carcinoma. Dig Dis Sci 2014; 1322–1329. [DOI] [PubMed]
- 23.Yan T.Ke Y, Chen Yet al. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19-9, globulin, eosinophils and hemoglobin. PLoS One 2017; 12: e0174735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012; 25: 1181–1192. [DOI] [PubMed] [Google Scholar]
- 25.Sah RP, Pannala R, Zhang L, et al. Eosinophilia and allergic disorders in autoimmune pancreatitis. Am J Gastroenterol 2010; 105: 2485–2491. [DOI] [PubMed] [Google Scholar]
- 26.Culver EL, Sadler R, Bateman AC, et al. Increases in IgE, eosinophils, and mast cells can be used in diagnosis and to predict relapse of IgG4-related disease. Clin Gastroenterol Hepatol 2017; 15: 1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zen Y. The pathology of IgG4-related disease in the bile duct and pancreas. Semin Liver Dis 2016; 36: 242–256. [DOI] [PubMed] [Google Scholar]
- 28.Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol 2011; 64: 237–243. [DOI] [PubMed] [Google Scholar]
- 29.Detlefsen S, Drewes A, Vyberg M, et al. Diagnosis of autoimmune pancreatitis by core needle biopsy: Application of six microscopic criteria. Virch Arch 2009; 454: 531–539. [DOI] [PubMed] [Google Scholar]
- 30.Bateman AC, Culver EL. IgG4-related disease-experience of 100 consecutive cases from a specialist centre. Histopathology 2017; 70: 798–813. [DOI] [PubMed] [Google Scholar]
- 31.Detlefsen S, Mortensen MB, Pless TK, et al. Laparoscopic and percutaneous core needle biopsy plays a central role for the diagnosis of autoimmune pancreatitis in a single-center study from denmark. Pancreas 2015; 44: 845–858. [DOI] [PubMed] [Google Scholar]
- 32.Arora K, Rivera M, Ting DT, et al. The histological diagnosis of IgG4-related disease on small biopsies: Challenges and pitfalls. Histopathology 2019; 74: 688–698. [DOI] [PubMed] [Google Scholar]
- 33.Detlefsen S, Lohr JM, Drewes AM, et al. Current concepts in the diagnosis and treatment of type 1 and type 2 autoimmune pancreatitis. Recent Pat Inflamm Allergy Drug Discov 2011; 5: 136–149. [DOI] [PubMed] [Google Scholar]
- 34.Sah RP.Chari ST, Pannala Ret al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology 2010; 139: 140–148. [DOI] [PubMed] [Google Scholar]
- 35.Negrelli R.Boninsegna E, Avesani Get al. Type 1 and Type 2 autoimmune pancreatitis: Distinctive clinical and pathological features, but are there any differences at magnetic resonance? Experience from a referral center. Pancreas 2018; 47: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 36.Deshpande V.Gupta R, Sainani Net al. Subclassification of autoimmune pancreatitis: A histologic classification with clinical significance. Am J Surg Pathol 2011; 35: 26–35. [DOI] [PubMed] [Google Scholar]
- 37.Negrelli R.Manfredi R, Pedrinolla Bet al. Pancreatic duct abnormalities in focal autoimmune pancreatitis: MR/MRCP imaging findings. Eur Radiol 2015; 25: 359–367. [DOI] [PubMed] [Google Scholar]
- 38.Manfredi R.Frulloni L, Mantovani Wet al. Autoimmune pancreatitis: Pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology 2011; 260: 428–436. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z.Wang Y, Guan Zet al. Utility of FDG-PET/CT in the diagnosis of IgG4-related diseases. Clin Exp Rheumatol 2016; 34: 119–125. [PubMed] [Google Scholar]
- 40.Kim HJ, Kim YK, Jeong WK, et al. Pancreatic duct “Icicle sign” on MRI for distinguishing autoimmune pancreatitis from pancreatic ductal adenocarcinoma in the proximal pancreas. Eur Radiol 2015; 25: 1551–1560. [DOI] [PubMed] [Google Scholar]
- 41.Furuhashi N.Suzuki K, Sakurai Yet al. Differentiation of focal-type autoimmune pancreatitis from pancreatic carcinoma: Assessment by multiphase contrast-enhanced CT. Eur Radiol 2015; 25: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 42.Sugumar A.Levy MJ, Kamisawa Tet al. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: An international multicentre study. Gut 2011; 60: 666–670. [DOI] [PubMed] [Google Scholar]
- 43.Kamisawa T.Ohara H, Kim MHet al. Role of endoscopy in the diagnosis of autoimmune pancreatitis and immunoglobulin G4-related sclerosing cholangitis. Dig Endosc 2014; 26: 627–635. [DOI] [PubMed] [Google Scholar]
- 44.Kanno A.Masamune A, Fujishima Fet al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: A prospective multicenter study. Gastrointest Endosc 2016; 84: 797–804. [DOI] [PubMed] [Google Scholar]
- 45.Moon SH, Kim MH. The role of endoscopy in the diagnosis of autoimmune pancreatitis. Gastrointest Endosc 2012; 76: 645–656. [DOI] [PubMed] [Google Scholar]
- 46.Kanno A, Masamune A, Shimosegawa T. Endoscopic approaches for the diagnosis of autoimmune pancreatitis. Dig Endosc 2015; 27: 250–258. [DOI] [PubMed] [Google Scholar]
- 47.Xiang P.Zhang X, Wang Cet al. Pancreatic tumor in type 1 autoimmune pancreatitis: A diagnostic challenge. BMC Cancer 2019; 19: 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asbun HJ.Conlon K, Fernandez-Cruz Let al. When to perform a pancreatoduodenectomy in the absence of positive histology? A consensus statement by the International Study Group of Pancreatic Surgery. Surgery 2014; 155: 887–892. [DOI] [PubMed] [Google Scholar]
- 49.van Heerde MJ.Biermann K, Zondervan PEet al. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. Dig Dis Sci 2012; 57: 2458–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Detlefsen S.Zamboni G, Frulloni Let al. Clinical features and relapse rates after surgery in type 1 autoimmune pancreatitis differ from type 2: A study of 114 surgically treated European patients. Pancreatology 2012; 12: 276–283. [DOI] [PubMed] [Google Scholar]
- 51.Clark CJ.Morales-Oyarvide V, Zaydfudim Vet al. Short-term and long-term outcomes for patients with autoimmune pancreatitis after pancreatectomy: A multi-institutional study. J Gastrointest Surg 2013; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 52.Miura F.Sano K, Amano Het al. Long-term surgical outcomes of patients with type 1 autoimmune pancreatitis. World J Surg 2013; 37: 162–168. [DOI] [PubMed] [Google Scholar]
- 53.Weber SM.Cubukcu-Dimopulo O, Palesty JAet al. Lymphoplasmacytic sclerosing pancreatitis: Inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg 2003; 7: 129–137; discussion 137–129. [DOI] [PubMed] [Google Scholar]
- 54.Löhr JM.Dominguez-Munoz E, Rosendahl Jet al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J 2017; 5: 153–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klöppel G, Lüttges J, Löhr M, et al. Autoimmune pancreatitis: Pathological, clinical, and immunological features. Pancreas 2003; 27: 14–19. [DOI] [PubMed] [Google Scholar]
- 56.Tacelli M.Celsa C, Magro Bet al. Risk factors for rate of relapse and effects of steroid maintenance therapy in patients with autoimmune pancreatitis: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019; 17: 1061–1072.e8. [DOI] [PubMed] [Google Scholar]
- 57.Frulloni L.Scattolini C, Falconi Met al. Autoimmune pancreatitis: Differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol 2009; 104: 2288–2294. [DOI] [PubMed] [Google Scholar]
- 58.van Buuren HR.Vleggaar FP, Willemien Erkelens Get al. Autoimmune pancreatocholangitis: A series of ten patients. Scand J Gastroenterol 2006; 41: 70–78. [DOI] [PubMed] [Google Scholar]
- 59.Church NI.Pereira SP, Deheragoda MGet al. Autoimmune pancreatitis: Slinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 2007; 102: 2417–2425. [DOI] [PubMed] [Google Scholar]
- 60.Czako L.Gyokeres T, Topa Let al. Autoimmune pancreatitis in Hungary: A multicenter nationwide study. Pancreatology 2011; 11: 261–267. [DOI] [PubMed] [Google Scholar]
- 61.Vujasinovic M.Valente R, Maier Pet al. Diagnosis, treatment and long-term outcome of autoimmune pancreatitis in Sweden. Pancreatology 2018; 18: 900–904. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Serrano A.Crespo J, Pascual Iet al. Diagnosis, treatment and long-term outcomes of autoimmune pancreatitis in Spain based on the International Consensus Diagnostic Criteria: A multi-centre study. Pancreatology 2016; 16: 382–390. [DOI] [PubMed] [Google Scholar]
- 63.Vujasinovic M.Hedstrom A, Maisonneuve Pet al. Zinc deficiency in patients with chronic pancreatitis. World J Gastroenterol 2019; 25: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindkvist B.Dominguez-Munoz JE, Luaces-Regueira Met al. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology 2012; 12: 305–310. [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Moneo E.Stigliano S, Hedstrom Aet al. Deficiency of fat-soluble vitamins in chronic pancreatitis: A systematic review and meta-analysis. Pancreatology 2016; 16: 988–994. [DOI] [PubMed] [Google Scholar]
- 66.Duggan SN.Smyth ND, O’Sullivan Met al. The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr Clin Pract 2014; 29: 348–354. [DOI] [PubMed] [Google Scholar]
- 67.Duggan SN.Smyth ND, Murphy Aet al. High prevalence of osteoporosis in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014; 12: 219–228. [DOI] [PubMed] [Google Scholar]
- 68.Dujsikova H, Dite P, Tomandl J, et al. Occurrence of metabolic osteopathy in patients with chronic pancreatitis. Pancreatology 2008; 8: 583–586. [DOI] [PubMed] [Google Scholar]
- 69.Ito T.Kawa S, Matsumoto Aet al. Risk factors for pancreatic stone formation in type 1 autoimmune pancreatitis: A long-term Japanese multicenter analysis of 624 patients. Pancreas 2019; 48: 49–54. [DOI] [PubMed] [Google Scholar]
- 70.Maruyama M.Arakura N, Ozaki Yet al. Type 1 autoimmune pancreatitis can transform into chronic pancreatitis: A long-term follow-up study of 73 Japanese patients. Int J Rheumatol 2013; 272595. [DOI] [PMC free article] [PubMed]
- 71.Bjornsson E, Chari ST, Smyrk TC, et al. Immunoglobulin G4 associated cholangitis: Description of an emerging clinical entity based on review of the literature. Hepatology 2007; 45: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 72.EASL. Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol 2015; 63: 971–1004. [DOI] [PubMed] [Google Scholar]
- 73.Ghazale A.Chari ST, Zhang Let al. Immunoglobulin G4-associated cholangitis: Clinical profile and response to therapy. Gastroenterology 2008; 134: 706–715. [DOI] [PubMed] [Google Scholar]
- 74.EASL. Clinical Practice Guidelines : Management of cholestatic liver diseases. J Hepatol 2009; 51: 237–267. [DOI] [PubMed] [Google Scholar]
- 75.Stone JH.Khosroshahi A, Deshpande Vet al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum 2012; 64: 3061–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamisawa T.Nakazawa T, Tazuma Set al. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci 2019; 26: 9–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beuers U.Gershwin ME, Gish RGet al. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Clin Res Hepatol Gastroenterol 2015; 39: e57–e59 [DOI] [PubMed] [Google Scholar]
- 78.Tanaka A.Tazuma S, Okazaki Ket al. Clinical features, response to treatment, and outcomes of IgG4-related sclerosing cholangitis. Clin Gastroenterol Hepatol 2017; 15: 920–926. [DOI] [PubMed] [Google Scholar]
- 79.de Vries E.Tielbeke F, Hubers Let al. IgG4/IgG RNA ratio does not accurately discriminate IgG4-related disease from pancreatobiliary cancer. J Hepatol Reports 2020; in press. [DOI] [PMC free article] [PubMed]
- 80.Nakazawa T. Ikeda Y, Kawaguchi Yet al. Isolated intrapancreatic IgG4-related sclerosing cholangitis. World J Gastroenterol 2015; 21: 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsubayashi H.Uesaka K, Sugiura Tet al. IgG4-related sclerosing cholangitis without obvious pancreatic lesion: Difficulty in differential diagnosis. J Dig Dis 2014; 15: 394–403. [DOI] [PubMed] [Google Scholar]
- 82.Vujasinovic M.Maier P, Maetzel Het al. Immunoglobulin G subtypes-1 and 2 (IgG1 and IgG2) can differentiate between autoimmune pancreatitis with associated cholangiopathy and primary sclerosing cholangitis. UEG J 2020; in press.
- 83.Nakazawa T.Naitoh I, Hayashi Ket al. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J Gastroenterol 2012; 47: 79–87. [DOI] [PubMed] [Google Scholar]
- 84.Huggett MT.Culver EL, Kumar Met al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol 2014; 109: 1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandanayake NS.Church NI, Chapman MHet al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol 2009; 7: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka A.Tazuma S, Okazaki Ket al. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci 2014; 21: 43–50. [DOI] [PubMed] [Google Scholar]
- 87.Xiao J.Xu P, Li Bet al. Analysis of clinical characteristics and treatment of immunoglobulin G4-associated cholangitis: A retrospective cohort study of 39 IAC patients. Medicine (Baltimore) 2018; 97: e9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W.Chen W, He Xet al. Poor response of initial steroid therapy for IgG4-related sclerosing cholangitis with multiple organs affected. Medicine (Baltimore) 2017; 96: e6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buijs J.van Heerde MJ, Rauws EAet al. Comparable efficacy of low- versus high-dose induction corticosteroid treatment in autoimmune pancreatitis. Pancreas 2014; 43: 261–267. [DOI] [PubMed] [Google Scholar]
- 90.Brito-Zeron P.Kostov B, Bosch Xet al. Therapeutic approach to IgG4-related disease: A systematic review. Medicine (Baltimore) 2016; 95: e4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Umemura T.Zen Y, Hamano Het al. Immunoglobin G4-hepatopathy: Association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology 2007; 46: 463–471 [DOI] [PubMed] [Google Scholar]
- 92.Umemura T.Zen Y, Hamano Het al. Clinical significance of immunoglobulin G4-associated autoimmune hepatitis. J Gastroenterol 2011; 46(Suppl 1): 48–55. [DOI] [PubMed] [Google Scholar]
- 93.Chung H.Watanabe T, Kudo Met al. Identification and characterization of IgG4-associated autoimmune hepatitis. Liver Int 2010; 30: 222–231. [DOI] [PubMed] [Google Scholar]
- 94.Canivet CM.Anty R, Patouraux Set al. Immunoglobulin G4-associated autoimmune hepatitis may be found in Western countries. Dig Liver Dis 2016; 48: 302–308. [DOI] [PubMed] [Google Scholar]
- 95.Castillo-Rama M.Sebagh M, Sasatomi Eet al. “Plasma cell hepatitis” in liver allografts: Identification and characterization of an IgG4-rich cohort. Am J Transplant 2013; 13: 2966–2977. [DOI] [PubMed] [Google Scholar]
- 96.Ahn KS.Kang KJ, Kim YHet al. Inflammatory pseudotumors mimicking intrahepatic cholangiocarcinoma of the liver: IgG4-positivity and its clinical significance. J Hepatobiliary Pancreat Sci 2012; 19: 405–412. [DOI] [PubMed] [Google Scholar]
- 97.Sheng RF.Zhai CW, Ji Yet al. Role of MR in the differentiation of IgG4-related from non-IgG4-related hepatic inflammatory pseudotumor. Hepatobiliary Pancreat Dis Int 2017; 16: 631–637. [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto H.Yamaguchi H, Aishima Set al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: A comparative clinicopathologic study. Am J Surg Pathol 2009; 33: 1330–1340. [DOI] [PubMed] [Google Scholar]
- 99.Zen Y.Harada K, Sasaki Met al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor and sclerosing pancreatitis-associated sclerosing cholangitis: Do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 2004; 28: 1193–1203. [DOI] [PubMed] [Google Scholar]
- 100.Ko Y.Woo JY, Kim JWet al. An immunoglobulin G4-related sclerosing disease of the small bowel: CT and small bowel series findings. Korean J Radiol 2013; 14: 776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ciccone F.Ciccone A, Di Ruscio Met al. IgG4-related disease mimicking Crohn’s disease: A case report and review of literature. Dig Dis Sci 2018; 63: 1072–1086. [DOI] [PubMed] [Google Scholar]
- 102.Bilal M, Gulati A, Clarke K. Immunoglobulin G4 IgG4)-associated pouchitis - Part of IgG4 related disease? A case series and review of the literature. Dig Liver Dis 2016; 48: 817–819. [DOI] [PubMed] [Google Scholar]
- 103.Obiorah I.Hussain A, Palese Cet al. IgG4-related disease involving the esophagus: A clinicopathological study. Dis Esophagus 2017; 30: 1–7. [DOI] [PubMed] [Google Scholar]
- 104.Notohara K, et al. Gastrointestinal manifestation of immunoglobulin G4-related disease: clarification through a multicenter survey. J Gastroenterol 2018; 53: 845–853. [DOI] [PubMed] [Google Scholar]
- 105.Topal F, et al. The prevalence of IgG4-positive plasma cell infiltrates in inflammatory bowel disease patients without autoimmune pancreatitis. Turk J Gastroenterol 2014; 25: 558–562. [DOI] [PubMed] [Google Scholar]
- 106.Notohara K.Kamisawa T, Uchida Ket al. Clinicopathological features of Type 2 autoimmune pancreatitis in Japan: Results of a multicenter survey. Pancreas 2015; 44: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 107.Choi S.B, Lim CH, Cha MG, et al. IgG4-related disease of the rectum. Ann Surg Treat Res 2016; 90: 292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fujita K.Naganuma M, Saito Eet al. Histologically confirmed IgG4-related small intestinal lesions diagnosed via double balloon enteroscopy. Dig Dis Sci 2012; 57: 3303–3306. [DOI] [PubMed] [Google Scholar]
- 109.Harada A, Torisu T, Sakuma T, et al. A case of duodenal bulb involvement of immunoglobulin G4 related disease complicated by ulcerative colitis. Dig Liver Dis 2018; 50: 515. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe A.Goto T, Kamo Het al. Resection of lesions in the ileum of patients with IgG4-related disease may ameliorate disease progression without steroid administration. Surg Case Rep 2018; 4: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abe A.Manabe T, Takizawa Net al. IgG4-related sclerosing mesenteritis causing bowel obstruction: A case report. Surg Case Rep 2016; 2: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Comtesse S, Friemel J, Fankhauser R, et al. Enterocolic lymphocytic phlebitis of the cecal pole and appendix vermiformis with increase of IgG4-positive plasma cells. Virchows Arch 2014; 464: 113–116. [DOI] [PubMed] [Google Scholar]
- 113.Kim HS, Kang WK, Chung DJ. Appendiceal immunoglobulin G4-related disease mimicking appendiceal tumor or appendicitis: A case report. Korean J Radiol 2016; 17: 56–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hiyoshi Y.Oki E, Zaitsu Yet al. IgG4-related disease of the ileocecal region mimicking malignancy: A case report. Int J Surg Case Rep 2014; 5: 669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fujita T, Ando T, Sakakibara M, et al. Refractory gastric ulcer with abundant IgG4-positive plasma cell infiltration: A case report. World J Gastroenterol 2010; 16: 2183–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wong DD.Pillai SR, Kumarasinghe MPet al. IgG4-related sclerosing disease of the small bowel presenting as necrotizing mesenteric arteritis and a solitary jejunal ulcer. Am J Surg Pathol 2012; 36: 929–934. [DOI] [PubMed] [Google Scholar]
- 117.Hasosah MY.Satti MB, Yousef YAet al. IgG4-related sclerosing mesenteritis in a 7-year-old Saudi girl. Saudi J Gastroenterol 2014; 20: 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coulier B, Montfort L, Beniuga G, et al. Small bowel obstruction caused by peritoneal immunoglobulin g4-related disease mimicking carcinomatosis: Case report. Korean J Radiol 2014; 15: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skorus U, Kenig J and, Mastalerz K. IgG4-related disease manifesting as an isolated gastric lesion – a literature review. Pol Przegl Chir 2018; 90: 41–45. [DOI] [PubMed] [Google Scholar]
- 120.Lin W.Lu S, Chen Het al. Clinical characteristics of immunoglobulin G4-related disease: A prospective study of 118 Chinese patients. Rheumatology Oxford) 2015; 54: 1982–1990. [DOI] [PubMed] [Google Scholar]
- 121.Wallace ZS.Deshpande V, Mattoo Het al. IgG4-related disease: Clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol 2015; 67: 2466–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wallace ZS.Zhang Y, Perugino CAet al. Clinical phenotypes of IgG4-related disease: An analysis of two international cross-sectional cohorts. Ann Rheum Dis 2019; 78: 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ebbo M.Grados A, Bernit Eet al. Pathologies associated with serum IgG4 elevation. Int J Rheumatol 2012; 602809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Campochiaro C.Ramirez GA, Bozzolo EPet al. IgG4-related disease in Italy: Clinical features and outcomes of a large cohort of patients. Scand J Rheumatol 2016; 45: 135–145. [DOI] [PubMed] [Google Scholar]
- 125.Martinez-Pimienta G, Noriega-Alvarez E, Simo-Perdigo M. Study of systemic disease IgG4. Usefulness of 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography for staging: Selection of biopsy site: evaluation of treatment response and follow-up. Eur J Rheumatol 2017; 4: 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Inoue D.Yoshida K, Yoneda Net al. IgG4-related disease: Dataset of 235 consecutive patients. Medicine (Baltimore) 2015; 94: e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sekiguchi H.Horie R, Kanai Met al. IgG4-related disease: Retrospective analysis of one hundred sixty-six patients. Arthritis Rheumatol 2016; 68: 2290–2299. [DOI] [PubMed] [Google Scholar]
- 128.Yamada K.Yamamoto M, Saeki Tet al. New clues to the nature of immunoglobulin G4-related disease: A retrospective Japanese multicenter study of baseline clinical features of 334 cases. Arthritis Res Ther 2017; 19: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Bommel EF, Jansen I, Hendriksz TR, et al. Idiopathic retroperitoneal fibrosis: Prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltimore ) 2009; 88: 193–201. [DOI] [PubMed] [Google Scholar]
- 130.Ebbo M.Daniel L, Pavic Met al. IgG4-related systemic disease: Features and treatment response in a French cohort: Results of a multicenter registry. Medicine (Baltimore) 2012; 91: 49–56. [DOI] [PubMed] [Google Scholar]
- 131.Khosroshahi A.Wallace ZS, Crowe JLet al. International Consensus Guidance Statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 2015; 67: 1688–1699. [DOI] [PubMed] [Google Scholar]
- 132.Berti A.Della-Torre E, Gallivanone Fet al. Quantitative measurement of 18F-FDG PET/CT uptake reflects the expansion of circulating plasmablasts in IgG4-related disease. Rheumatology Oxford) 2017; 56: 2084–2092. [DOI] [PubMed] [Google Scholar]
- 133.Mattoo H.Mahajan VS, Della-Torre Eet al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014; 134: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grados A.Ebbo M, Piperoglou Cet al. T cell polarization toward TH2/TFH2 and TH17/TFH17 in patients with IgG4-related disease. Front Immunol 2017; 8: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akiyama M.Suzuki K, Yamaoka Ket al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol 2015; 67: 2476–2481. [DOI] [PubMed] [Google Scholar]
- 136.Takahashi H.Yamashita H, Morooka Met al. The utility of FDG-PET/CT and other imaging techniques in the evaluation of IgG4-related disease. Joint Bone Spine 2014; 81: 331–336. [DOI] [PubMed] [Google Scholar]
- 137.Zhang J.Chen H, Ma Yet al. Characterizing IgG4-related disease with 1)8)F-FDG PET/CT: A prospective cohort study. Eur J Nucl Med Mol Imaging 2014; 41: 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ebbo M.Grados A, Guedj Eet al. Usefulness of 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography for staging and evaluation of treatment response in IgG4-related disease: A retrospective multicenter study. Arthritis Care Res (Hoboken) 2014; 66: 86–96. [DOI] [PubMed] [Google Scholar]
- 139.Matsubayashi H.Furukawa H, Maeda Aet al. Usefulness of positron emission tomography in the evaluation of distribution and activity of systemic lesions associated with autoimmune pancreatitis. Pancreatology 2009; 9: 694–699. [DOI] [PubMed] [Google Scholar]
- 140.Wallace ZS.Khosroshahi A, Carruthers MDet al. An international multispecialty validation study of the IgG4-Related Disease Responder Index. Arthritis Care Res 2018; 70: 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carruthers MN, Khosroshahi A, Augustin T, et al. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015; 74: 14–18. [DOI] [PubMed] [Google Scholar]
- 142.Ghazale A.Chari ST, Smyrk TC,et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007; 102: 1646–1653. [DOI] [PubMed] [Google Scholar]
- 143.Della-Torre E, Lanzillotta M and, Doglioni C. Immunology of IgG4-related disease. Clin Exp Immunol 2015; 181: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carruthers MN.Topazian MD, Khosroshahi Aet al. Rituximab for IgG4-related disease: A prospective: open-label trial. Ann Rheum Dis 2015; 74: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 145.Della-Torre E.Galli L, Franciotta Det al. Diagnostic value of IgG4 Indices in IgG4-related hypertrophic pachymeningitis. J Neuroimmunol 2014; 266: 82–86. [DOI] [PubMed] [Google Scholar]
- 146.Della-Torre E.Passerini G, Furlan Ret al. Cerebrospinal fluid analysis in immunoglobulin G4-related hypertrophic pachymeningitis. J Rheumatol 2013; 40: 1927–1929. [DOI] [PubMed] [Google Scholar]
- 147.Lu LX, Della-Torre E, Stone JH, et al. IgG4-related hypertrophic pachymeningitis: Clinical features, diagnostic criteria, and treatment. JAMA Neurol 2014; 71: 785–793. [DOI] [PubMed] [Google Scholar]
- 148.Lanzillotta M.Della-Torre E, Milani Ret al. Increase of circulating memory B cells after glucocorticoid-induced remission identifies patients at risk of IgG4-related disease relapse. Arthritis Res Ther 2018; 20: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lanzillotta M.Della-Torre E, Milani Ret al. Effects of glucocorticoids on B-cell subpopulations in patients with IgG4-related disease. Clin Exp Rheumatol 2019; 37 (Suppl. 118): S159–S166. [PubMed]
- 150.Thompson A and, Whyte A. Imaging of IgG4-related disease of the head and neck. Clin Radiol 2018; 73: 106–120. [DOI] [PubMed] [Google Scholar]
- 151.Narayan AK, Baer A, Fradin J. Sonographic findings of IgG4-related disease of the salivary glands: Case report and review of the literature. J Clin Ultrasound 2018; 46: 73–77. [DOI] [PubMed] [Google Scholar]
- 152.George V, Tammisetti VS, Surabhi VR, et al. Chronic fibrosing conditions in abdominal imaging. Radiographics 2013; 33: 1053–1080. [DOI] [PubMed] [Google Scholar]
- 153.Kim JH, Byun JH, Lee SS, et al. Atypical manifestations of IgG4-related sclerosing disease in the abdomen: Imaging findings and pathologic correlations. AJR Am J Roentgenol 2013; 200: 102–112. [DOI] [PubMed] [Google Scholar]
- 154.Nakatani K, Nakamoto Y, Togashi K. Utility of FDG PET/CT in IgG4-related systemic disease. Clin Radiol 2012; 67: 297–305. [DOI] [PubMed] [Google Scholar]
- 155.Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet 2015; 385: 1460–1471. [DOI] [PubMed] [Google Scholar]
- 156.Vlachou PA.Khalili K, Jang HJet al. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics 2011; 31: 1379–1402. [DOI] [PubMed] [Google Scholar]
- 157.Karim F.Loeffen J, Bramer Wet al. IgG4-related disease: A systematic review of this unrecognized disease in pediatrics. Pediatr Rheumatol Online J 2016; 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scheers I.Palermo JJ, Freedman Set al. Autoimmune pancreatitis in children: Characteristic features, diagnosis, and management. Am J Gastroenterol 2017; 112: 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kolodziejczyk E, Wejnarska K, Oracz G. Autoimmune pancreatitis in the paediatric population - review of the literature and own experience. Dev Period Med 2016; 20: 279–286. [PubMed] [Google Scholar]
- 160.Lee HM.Deheragoda M, Harrison Pet al. Autoimmune pancreatitis in children: A single centre experience in diagnosis, management and long term follow up. Pancreatology 2019; 19: 169–176. [DOI] [PubMed] [Google Scholar]
- 161.Aydemir Y.Akcoren Z, Demir Het al. Clinical and histopathological features of immunoglobulin G4-associated autoimmune hepatitis in children. J Gastroenterol Hepatol 2019; 34: 742–746. [DOI] [PubMed] [Google Scholar]
- 162.Wolfson AR, Hamilos DL. Recent advances in understanding and managing IgG4-related disease. F1000Res 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gregorio GV.Portmann B, Karani Jet al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: A 16-year prospective study. Hepatology 2001; 33: 544–553. [DOI] [PubMed] [Google Scholar]
- 164.Mieli-Vergani G.Vergani D, Baumann Uet al. Diagnosis and management of pediatric autoimmune liver disease: ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr 2018; 66: 345–360. [DOI] [PubMed] [Google Scholar]
- 165.Smolka V.Karaskova E, Tkachyk Oet al. Long-term follow-up of children and adolescents with primary sclerosing cholangitis and autoimmune sclerosing cholangitis. Hepatobiliary Pancreat Dis Int 2016; 15: 412–418. [DOI] [PubMed] [Google Scholar]
- 166.Scheers I.Palermo JJ, Freedman Set al. Recommendations for diagnosis and management of autoimmune pancreatitis in childhood: Consensus from INSPPIRE. J Pediatr Gastroenterol Nutr 2018; 67: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Parniczky A.Abu-El-Haija M, Husain Set al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology 2018; 18: 146–160. [DOI] [PubMed] [Google Scholar]
- 168.Fujii LL.Chari ST, El-Youssef Met al. Pediatric pancreatic EUS-guided trucut biopsy for evaluation of autoimmune pancreatitis. Gastrointest Endosc 2013; 77: 824–828. [DOI] [PubMed] [Google Scholar]
- 169.Scandavini C.Valente R, Rangelova Eet al. Pancreatectomies for pancreatic neoplasms in pediatric and adolescent age: A single institution experience. Pancreatology 2018; 18: 204–207. [DOI] [PubMed] [Google Scholar]
- 170.Kanno A.Nishimori I, Masamune Aet al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan. Pancreas 2012; 41: 835–839. [DOI] [PubMed] [Google Scholar]
- 171.Nishimori I, Tamakoshi A, Otsuki M. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol 2007; 42(Suppl 18): 6–8. [DOI] [PubMed] [Google Scholar]
- 172.Ito T.Nakamura T, Fujimori Net al. Characteristics of pancreatic diabetes in patients with autoimmune pancreatitis. J Dig Dis 2011; 12: 210–216. [DOI] [PubMed] [Google Scholar]
- 173.Miyazawa M.Takatori H, Shimakami Tet al. Prognosis of type 1 autoimmune pancreatitis after corticosteroid therapy-induced remission in terms of relapse and diabetes mellitus. PLoS One 2017; 12: e0188549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ito N.Yagi K, Kawano Met al. Analysis of pancreatic endocrine function in patients with IgG4-related diseases in whom autoimmune pancreatitis was ruled out by diagnostic imaging. Endocr J 2014; 61: 765–772. [DOI] [PubMed] [Google Scholar]
- 175.Frulloni L.Scattolini C, Katsotourchi AMet al. Exocrine and endocrine pancreatic function in 21 patients suffering from autoimmune pancreatitis before and after steroid treatment. Pancreatology 2010; 10: 129–133. [DOI] [PubMed] [Google Scholar]
- 176.Maire F.Rebours V, Vullierme MPet al. Does tobacco influence the natural history of autoimmune pancreatitis? Pancreatology 2014; 14: 284–288. [DOI] [PubMed] [Google Scholar]
- 177.Masuda A.Shiomi H, Matsuda Tet al. The relationship between pancreatic atrophy after steroid therapy and diabetes mellitus in patients with autoimmune pancreatitis. Pancreatology 2014; 14: 361–365. [DOI] [PubMed] [Google Scholar]
- 178.Nishimori I.Tamakoshi A, Kawa Set al. Influence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis: Findings from a nationwide survey in Japan. Pancreas 2006; 32: 244–248. [DOI] [PubMed] [Google Scholar]
- 179.Miyamoto Y.Kamisawa T, Tabata Tet al. Short and long-term outcomes of diabetes mellitus in patients with autoimmune pancreatitis after steroid therapy. Gut Liver 2012; 6: 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hirano K.Tada M, Sasahira Net al. Incidence of malignancies in patients with IgG4-related disease. Intern Med 2014; 53: 171–176. [DOI] [PubMed] [Google Scholar]
- 181.Yamamoto M.Takahashi H, Tabeya Tet al. Risk of malignancies in IgG4-related disease. Mod Rheumatol 2012; 22: 414–418. [DOI] [PubMed] [Google Scholar]
- 182.Asano J.Watanabe T, Oguchi Tet al. Association between immunoglobulin G4-related disease and malignancy within 12 years after diagnosis: An analysis after longterm followup. J Rheumatol 2015; 42: 2135–2142. [DOI] [PubMed] [Google Scholar]
- 183.Wallace ZS, Wallace CJ, Lu N, et al. Association of IgG4-related disease with history of malignancy. Arthritis Rheumatol 2016; 68: 2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ahn SS, Song JJ, Park YB, et al. Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis 2017; 20: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 185.Shiokawa M.Kodama Y, Yoshimura Ket al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 2013; 108: 610–617. [DOI] [PubMed] [Google Scholar]
- 186.Schneider A.Kodama Y, Yoshimura Ket al. Risk of cancer in patients with autoimmune pancreatitis: A single-center experience from Germany. Digestion 2017; 95: 172–180. [DOI] [PubMed] [Google Scholar]
- 187.Hart PA.Law RJ, Dierkhising RAet al. Risk of cancer in autoimmune pancreatitis: A case-control study and review of the literature. Pancreas 2014; 43: 417–421. [DOI] [PubMed] [Google Scholar]
- 188.Lee HW.Moon SH, Kim MHet al. Relapse rate and predictors of relapse in a large single center cohort of type 1 autoimmune pancreatitis: Long-term follow-up results after steroid therapy with short-duration maintenance treatment. J Gastroenterol 2018; 53: 967–977. [DOI] [PubMed] [Google Scholar]
- 189.Okamoto A, Watanabe T, Kamata K, et al. Recent updates on the relationship between cancer and autoimmune pancreatitis. Intern Med 2019; 58: 1533–1539. [DOI] [PMC free article] [PubMed]
- 190.Choi S-Y.Kim SH, Kang TWet al. Differentiating mass-forming autoimmune pancreatitis from pancreatic ductal adenocarcinoma on the basis of contrast-enhanced MRI and DWI findings. Am J Roentgenol 2016; 206: 291–300. [DOI] [PubMed] [Google Scholar]
- 191.Hur BY.Lee JM, Lee JEet al. Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: Comparison with pancreatic adenocarcinoma. J Magn Reson Imaging 2012; 36: 188–197. [DOI] [PubMed] [Google Scholar]
- 192.Yata M.Suzuki K, Furuhashi Net al. Comparison of the multidetector-row computed tomography findings of IgG4-related sclerosing cholangitis and extrahepatic cholangiocarcinoma. Clin Radiol 2016; 71: 203–210. [DOI] [PubMed] [Google Scholar]
- 193.Lee TY.Kim MH, Park DHet al. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol 2009; 193: 343–348. [DOI] [PubMed] [Google Scholar]
- 194.Imai K.Matsubayashi H, Fukutomi Aet al. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis 2011; 43: 869–874. [DOI] [PubMed] [Google Scholar]
- 195.Ishikawa T.Itoh A, Kawashima Het al. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J Gastroenterol 2012; 18: 3883–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Morishima T.Kawashima H, Ohno Eet al. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest Endosc 2016; 84: 241–248. [DOI] [PubMed] [Google Scholar]
- 197.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016; 48: 339–349. [DOI] [PubMed] [Google Scholar]
- 198.Khan MA.Grimm IS, Ali Bet al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open 2017; 5: E363–E375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Karadsheh Z, Al-Haddad M. Endoscopic ultrasound-guided fine-needle aspiration needles: Which one and in what situation? Gastrointest Endosc Clin N Am 2014; 24: 57–69. [DOI] [PubMed] [Google Scholar]
- 200.Bateman AC, Culver EL, Sommerlad M, et al. Intraduct papillary mucinous neoplasm of the pancreas: A tumour linked with IgG4-related disease? J Clin Pathol 2013; 66: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: Mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 2012; 143: 550–563. [DOI] [PubMed] [Google Scholar]
- 202.Kamisawa T.Horiguchi S, Hayashi Yet al. K-ras mutation in the major duodenal papilla and gastric and colonic mucosa in patients with autoimmune pancreatitis. J Gastroenterol 2010; 45: 771–778. [DOI] [PubMed] [Google Scholar]
- 203.Karagiannis P.Gilbert AE, Josephs DHet al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest 2013; 123: 1457–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Okazaki K.Chari ST, Frulloni Let al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology 2017; 17: 1–6. [DOI] [PubMed] [Google Scholar]
- 205.Ozden I, Dizdaroglu F, Poyanli A, et al. Spontaneous regression of a pancreatic head mass and biliary obstruction due to autoimmune pancreatitis. Pancreatology 2005; 5: 300–303. [DOI] [PubMed] [Google Scholar]
- 206.Maire F.Le Baleur Y, Rebours Vet al. Outcome of patients with type 1 or 2 autoimmune pancreatitis. Am J Gastroenterol 2011; 106: 151–156. [DOI] [PubMed] [Google Scholar]
- 207.Matsubayashi H.Kishida Y, Iwai Tet al. Transpapillary biliary stenting is a risk factor for pancreatic stones in patients with autoimmune pancreatitis. Endosc Int Open 2016; 4: E912–E917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yukutake M.Sasaki T, Serikawa Met al. Timing of radiological improvement after steroid therapy in patients with autoimmune pancreatitis. Scand J Gastroenterol 2014; 49: 727–733. [DOI] [PubMed] [Google Scholar]
- 209.Sahani DV.Kalva SP, Farrell Jet al. Autoimmune pancreatitis: Imaging features. Radiology 2004; 233: 345–352. [DOI] [PubMed] [Google Scholar]
- 210.Matsushita M.Yamashina M, Ikeura Tet al. Effective steroid pulse therapy for the biliary stenosis caused by autoimmune pancreatitis. Am J Gastroenterol 2007; 102: 220–221. [PubMed] [Google Scholar]
- 211.Masamune A.Nishimori I, Kikuta Ket al. Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut 2017; 66: 487–494. [DOI] [PubMed] [Google Scholar]
- 212.Kawa S.Okazaki K, Kamisawa Tet al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis: 2013 II. Extrapancreatic lesions: differential diagnosis. J Gastroenterol 2014; 49: 765–784. [DOI] [PubMed] [Google Scholar]
- 213.Kubota K.Kamisawa T, Okazaki Ket al. Low-dose maintenance steroid treatment could reduce the relapse rate in patients with type 1 autoimmune pancreatitis: A long-term Japanese multicenter analysis of 510 patients. J Gastroenterol 2017; 52: 955–964. [DOI] [PubMed] [Google Scholar]
- 214.Topazian M.Witzig TE, Smyrk TCet al. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol 2008; 6: 364–366. [DOI] [PubMed] [Google Scholar]
- 215.Khosroshahi A, Bloch DB, Deshpande V, et al. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum 2010; 62: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 216.Soliman H.Vullierme MP, Maire Fet al. Risk factors and treatment of relapses in autoimmune pancreatitis: Rituximab is safe and effective. United European Gastroenterol J 2019; 7: 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Naitoh I.Nakazawa T, Ohara Het al. Autoimmune pancreatitis associated with various extrapancreatic lesions during a long-term clinical course successfully treated with azathioprine and corticosteroid maintenance therapy. Intern Med 2009; 48: 2003–2007. [DOI] [PubMed] [Google Scholar]
- 218.Rovati L.Lanzillotta M, Bozzolo Eet al. Methotrexate as induction of remission therapy for type 1 autoimmune pancreatitis. Am J Gastroenterol 2019; 114: 831–833. [DOI] [PubMed] [Google Scholar]
- 219.Della-Torre E.Campochiaro C, Bozzolo EPet al. Methotrexate for maintenance of remission in IgG4-related disease. Rheumatology (Oxford) 2015; 54: 1934–1936. [DOI] [PubMed] [Google Scholar]
- 220.Beaugerie L.Brousse N, Bouvier AMet al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet 2009; 374: 1617–1625. [DOI] [PubMed] [Google Scholar]
- 221.Yunyun F.Yu C, Panpan Zet al. Efficacy and safety of low dose Mycophenolate mofetil treatment for immunoglobulin G4-related disease: A randomized clinical trial. Rheumatology (Oxford) 2019; 58: 52–60. [DOI] [PubMed] [Google Scholar]
- 222.Yunyun F.Yu C, Panpan Zet al. Efficacy of Cyclophosphamide treatment for immunoglobulin G4-related disease with addition of glucocorticoids. Sci Rep 2017; 7: 6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Park S.Chu LC, Hruban RHet al. Differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma with CT radiomics features. Diagn Interv Imaging 2020; Epub april 8, 2020 10.1016/j.diii.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 224.Lanzillotta M.Vinge-Holmquist O, Overbeek KAet al. PrescrAIP: A Pan-European Study on Current Treatment Regimens of Auto-Immune Pancreatitis. Frontiers Med 2020; in press. [DOI] [PMC free article] [PubMed]
- 225.Vujasinovic M.Pozzi Mucelli RM, Valente Ret al. Kidney involvement in patients with type 1 autoimmune pancreatitis. J Clin Med 2019; 8: 258; 10.3390/jcm8020258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang L.Chari S, Smyrk TCet al. Autoimmune pancreatitis AIP) type 1 and type 2: An international consensus study on histopathologic diagnostic criteria. Pancreas 2011; 40: 1172–1179. [DOI] [PubMed] [Google Scholar]
- 227.Asano J.Watanabe T, Oguchi Tet al. Association between immunoglobulin G4-related disease and malignancy within 12 years after diagnosis: An analysis after longterm followup. J Rheumatol 2015; 42: 2135–2142. [DOI] [PubMed] [Google Scholar]
- 228.Buijs J.Cahen DL, van Heerde MJet al. The long-term impact of autoimmune pancreatitis on pancreatic function: Quality of life, and life expectancy. Pancreas 2015; 44: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 229.Schneider A.Hirth M, Munch Met al. Risk of cancer in patients with autoimmune pancreatitis: A single-center experience from Germany. Digestion 2017; 95: 172–180. [DOI] [PubMed] [Google Scholar]
- 230.Ahn SS, Song JJ, Park YB, et al. Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis 2017; 20(8): 1028–1035. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620934911 for European Guideline on IgG4-related digestive disease – UEG and SGF evidence-based recommendations by J-Matthias Löhr, Ulrich Beuers, Miroslav Vujasinovic, Domenico Alvaro, Jens Brøndum Frøkjær, Frank Buttgereit, Gabriele Capurso, Emma L Culver, Enrique de-Madaria, Emanuel Della-Torre, Sönke Detlefsen, Enrique Dominguez-Muñoz, Piotr Czubkowski, Nils Ewald, Luca Frulloni, Natalya Gubergrits, Deniz Guney Duman, Thilo Hackert, Julio Iglesias-Garcia, Nikolaos Kartalis, Andrea Laghi, Frank Lammert, Fredrik Lindgren, Alexey Okhlobystin, Grzegorz Oracz, Andrea Parniczky, Raffaella Maria Pozzi Mucelli, Vinciane Rebours, Jonas Rosendahl, Nicolas Schleinitz, Alexander Schneider, Eric FH van Bommel, Caroline Sophie Verbeke, Marie Pierre Vullierme, Heiko Witt and the UEG guideline working group in United European Gastroenterology Journal