Abstract
Background
Glucagon-like peptide-1 receptor agonists, such as liraglutide, reduce hyperglycaemia and induce weight loss and are used as a treatment in diabetes. However, common adverse effects include nausea, loss of appetite and prolonged gastric emptying. It is not known whether these changes are centrally generated or if liraglutide alters the enteric motility.
Objective
To investigate the effects of liraglutide on gastrointestinal function and symptoms.
Methods
A total of 48 adults with type 1 diabetes and confirmed distal symmetric polyneuropathy were randomised to receive liraglutide 1.8 mg/day or placebo for 26 weeks. Regional transit times and motility indexes were assessed with a wireless motility capsule, whereas symptoms were evaluated using the validated gastroparesis cardinal symptom index.
Results
Liraglutide treatment reduced large bowel transit time (31.7%, p = 0.04) and decreased motility index (6.1%, p = 0.04) compared to placebo, whereas the groups did not differ in gastric emptying or small-bowel transit times. Liraglutide increased postprandial fullness with 29% (p = 0.01). Increased small bowel transit time was associated with decreased bloating (p = 0.008).
Conclusion
Liraglutide accelerates large bowel transit and decreases motility index, which may indicate better coordination of propulsive motility. This potentially improves the function of the enteric nervous system, leading to normalised colonic function and positive effects in type 1 diabetes.
Keywords: Diabetes mellitus type 1, polyneuropathies, liraglutide, gastrointestinal transit, gastrointestinal motility, digestive signs and symptoms
Introduction
Type 1 diabetes is characterised by hyperglycaemia caused by insulin deficiency. Prolonged hyperglycaemia results in microvascular complications, of which neuropathy is amongst the most common affecting both peripheral and autonomic nerves, leading to reduced neuronal function.1 Disturbances of the enteric nerves results in gastro-enteropathy, accompanied by disabling symptoms of nausea, vomiting, bloating, early satiety, abdominal pain and diarrhoea or constipation, which is experienced by more than 50% of patients.1,2 Yet, gastro-enteropathy is frequently underdiagnosed and effective management is challenging because symptoms rarely associate with impaired function.3 Treatment challenges may also be a result of the underlying complexity of gastrointestinal dysfunction, as any disruption may disturb the entire regulatory balance.2 As an example, normal gastric emptying is the result of coordinated motility of the stomach, duodenum and the remaining gastrointestinal tract, induced by complex somatosensory signalling by the autonomic nervous system and central nervous system, collectively referred to as the gut-brain axis.2 Furthermore, emptying is influenced by a number of factors including the characteristics of food contents in the stomach, neurotransmitters evoking gastrointestinal motility and migrating motor complexes.4
The glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide is an analogue to the human native GLP-1 hormone released from the intestinal L cells with an increased plasma half-life compared with the natural hormone. Liraglutide reduces hyperglycaemia and induces weight loss in people with type 2 diabetes.5 The efficacy of long-term liraglutide on hyperglycaemia in type 1 diabetes is ambiguous, however, the development of adverse effects including nausea, appetite loss and weight loss appear more consistent.6–8 It has been shown that GLP-1 administration delays gastric emptying time and induces nausea in people with type 1 diabetes6,7 and delays gastric emptying in people with type 2 diabetes or obesity.9–11 These adverse effects often limit the intended therapeutic improvement of hyperglycaemia and weight loss due to discontinuation of the treatment. However, it is not fully known how or to what degree liraglutide elicits changes on enteric motility or how it induces gastrointestinal symptoms. The wireless motility capsule provides objective measures of temperature, pressure and pH as it traverses the gastrointestinal tract. Based on these measures, segmental transit times and motility indices (useful composite measures that incorporate both contraction frequency and amplitude) can be estimated.
Hitherto, studies investigating gastric emptying and gastrointestinal symptoms are sparse and show ambiguous results.6 To our knowledge an association between objective measures of gastrointestinal function such as segmental transit or motility and the concomitant gastrointestinal symptoms have not been investigated during GLP-1 treatment. We hypothesised that liraglutide would exert an effect on all gut segments in adults with type 1 diabetes and confirmed distal symmetrical polyneuropathy. Therefore, the aims of the present study were to investigate whether liraglutide administration induced (a) increased gastric emptying time, (b) changes in regional gastrointestinal transit time and motility and (c) change in self-reported gastrointestinal symptoms. Lastly, we aimed to investigate how changes in these measures correlate.
Materials and methods
Study population
This paper is based on post-hoc analysis of a prospective, randomised, double-blinded, parallel-group, placebo-controlled trial investigating the efficacy of liraglutide on inflammation and neuronal function, conducted at Aalborg University Hospital from June 2014 to January 2017 (Treatment of Diabetic Neuropathy with Liraglutide (TODINELI), ethical review board (Den Videnskabsetiske Komité for Region Nordjylland): N-20130077 (17 December 2013), EUDRACT: 2013-004375-12, clinicaltrials.gov: NCT02138045). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee (Den Videnskabsetiske Komité for Region Nordjylland: N-20130077 (17 December 2013)). Potential eligible participants were recruited at the Department of Endocrinology and pre-screened on the basis of biothesiometry with a recorded vibration perception threshold above 18 volts. Distal symmetric polyneuropathy was verified by nerve conduction tests, according to the Toronto criteria. Additional inclusion criteria were age above 18 years, a verified diagnosis of type 1 diabetes for a minimum of 2 years: HbA1c ≥6.5% (>48 mmol/mol), stable medication and body mass index >22. Exclusion criteria included type 2 diabetes, other neurological disorders than distal symmetric polyneuropathy, estimated glomerular filtration rate <60 ml/min/1.73 m2; calcitonin >25 ng/l, HbA1c level <6.5%, use of GLP-1 analogues or DPP-4 inhibitors as described in detail previously.12 All participants gave their written informed consent prior to entry.
Randomisation
Treatments (active vs placebo) were masked and appeared identical as injectable pens designed to be used with disposable needles. Participants were randomly assigned 1:1 to receive liraglutide or placebo in blocks of eight, generated from a randomisation list provided by the drug supplier, Novo Nordisk. Dropouts were mirror randomised. The intervention was titrated over a 6-week period to a dose minimum 1.2–1.8 mg/day, depending on tolerability. The treatment was continued for further 26 weeks. All participants underwent multiple tests over a period of 3 study days at baseline and all experimental procedures were performed between 8am and noon. Follow-up was performed after 26 weeks of intervention.
Symptom assessment
Symptoms from the upper gastrointestinal tract was examined using the Gastroparesis Cardinal Symptom Index (GCSI) questionnaire. The GCSI is derived from the 20-item Patient Assessment of Upper Gastrointestinal Symptom Severity Index questionnaire and consists of three subcategories, namely nausea/vomiting, postprandial fullness and bloating. Scores are based on the sum of two to four items scored from no symptoms (0) to very severe symptoms (5).
Gastrointestinal assessment
Gastrointestinal components of transit times and motility index in the stomach, small bowel and large bowel regions were investigated using the standardised wireless motility capsule (SmartPill®, Medtronic, Minneapolis, USA). The non-invasive ambulatory wireless motility capsule is a validated method to measure pressure, pH and temperature as it traverses the gastrointestinal tract. Even though the wireless motility capsule is known to empty with the return of a fasting migrating motor complex, there is a high agreeability between this method and measurements from scintigraphy and radio-labelled markers on regional transit times,13–15 also in diabetic patients.16 Before initiating the test, participants were instructed to fast overnight and to discontinue drugs influencing the gastrointestinal tract including acid suppressants 3 days prior to investigation. The capsule was ingested accompanied by a standardised meal consisting of a Smartbar (Medtronic, Minneapolis, USA) and 50 mL of water. Data from the capsule were recorded over 1–7 days on a portable receiver and capsule expulsion was confirmed by a drop in temperature or a persistent lack of connection between the capsule and receiver after bowel movements. Data from the receiver were analysed using MotiliGI software (version 3.0, Medtronic, Minneapolis, USA) to assess transit times and GIMS data viewer (version 3.0, Medtronic, Minneapolis, USA) was used to retrieve motility index measurements. The regional gastrointestinal transit times were based on identification of stereotypical changes in the pH defined by Sarosiek et al.17 The motility index is a useful composite measure that incorporates both contraction frequency and amplitude and is calculated as ln(sum of amplitudes x number of contractions + 1)18 assessed on contractions between 10–300 mmHg. Additionally, extensive normative values datasets have been published, making gastrointestinal dysfunction easier to quantify.19,20
Statistics
Participant characteristics are presented as means with standard deviations, medians with interquartile range or percentages, as appropriate. Effects of liraglutide versus placebo were modelled by linear regression adjusting for baseline variables of study outcomes. Also, the modifying effect on gastrointestinal symptoms of liraglutide-induced changes in measures of transit time and the motility index during the trial were modelled by linear regression with transit time and motility index as determinants and gastrointestinal symptoms as outcomes. Models were adjusted for baseline variables of gastrointestinal symptoms.
All analyses were performed as per-protocol analysis. To fulfil the requirement of a normal distribution of the model residuals, outcomes were log-transformed. Thus, estimates are presented as percentage changes due to subsequent back transformation of results. Statistical significance was inferred at a two-tailed p value <0.05. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 48 people was randomised and included in the study. Baseline demographics for the liraglutide and placebo group of the participants who completed the trial are presented in Table 1. There was no significant demographic difference between the groups, except that more participants in the placebo group used insulin pumps rather than pens (11% vs 40%, p = 0.04). Additionally, the wireless motility capsule measurements and symptoms were similar between the groups at baseline (p > 0.05), which have previously been published.21,22 No differences were found between dropouts and replacements and details can be found in the supplementary materials.
Table 1.
Baseline demographics.
| Liraglutide (n = 19) | Placebo (n = 20) | p value | |
|---|---|---|---|
| Sex (male) | 17/89% | 14/70% | 0.132 |
| Age (years) | 51 ± 10 | 50 ± 8 | 0.659 |
| Disease duration (years) | 32 ± 11 | 32 ± 7 | 0.965 |
| Weight (kilogram) | 92.4 ± 16.3 | 92.3 ± 16.7 | 0.986 |
| Height (cm) | 179 ± 7 | 178 ± 10 | 0.632 |
| Body mass index | 28.7 ± 4.1 | 28 (26.0–32.5) | 0.672 |
| HbA1c (mmol/mol) (NGSP units) | 69 ± 12 (8.5 ± 3.2) | 64 ± 10 (8.0 ± 3.1) | 0.141 |
| Vibration threshold (volt) | 37 ± 13 | 31 ± 13 | 0.197 |
| RR interval | 776 ± 123 | 758 ± 69 | 0.581 |
| Cardiac vagal tone | 1.56 (1.02–2.38) | 2.76 ± 1.37 | 0.160 |
| Orthostatic hypotension | 18/95% | 17/85% | 0.310 |
| Fast-acting insulin use | 17/90% | 17/85% | 0.675 |
| Fast-acting insulin dose | 32 (26–46) | 33 (24–47) | 0.852 |
| Long-acting insulin use | 9/47% | 9/45% | 0.882 |
| Long-acting insulin dose | 24 (20–40) | 33 (20–40) | 0.545 |
| Pump use | 2/11% | 8/40% | 0.035 |
| Smoking | 4/21% | 4/20% | 0.935 |
| Nausea/vomiting | 0.00 (0.00–0.33) | 0.00 (0.00–0.17) | 0.545 |
| Postprandial fullness | 0.25 (0.00–0.75) | 0.50 (0.00–1.25) | 0.802 |
| Bloating | 0.00 (0.00–1.50) | 0.50 (0.00–2.00) | 0.161 |
| Gastroparesis cardinal symptoms index | 0.17 (0.08–1.00) | 0.33 (0.08–1.36) | 0.452 |
| Gastric emptying | 202 (147–240) | 195 (170–265) | 0.447 |
| Small bowel transit | 296 (210–337) | 266 (228–352) | 0.726 |
| Large bowel transit | 1871 (1153–2368) | 2000 (1216–3406) | 0.619 |
| Gastric motility index | 11.6 (10.8–12.9) | 11.1 (9.7–12.6) | 0.421 |
| Small bowel motility index | 13.7 (12.9–15.2) | 13.5 (12.0–15.1) | 0.640 |
| Large bowel motility index | 16.9 (14.3–17.9) | 16.5 (15.4–17.5) | 0.907 |
Data displayed as means ± standard deviation, medians (interquartile range) or number/percentage.
Vibration threshold is average of left and right and can only be measured up to 50 volts. RR: oscillation between successive sinus beats; NGSP: National Glycohemoglobin Standardization Program.
Gastric emptying and motility
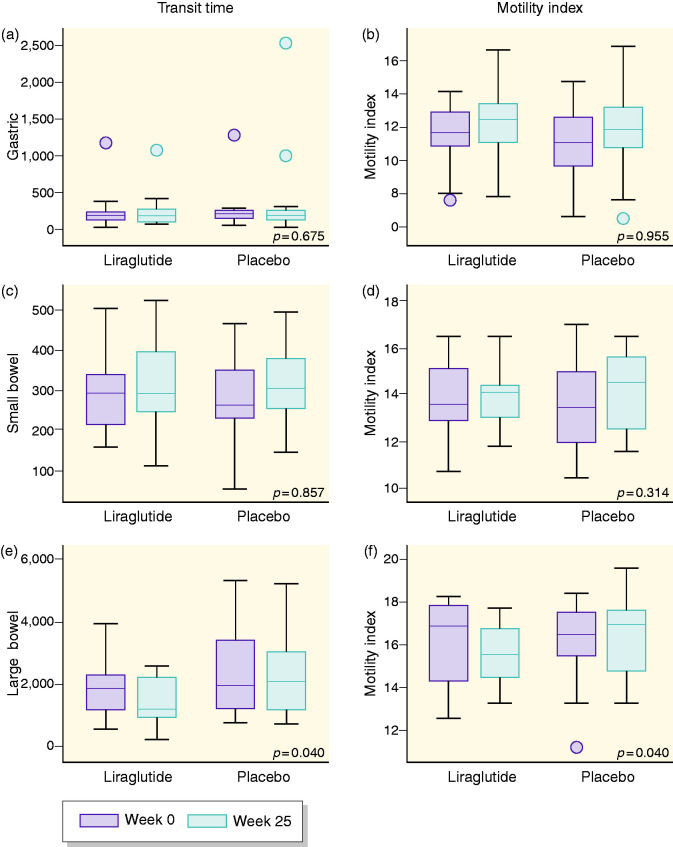
In comparison to placebo, 26 weeks of liraglutide treatment did not induce changes in gastric emptying (9.5% increase (95% confidence interval (CI) -28.28; 67.14, p = 0.68) or the gastric motility index (0.34% decrease (95% CI -11.46; 12.18), p = 0.96) (see Figure 1).
Figure 1.
Effect of liraglutide and placebo on gastrointestinal parameters. Graphs are shown as boxplots between baseline (dark grey) and after 26 weeks (light grey) for the respective trial groups. Graphs are as follows: (a) gastric transit time, (b) gastric motility index, (c) small bowel transit time, (d) small bowel motility index, (e) large bowel transit time and (f) large bowel motility index. No change was found between liraglutide and placebo in graphs (a) to (d); however, a significant decrease in large bowel transit time motility index in the liraglutide group was found in (e) and (f).
Small bowel transit and motility
In comparison to placebo, 26 weeks of liraglutide treatment did not induce changes in small bowel transit time (1.96% decrease (95% CI -20.95; 21.59), p = 0.86), or motility index (2.77% decrease (95% CI −7.94; 2.69), p = 0.31) (see Figure 1).
Large bowel transit and motility
In comparison to placebo, 26 weeks of liraglutide treatment shortened large bowel transit time with 31.7% (95% CI −52.5; −1.7, p = 0.04) and decreased motility index with 6.1% (95% CI −11.5; −0.3, p = 0.04) (see Figure 1).
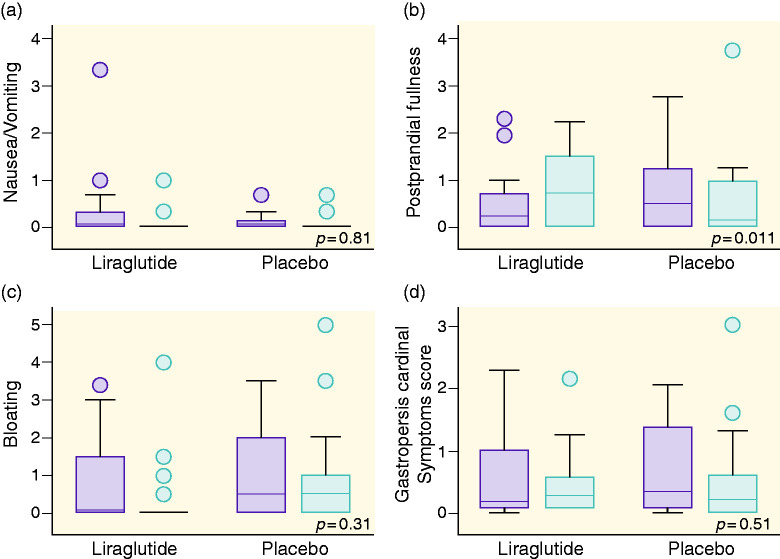
Symptom severity score
At randomisation, there was no difference in total GCSI or sub-scores between the groups assigned to placebo and liraglutide treatment. In comparison to placebo, 26 weeks of liraglutide treatment increased postprandial fullness by 28.8% (95% CI 5.8; 54.8, p = 0.01) (see Figure 2). Liraglutide did not induce significant changes in any of the other scores; GCSI 5.3% increase (95% CI −9.5; 22.4, p = 0.51), nausea/vomiting 1.2% increase (95% CI −10.2; 14.1, p = 0.81) and bloating 11.5% decrease (95% CI −30.1; 12.1, p = 0.31).
Figure 2.
Effect of liraglutide and placebo on symptom severity. Graphs are shown as boxplots between baseline (dark grey) and after 26 weeks (light grey) for the respective trial groups. Graphs are as follows: (a) nausea and vomiting, (b) postprandial fullness, (c) bloating and (d) gastrointestinal cardinal symptom score. An increase in symptom score was found in (b).
Correlations between transit time or motility index and clinical parameters
In the liraglutide-treated group, increased small bowel transit time was associated with decreased bloating (p = 0.008) (see Table 2), specifically, a 1-minute increase in small bowel transit time led to a 0.11% reduction in bloating symptoms (95% CI 0.19; 0.02, p = 0.002). This was not seen during placebo treatment (0.08% (95% CI: -0.02; 0.19) p = 0.12). No other associations were found.
Table 2.
Gastric motility and associations to gastrointestinal symptoms: liraglutide group.
| Nausea and vomiting | Postprandial fullness | Bloating | Gastroparesis cardinal symptoms index | |
|---|---|---|---|---|
| Gastric emptying | 0.02% (−0.01; 0.05) [0.256] | −0.04% (−0.09; 0.02) [0.201] | −0.02% (−0.08; 0.04) [0.619] | −0.01% (−0.05; 0.04) [0.778] |
| Small bowel transit | −0.07% (−0.15; 0.01) [0.099] | −0.05% (−0.22; 0.12) [0.544] | −0.18% (−0.31; -0.05) [0.008]* | −0.1% (0.21; 0.01) [0.077] |
| Large bowel transit | −0.01% (−0.01; 0.00) [0.224] | −0.01% (−0.02; 0.01) [0.548] | 0.01% (−0.01; 0.03) [0.262] | 0% (−0.01; 0.01) [0.898] |
| Gastric motility index | 3.73% (−1.48; 9.22) [0.163] | 2.87% (−6.53; 13.21) [0.563] | −2.13% (−11.11; 7.74) [0.660] | 1.93% (−4.99; 9.36) [0.594] |
| Small bowel motility index | −5.97% (−12.73; 1.31) [0.106] | −8.82% (−20.69; 4.82) [0.194] | −0.51% (−13.68; 14.67) [0.943] | −3.9% (−13.47; 6.73) [0.457] |
| Large bowel motility index | 1.27% (−4.06; 6.90) [0.648] | −0.81% (−10.28; 9.66) [0.875] | 5.74% (−3.33; 15.65) [0.223] | 2.03% (−4.91; 9.49) [0.576] |
Gastrointestinal symptoms change in percentage from baseline to follow-up in the liraglutide group with 95% confidence intervals. Results are estimates of a one-unit increase in measures of gastrointestinal transit or motility index during trial. Estimates are percentages (95% confidence intervals) [p value].
*p<0.05 for group difference (liraglutide vs. placebo).
Discussion
This study explored the effect of 26 weeks of liraglutide treatment on gastrointestinal function and symptoms in people with type 1 diabetes and verified distal symmetric polyneuropathy. We showed a shortened large bowel transit time and a decreased large bowel motility index. Furthermore, the subjective perception of postprandial fullness increased in the liraglutide group, but this was not the case for perception of nausea/vomiting or bloating, indicating the main adverse effects of liraglutide are temporary in people with type 1 diabetes. Additionally, in the liraglutide-treated group, an association between bloating and small bowel transit was shown.
Long-term effect of liraglutide on gastrointestinal function
Transit time
It is well established that short-acting GLP-1 receptor agonist induces delayed gastric emptying,23 whereas the long-acting GLP-1 receptor agonist liraglutide induces delayed gastric emptying in people with type 2 diabetes or obesity.9,10,24 In type 1 diabetes, Dejgaard et al. found slower gastric emptying after both 3 and 24 weeks of liraglutide, assessed with paracetamol in a liquid meal.6 In contrast, we showed a 10% increase in gastric emptying time in the liraglutide group, although no significant differences from placebo were observed. We have previously shown that 90% of our cohort not only exhibits distal symmetric polyneuropathy, but concomitantly suffers from orthostatic hypotension and decreased vagal tone indicative of autonomic neuropathy;12,25 however, this was equally distributed between the groups. Additionally, GLP-1 agonists have been suggested to directly diminish the vagal tone and in turn inhibit gastric emptying.26 Alternatively, this effect is caused by tachyphylaxis in the effect of liraglutide on gastric emptying due to continuous activation of liraglutide on the GLP-1 receptor, effectively inducing tolerance.24 Then again, the observed differences may be a consequence of the differences in assessment methodology applied in different studies. It is generally accepted that the wireless motility capsule is not emptied from the stomach until a fasted state is reached and the first migrating motor complexes are generated.2,15 Finally, the discrepancy can be explained by reports from Schirra et al., who showed that in response to GLP-1 administration gastric emptying was initially delayed then was followed by an accelerated emptying, which created a lag period, without affecting the total gastric emptying time in type 2 diabetes. A similar pattern may be present in type 1 diabetes.27 Due to the nature of the wireless motility capsule, however, we could not observe this lag period in our data.
In the current study, we found a 32% decrease in large bowel transit time corresponding to an average reduction of 10 hours. Previous studies have reported ambiguous results on large bowel transit times in response to administration of GLP analogues and thus both accelerated or delayed transit times exist.28,29 Nakatani et al. assessed colonic transit time with the capsule endoscope PillCam – a method resembling the wireless motility capsule – in type 2 diabetes presented with or without neuropathy. They reported that the presence of neuropathy decreased gastric emptying, but did not affect small bowel or large bowel transit.30 We believe that even though differences exist between the two studies, they still suggest a compartmentalisation of the gastrointestinal tract, focusing on the entire diabetic gastro-enteropathy and not exclusively the gastric emptying as this clearly only represents part of the syndrome.31 Furthermore, we know from previous investigations of the current cohort that they had prolonged large bowel transit time at baseline,32 therefore, this reduction in transit time is an improvement in colonic function and may in itself explain the reduction in experienced upper gastrointestinal symptoms.
Motility
The response to GLP-1 administration on gastrointestinal motility is largely unknown in humans, but animal studies have shown GLP-1 agonists possess an inhibitory effect on migrating motor complexes mediated via the vagal nerve. Consequently, an increased cycle length affects the myotonic tonus of the stomach and disrupts the myoelectric activity in the upper gastrointestinal tract.33 In the current study, we showed a 6% decrease in the large bowel motility index, but no changes in the stomach and small bowel motility indices. The motility index is a composite score describing the contractility patterns, more specifically contraction frequency and amplitude, meaning that decreased motility index corresponds to decreased myoelectric activity. Although not widely used for looking at motility in research, the motility index correlates well with other contractility indices.19 Although the measure cannot be referred directly regarding changes in peristalsis, it is plausible to conclude the motility pattern is more propulsive and better coordinated during liraglutide treatment in comparison to placebo because transit time also decreased.
Long-term effects of liraglutide on nausea and gastrointestinal symptoms
It is well known that nausea is a dose-dependent adverse effect of the administration of GLP-1 analogues such as liraglutide,34 which often results in discontinuations or reductions in the prescribed dose. Nausea is generated centrally and it has been suggested the perception of nausea in response to GLP-1 is transient and resolves within weeks.7,8 Even though the study dose was titrated relatively slowly (0.6 mg/2 weeks), the participants initially observed more and typically longer-lasting episodes of nausea and vomiting, which resulted in discontinuation of the study in 9/28 people (32%). However, following 26 weeks of treatment there was no difference in reported nausea/vomiting between the liraglutide and placebo groups, supporting central tolerance. The number of discontinued participants may have been smaller if doses had been individualised, as it has been shown that greater doses of liraglutide induce more nausea.11
The postprandial fullness subscale encompasses stomach fullness, early satiety, loss of appetite and postprandial fullness, contributing to decreased appetite, which is a well-known effect of liraglutide.6,7 The effect has been attributed to centrally mediated appetite regulation effects, such as through the vagal nerve.26 In concurrence with previous studies, we observed an increase in the postprandial fullness score in the liraglutide-treated group, possibly due to disrupted central coordination. This is supported by Brock et al., who showed increased postprandial fullness was associated with a decreased dipole shift in the cingulate cortex suggested to be linked to food-specific satiety.35 Additionally we found weight loss (data previously published)12 but based on the data we cannot distinguish the underlying mechanism.
Symptom generation and gastrointestinal function
We investigated the associations between gastrointestinal symptomatology and gastrointestinal function. We found that during liraglutide treatment, small bowel transit reduced the perceived bloating score. Usually longer small bowel transit times are associated with increased sensation of bloating in the presence of small bowel bacterial overgrowth. However, an alteration of the gastrointestinal microbiota composition towards, for example, less gas-producing flora, due to liraglutide and its influence the incretin releasing enteroendocrine cells directly through fermentation of nutrients and short-chain fatty acid or food intake, may still exist.36 However, the bacterial composition was not measured in the current study.
Limitations
First, the relatively large dropout percentage in the liraglutide-treated group may have skewed the results, especially when reporting symptom severity as those with the highest symptom burden discontinued the study. Second, even though GCSI is a validated questionnaire for assessing gastroparesis, it is not specifically developed for diabetes and it is vulnerable to recall bias. Third, the wireless motility capsule is a solid cylinder and does not resemble the texture of a food bolus or a liquid meal, so may have influenced our data. Fourth, this study investigated 1.8 mg liraglutide, contrary to the commonly used 3.0 mg in obesity, however, studies have shown no difference on gastric emptying between the two dosages, whereas an increased dose induces greater weight loss.24 Fifth, the bacterial composition was not measured in the current study, which could help explain the changes in bloating we uncovered associated with small bowel transit time. Last, the generalisability of these findings is moderate and based on the data we cannot distinguish between long-term exposure to hyperglycaemia and the presence of distal symmetric polyneuropathy.
Conclusion
Liraglutide shortened large bowel transit time and decreased the motility index, which indicates improved coordination of propulsive motility. Thus, despite the negative reputation for induction of nausea and prolonged gastric emptying time, these data show that in people with type 1 diabetes, liraglutide may potentially improve the function of the enteric nervous system. The key points are summarized below:
Liraglutide is known to induce weight loss, prolong gastric emptying and produce gastrointestinal symptoms such as nausea.
26 weeks of liraglutide treatment did not prolong gastric emptying but shortened large bowel transit time and decreased the motility index. Nausea appeared transient whereas postprandial fullness was persistently increased after 26 weeks.
Liraglutide may improve the function of the enteric nervous system, leading to normalised large bowel function and enhanced postprandial fullness inconsequential of gastroparesis, which could have a positive effect in type 1 diabetes.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620925968 for Liraglutide accelerates colonic transit in people with type 1 diabetes and polyneuropathy: A randomised, double-blind, placebo-controlled trial by Anne-Marie Langmach Wegeberg, Christian Stevns Hansen, Adam D Farmer, Jesper Scott Karmisholt, Asbjorn M Drewes, Poul Erik Jakobsen, Birgitte Brock and Christina Brock in United European Gastroenterology Journal
Author contributions
The study design and original idea came from CB, AMD and BB. CB, PEJ and JK collected data. ALW, ADF and CSH analysed the data and all authors interpreted it. ALW wrote first draft, but all authors contributed to the final manuscript and critically reviewed the manuscript for intellectual content. CB is the guarantor of the work, with full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of conflicting interest
None declared.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Novo Nordisk Scandinavia AS and Empowering Industry and Research, Northern Jutland supported this investigator-initiated and -driven trial. CB received funding from the Talent Programme, Aalborg University. No funding source had any role in study design, data collection, data analysis, data interpretation, or the preparation of this article. The authors had full access to all data in the study and final responsibility for the decision to submit for publication.
Ethics approval
This study was approved on December 7th 2013 by The North Denmark Region Committee on Health Research Ethics (N2013-0077), and registered in public databases (EUDRA CT, ref 2013-004375-12; and clinicaltrials.gov, ref NCT02138045).
Informed consent
Written, informed consent was obtained from each participant included in the study.
ORCID iD
Anne-Marie Langmach Wegeberg https://orcid.org/0000-0002-8323-4843
Supplemental material
Supplemental material for this article is available online.
References
- 1.Krishnan B, Babu S, Walker J, et al. Gastrointestinal complications of diabetes mellitus. World J Diabetes 2013; 4: 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azpiroz F, Malagelada C. Diabetic neuropathy in the gut: Pathogenesis and diagnosis. Diabetologia 2016; 59: 404–408. [DOI] [PubMed] [Google Scholar]
- 3.Cassilly DW, Wang YR, Friedenberg FK, et al. Symptoms of gastroparesis: Use of the gastroparesis cardinal symptom index in symptomatic patients referred for gastric emptying scintigraphy. Digestion 2008; 78: 144–151. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Chedid V, Ford AC, et al . Gastroparesis. Nat Rev Dis Prim 2018; 4: 41. [DOI] [PubMed] [Google Scholar]
- 5.Feinglos MN, Saad MF, Pi-Sunyer FX, et al. Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with type 2 diabetes. Diabet Med 2005; 22: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 6.Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2016; 4: 221–232. [DOI] [PubMed] [Google Scholar]
- 7.Frandsen CS, Dejgaard TF, Holst JJ, et al. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: A randomized, placebo- controlled, double-blind parallel study. Diabetes Care 2015; 38: 2250–2257. [DOI] [PubMed] [Google Scholar]
- 8.Harrison LB, Mora PF, Clark GO, et al. Type 1 diabetes treatment beyond insulin. J Investig Med 2013; 61: 40–44. [DOI] [PubMed] [Google Scholar]
- 9.Meier JJ, Rosenstock J, Hincelin-Méry A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: A randomized, open-label trial. Diabetes Care 2015; 38: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 10.Nagai Y, Hashimoto E, Oikawa R, et al. Differing effects of liraglutide on gastric emptying in Japanese patients with type 2 diabetes. Diabetes, Obes Metab 2014; 16: 573–576. [DOI] [PubMed] [Google Scholar]
- 11.Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: A randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2017; 2: 890–899. [DOI] [PubMed] [Google Scholar]
- 12.Brock C, Hansen CS, Karmisholt J, et al. Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol 2019; bcp.14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: Comparison of the smartPill® GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 2009; 54: 2167–2174. [DOI] [PubMed] [Google Scholar]
- 14.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008; 27: 186–196. [DOI] [PubMed] [Google Scholar]
- 15.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: Assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008; 20: 311–319. [DOI] [PubMed] [Google Scholar]
- 16.Sangnes DA, Bekkelund M, Søfteland E, et al. Wireless motility capsule compared with gastric emptying scintigraphy in the assessment of diabetic gastroparesis. Neurogastroenterol Motil 2019; 31: 77. [DOI] [PubMed] [Google Scholar]
- 17.Sarosiek I, Selover KH, Katz LA, et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther 2010; 31: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest 1984; 14: 420–427. [DOI] [PubMed] [Google Scholar]
- 19.Farmer AD, Wegeberg A-ML, Brock B, et al. Regional gastrointestinal contractility parameters using the wireless motility capsule: inter-observer reproducibility and influence of age, gender and study country. Aliment Pharmacol Ther 2018; 47: 391–400. [DOI] [PubMed] [Google Scholar]
- 20.Wang YT, Mohammed SD, Farmer AD, et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther 2015; 42: 761–772. [DOI] [PubMed] [Google Scholar]
- 21.Farmer AD, Pedersen AG, Brock B, et al. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of gastrointestinal transit times and an altered caecal pH profile. Diabetologia 2017; 60: 709–718. [DOI] [PubMed] [Google Scholar]
- 22.Wegeberg A-ML, Brock C, Brock B, et al. Regional gastrointestinal pH profile is altered in patients with type 1 diabetes and peripheral neuropathy. Neurogastroenterol Motil 2018; 30: e13407. [DOI] [PubMed] [Google Scholar]
- 23.Gentilella R, Pechtner V, Corcos A, et al. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: Are they all the same? Diabetes Metab Res Rev 2019; 35: 1–21. [DOI] [PubMed] [Google Scholar]
- 24.Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: A randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2017; 2: 890–899. [DOI] [PubMed] [Google Scholar]
- 25.Brock C, Jessen N, Brock B, et al. Cardiac vagal tone, a non-invasive measure of parasympathetic tone, is a clinically relevant tool in Type 1 diabetes mellitus. Diabet Med 2017; 34: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 26.Holst JJ. Incretin hormones and the satiation signal. Int J Obes 2013; 37: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schirra J, Leicht P, Hildebrand P, et al. Mechanisms of the antidiabetic action of subcutaneous glucagon-like peptide-1(7-36)amide in non-insulin dependent diabetes mellitus. J Endocrinol 1998; 156: 177–186. [DOI] [PubMed] [Google Scholar]
- 28.Camilleri M, Vazquez-Roque M, Iturrino J, et al. Effect of a glucagon-like peptide 1 analog, ROSE-010, on GI motor functions in female patients with constipation-predominant irritable bowel syndrome. AJP Gastrointest Liver Physiol 2012; 303: G120–G128. [DOI] [PubMed] [Google Scholar]
- 29.Thazhath SS, Marathe CS, Wu T, et al. The glucagon-like peptide 1 receptor agonist exenatide inhibits small intestinal motility, flow, transit, and absorption of glucose in healthy subjects and patients with type 2 diabetes: A randomized controlled trial. Diabetes 2016; 65: 269–275. [DOI] [PubMed] [Google Scholar]
- 30.Nakatani Y, Maeda M, Matsumura M, et al. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab 2017; 43: 430–437. [DOI] [PubMed] [Google Scholar]
- 31.Cogliandro RF, Rizzoli G, Bellacosa L, et al. Is gastroparesis a gastric disease? Neurogastroenterol Motil 2019; 31: e13562. [DOI] [PubMed] [Google Scholar]
- 32.Farmer AD, Pedersen A, Brock B, et al. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of transit times and heightened cecal fermentation. Gastroenterol 2016; 150: S214. [DOI] [PubMed] [Google Scholar]
- 33.Bozkurt A, Näslund E, Holst JJ, et al. GLP-1 and GLP-2 act in concert to inhibit fasted, but not fed, small bowel motility in the rat. Regul Pept 2002; 107: 129–135. [DOI] [PubMed] [Google Scholar]
- 34.Agersø H, Jensen LB, Elbrønd B, et al. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 2002; 45: 195–202. [DOI] [PubMed] [Google Scholar]
- 35.Brock C, Søfteland E, Gunterberg V, et al. Diabetic autonomic neuropathy affects symptom generation and brain-gut axis. Diabetes Care 2013; 36: 3698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drucker DJ. Evolving concepts and translational relevance of enteroendocrine cell biology. J Clin Endocrinol Metab 2016; 101: 778–786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620925968 for Liraglutide accelerates colonic transit in people with type 1 diabetes and polyneuropathy: A randomised, double-blind, placebo-controlled trial by Anne-Marie Langmach Wegeberg, Christian Stevns Hansen, Adam D Farmer, Jesper Scott Karmisholt, Asbjorn M Drewes, Poul Erik Jakobsen, Birgitte Brock and Christina Brock in United European Gastroenterology Journal