Abstract
Background
The current target oxygen saturation range for patients with COVID-19 recommended by the National Institutes of Health is 92–96%.
Main body
This article critically examines the evidence guiding current target oxygen saturation recommendation for COVID-19 patients, and raises important concerns in the extrapolation of data from the two studies stated to be guiding the recommendation. Next, it examines the influence of hypoxia on upregulation of ACE2 (target receptor for SARS-CoV-2 entry) expression, with supporting transcriptomic analysis of a publicly available gene expression profile dataset of human renal proximal tubular epithelial cells cultured in normoxic or hypoxic conditions. Finally, it discusses potential implications of specific clinical observations and considerations in COVID-19 patients on target oxygen saturation, such as diffuse systemic endothelitis and microthrombi playing an important pathogenic role in the wide range of systemic manifestations, exacerbation of hypoxic pulmonary vasoconstriction in the setting of pulmonary vascular endothelitis/microthrombi, the phenomenon of “silent hypoxemia” with some patients presenting to the hospital with severe hypoxemia disproportional to symptoms, and overburdened health systems and public health resources in many parts of the world with adverse implications on outpatient monitoring and early institution of oxygen supplementation.
Conclusions
The above factors and analyses, put together, call for an urgent exploration and re-evaluation of target oxygen saturation in COVID-19 patients, both in the inpatient and outpatient settings. Until data from such trials become available, where possible, it may be prudent to target an oxygen saturation at least at the upper end of the recommended 92–96% range in COVID-19 patients both in the inpatient and outpatient settings (in patients that are normoxemic at pre-COVID baseline). Home pulse oximetry, tele-monitoring, and earlier institution of oxygen supplementation for hypoxemic COVID-19 outpatients could be beneficial, where public health resources allow for their implementation.
Keywords: SARS-CoV-2, COVID-19, Hypoxemia, Hypoxia, ACE2
Background
The current target oxygen saturation range for patients with COVID-19 recommended by the NIH is 92–96%. “The use of supplemental oxygen in adults with COVID-19 has not been studied, but indirect evidence from other critical illnesses suggests the optimal oxygen target is an SpO2 between 92% and 96%” (https://www.covid19treatmentguidelines.nih.gov/critical-care/oxygenation-and-ventilation/). The indirect evidence refers to the following two studies:
A meta-analysis of 25 RCTs (randomized controlled trials) in 16,037 acutely ill patients [1], which concluded that liberal oxygenation (median 96%, range 94–99%) was associated with increased mortality (relative risk 1·21, 95% CI 1·03–1·43) when compared with conservative oxygenation.
The LOCO-2 trial [2] where ARDS (acute respiratory distress syndrome) patients were randomized to conservative (target partial pressure of arterial oxygen [PaO2], 55 to 70 mmHg; oxygen saturation as measured by pulse oximetry [SpO2], 88–92%) vs liberal (target PaO2, 90 to 105 mmHg; SpO2, ≥ 96%) oxygen arms. The trial was stopped early due to increased deaths in the conservative arm. At day 90, 44.4% of patients in the conservative-oxygen group and 30.4% of patients in the liberal-oxygen group had died (difference, 14.0 percentage points; 95% CI, 0.7 to 27.2).
Main body
Here, we examine the above two studies guiding current target oxygen saturation recommendations for COVID-19; discuss, with supporting transcriptomic analyses, the influence of hypoxia on ACE2 (angiotensin converting enzyme-2, target receptor for SARS-CoV-2 entry) expression; reflect on relevant clinical observations and considerations in COVID-19 patients; and propose a re-evaluation of target oxygen saturation in these patients—both in the inpatient and outpatient settings.
Critical analysis of studies guiding current target oxygen saturation recommendation
First, a closer look at the two studies on which the current recommendations are based:
The 2018 meta-analysis was not specific to ARDS (or even hypoxemia). RCTs in non-hypoxemic stroke patients exploring supplemental oxygen vs room air were included in the analysis, with supplemental oxygen being grouped in the overall “liberal oxygenation” arm and room-air oxygenation in non-hypoxemic patients grouped under the overall “conservative oxygenation” arm. Non-hypoxemic stroke patients receiving room air, i.e., “conservative oxygenation,” had a lower death rate. Similarly, RCTs of supplemental oxygen vs room air in largely normoxemic patients with myocardial infarction were also included in the analyses. Extrapolating these data to patients with ARDS raises significant concerns of relevance. Next, one of the RCTs included in the meta-analysis, the Oxygen-ICU Randomized Clinical Trial in critically ill patients [3], had a significant influence on the final analysis with a death rate of 80/243 vs 58/235 in liberal vs conservative oxygenation. In that study, however, “conservative oxygenation” was defined as an SpO2 of 94–98% or PaO2 between 70 and 100 mmHg, whereas conventional/liberal oxygenation was defined as an SpO2 of 97–100%, allowing PaO2 values up to 150 mmHg [3]. Therefore, what was considered “conservative” in that study had overlapping saturation ranges with the definition of “liberal” in the overall analysis. In addition, patients in the “liberal” arm in that study were allowed very high non-physiologic PaO2 levels.
Prior to the LOCO-2 trial, the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network recommended a target PaO2 between 55 and 80 mmHg (SpO2 88–95%). In fact, the LOCO-2 trial was conducted with the hypothesis that the lower limits of that range (PaO2 between 55 and 70 mmHg) would improve outcomes in comparison with target PaO2 between 90 and 105 mmHg. The opposite was true (adjusted hazard ratio for 90-day mortality of 1.62; 95% CI 1.02 to 2.56), and the trial was stopped early. Five mesenteric ischemic events were reported in the conservative-oxygen group.
Put together, RCT data in ARDS patients evaluating target SpO2 ≥ 96% (with a target upper PaO2 limit of 105 mmHg) vs target SpO2 92–95% are lacking. RCT data in ARDS has demonstrated that SpO2 ≥ 96% is significantly better than SpO2 88–92%. Basing oxygen saturation recommendations in ARDS patients, in part, on the 2018 meta-analysis, raises important concerns as detailed above.
ACE2 and hypoxia
Second, the role of ACE2 in SARS-CoV-2 pathogenesis and progression as a target receptor for viral entry as well as the influence of hypoxia on ACE2 expression merits particular consideration. ACE2 is a negative regulator of the angiotensin system and a counter-regulatory enzyme of ACE. While ACE coverts angiotensin I to angiotensin II, ACE2 degrades angiotensin II to angiotensin-(1-7). ACE2 expression and its catalytic product angiotensin-(1-7) have been shown to be protective against lung injury and ARDS by opposing the proliferative, hypertrophic, and fibrotic effects of angiotensin II [4–10].
SARS-CoV-2, by targeting (using as an entry receptor) the very protein that is protective against the above deleterious effects, poses unique challenges. The binding affinity of SARS-CoV-2 Spike protein to ACE2 receptor has been reported to be 10–20 times higher than that with SARS-CoV Spike protein [11], likely playing a key role in the markedly enhanced virulence. ACE2 knockout mice had significantly lower lung injury scores and SARS-CoV Spike RNA from SARS-CoV infection compared to wild type [12].
In humans, ACE2 is expressed abundantly on the surface of lung alveolar epithelial cells and enterocytes. It is also expressed in arterial and venous endothelial cells as well as arterial smooth muscle cells within multiple organs (lung, stomach, intestines, kidney, brain, bone marrow, spleen, etc.) [13]. This widespread expression of ACE2, and its high affinity with the SARS-CoV-2 Spike protein, possibly accounts for the range of severe clinical manifestations apart from ARDS, including acute renal failure and encephalopathy, with the pathogenic mechanism being diffuse endothelitis and microthrombi [14–16].
Intriguingly, pulmonary artery smooth muscle cells (PASMC) in rats have been shown to increase the expression of ACE2 with hypoxia, both at the transcript and protein levels [17]. In the experiment, the cells were incubated at 3% oxygen concentration for 0, 6, 12, 24, and 48 h. The normalized ACE2 transcript reached a maximum of 3-fold at the 12-h timepoint, and the normalized ACE2 protein expression reached a maximum of 2-fold at the 24-h timepoint, both with high statistical significance (Fig. 1C, 1D of ref. [17]). Similar effect of hypoxia on upregulation of ACE2 expression, both at the transcript and protein levels, has also been demonstrated in human pulmonary artery smooth muscle cells (Fig. 1A-E of ref. [18]).
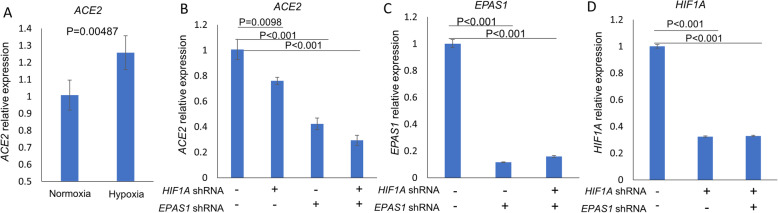
We therefore sought to determine if the same trend could also be observed in other human cells, by analyzing transcriptomic datasets deposited in Gene Expression Omnibus (GEO). Indeed, we found that human renal proximal tubular epithelial (HK2) cells cultured in hypoxic conditions for 24 h had an increase in the ACE2 transcript (raw p value = 0.0048, adjusted p value < 0.05, Fig. 1a) [19]. Furthermore, knockdown of hypoxia inducible factors 1A and 2A (encoded by HIF1A and EPAS1) in hypoxic HK2 cells reduced ACE2 transcript (Fig. 1b–d) [19], indicating that hypoxia-induced upregulation of ACE2 in these cells is likely mediated through the hypoxia inducible factors.
Fig. 1.
a Human renal proximal tubular epithelial (HK2) cells were cultured simultaneously under either normoxic (20% O2) or hypoxic (1% O2) conditions for 24 h. Hypoxia increased ACE2 expression (b–d). HK2 cells stably expressing shRNA (short hairpin RNA) targeting HIF1A and/or EPAS1 were cultured under hypoxic (1% O2) conditions for 24 h. (b). Under hypoxic conditions, knockdown of EPAS1 and HIF1A, alone and in combination, reduced ACE2 expression (c, d). shRNA knockdown of EPAS1 and HIF1A gene expression was confirmed. Data expressed as mean ± SE, with 3 replicates per group (n = 3) [19]. The gene expression profile of harvested cells was analyzed by microarray. Data were accessed through the Gene Expression Omnibus, GSE99324, and processed using affy and limma packages [20–22]. [In summary, hypoxia increased expression of ACE2 transcript in human renal proximal tubular epithelial (HK2) cells. Knockdown of hypoxia inducible factors 1A and 2A (encoded by HIF1A and EPAS1) with shRNA in hypoxic HK2 cells reduced ACE2 transcript, indicating that hypoxia-induced upregulation of ACE2 transcript in these cells is likely mediated through the hypoxia inducible factors. Hypoxia➔ ↑HIF1A and ↑HIF2A ➔ ↑ACE2] [Abbreviations: HIF1A, hypoxia inducible factor-1-alpha; EPAS1, endothelial PAS domain-containing protein 1; GEO, Gene Expression Omnibus; shRNA, short hairpin RNA—artificial RNA molecule with a tight hairpin turn that can be used to silence target gene expression via RNA interference (RNAi)]
Put together, cellular hypoxia, via upregulating the target receptor for viral entry, could potentially further contribute to an increase in the severity of SARS-CoV-2 clinical manifestations. This is yet to be tested in an in vivo model or in humans. It may be useful to determine the effect of hypoxemia on soluble ACE2 receptor levels in COVID-19 patients.
Relevant clinical observations and considerations
Third, a few clinical considerations:
Hypoxic pulmonary vasoconstriction is a well-recognized phenomenon [23, 24]. With clinical observations of several COVID-19 patients having a marked hypoxemia disproportional to the degree of infiltrates, pulmonary vasculature endothelitis and microthrombi which were suspected clinically have now been shown to be a prominent feature of COVID-19 lung pathology [25]. Any component of hypoxic pulmonary vasoconstriction and further exacerbation of pulmonary hypertension in this setting is best avoided. Further to this point, nocturnal drop in oxygen saturation is a well-known phenomenon [26], is common in patients with primary pulmonary hypertension [27], and has also been demonstrated in patients with pneumonia and sepsis [28]. Nocturnal hypoxemia could therefore potentially further exacerbate reflex pulmonary vasoconstriction as well as peripheral tissue hypoxia in patients with COVID-19 pneumonia. Patients in regular inpatient wards or at home who maintain an SpO2 of 92–94% during the day, with or without O2 supplementation, can have nocturnal drops into the 80s, with higher drops in patients with obstructive sleep apnea—a highly prevalent morbidity in obese patients.
Next, diffuse systemic endothelitis and microthrombi play an important pathogenic role in the wide range of systemic manifestations (such as acute renal failure, encephalopathy, cardiovascular complications) seen in COVID-19 patients [14–16, 29], explaining the improved outcomes associated with systemic anticoagulation [29]. In the presence of these systemic microthrombi, hypoxemia would be expected to result in a higher degree of peripheral tissue hypoxia/injury. This is another reason why the optimal oxygen saturation in COVID-19 ARDS may be higher than that in ARDS of other etiologies.
The phenomenon of “silent hypoxemia” resulting in some COVID-19 patients presenting to the hospital with severe hypoxemia disproportional to symptoms is now being increasingly noted [30–32], and albeit not fully understood at this stage, may be a harbinger for clinical deterioration [30], and further supports outpatient monitoring with pulse oximetry and earlier institution of oxygen supplementation.
Lastly, with overburdened health systems around the world and viral transmission considerations, COVID-19 patients in the outpatient setting (suspected and confirmed) are instructed to come in to the hospital if their respiratory status deteriorates, most often with no oxygen saturation monitoring at home. While this approach may be essential in managing burdened health system resources and caring for the critically sick, it risks a significant delay in oxygen supplementation for patients in the outpatient setting. With the lack of strikingly effective therapeutic modalities to date, inpatient mortality numbers and percentages for COVID-19 patients around the world have been staggering [33–37]. (It is of relevance to note here that even in non-COVID-19 pneumonia outpatients, oxygen saturations less than 92% are known to be associated with major adverse events [38].)
Put together, while the effects of the degree/duration of hypoxemia in COVID-19 patients have not been comprehensively studied, the concern of its potential adverse effects (above that in pneumonia/ARDS of other etiologies) is based on the above-detailed specific considerations and well-known principles in respiratory/internal medicine. If maintaining a higher oxygen saturation in hypoxemic COVID-19 patients in the outpatient setting could have a role in decreasing the severity of disease progression and complications, earlier institution of oxygen supplementation at home and tele-monitoring could potentially be beneficial.
Conclusions
The above considerations, put together, call for an urgent exploration and re-evaluation of target oxygen saturation in COVID-19 patients, both in the inpatient and outpatient settings. While conducting randomized controlled trials in the inpatient setting exploring a target SpO2 ≥ 96% (target upper PaO2 limit of 105 mmHg) vs target SpO2 92–95% would be relatively less complex in terms of execution and logistics, the outpatient setting would require special considerations such as frequent tele-visits and pulse oximetry recordings, home oxygen supplementation as needed to meet target oxygen saturation, and patient compliance. Until data from such trials become available, it may be prudent to target an oxygen saturation at least at the upper end of the recommended 92–96% range in COVID-19 patients both in the inpatient and outpatient settings (in patients that are normoxemic at pre-COVID baseline). Home pulse oximetry, tele-monitoring, and earlier institution of oxygen supplementation for hypoxemic COVID-19 outpatients could be beneficial but should be studied systematically given the significant public health resource implications.
Acknowledgements
Not applicable
Abbreviations
- ACE2
Angiotensin converting enzyme-2
- ARDS
Acute respiratory distress syndrome
- SpO2
Oxygen saturation
- PaO2
Partial pressure of oxygen
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- PASMC
Pulmonary artery smooth muscle cells
- HIF1A
Hypoxia inducible factor-1-alpha
- EPAS1
Endothelial PAS domain-containing protein 1
- RCT
Randomized controlled trial
- GEO
Gene Expression Omnibus
- shRNA
Short hairpin RNA
Authors’ contributions
N.S.: conceptualization (after experience with management of inpatient COVID-19 patients), manuscript writing, literature review, data acquisition, and overall analysis. R.L.: ACE2 data acquisition. P.G.: manuscript editing (experience with management of critically ill patients with COVID-19). All authors read and approved the final manuscript.
Authors’ information
N.S. is a faculty physician-scientist (Assistant Professor) in the Department of Medicine (Oncology) at Albert Einstein College of Medicine/Montefiore and is American Board certified in Internal Medicine, Hematology, and Oncology. R.L. is a faculty scientist (Research Assistant Professor) in the Department of Medicine (Oncology) at Albert Einstein College of Medicine/Montefiore. P.G. is a faculty physician (Assistant Professor) in the Department of Medicine (Critical Care Medicine) at Albert Einstein College of Medicine/Jacobi and is American Board certified in Internal Medicine and Critical Care Medicine.
Funding
Dr. Shenoy’s research is supported by the Albert Einstein Cancer Center core grant (2P30CA013330-47).
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, Szczeklik W, Schunemann HJ, Neary JD, Alhazzani W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 2.Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot JP, Pili-Floury S, Bouhemad B, Louis G, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 3.Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316(15):1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 4.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu H, Xie Z, Li T, Zhang S, Lai C, Zhu P, Wang K, Han L, Duan Y, Zhao Z, et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;6:19840. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wosten-van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic J, Florquin S, Bos AP. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 7.Santos RA, Ferreira AJ, Simoes ESAC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93(5):519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 8.Morrell NW, Upton PD, Kotecha S, Huntley A, Yacoub MH, Polak JM, Wharton J. Angiotensin II activates MAPK and stimulates growth of human pulmonary artery smooth muscle via AT1 receptors. Am J Phys. 1999;277(3):L440–L448. doi: 10.1152/ajplung.1999.277.3.L440. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Zagariya A, Ibarra-Sunga O, Gidea C, Ang E, Deshmukh S, Chaudhary G, Baraboutis J, Filippatos G, Uhal BD. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Phys. 1999;276(5):L885–L889. doi: 10.1152/ajplung.1999.276.5.L885. [DOI] [PubMed] [Google Scholar]
- 10.Shenoy V, Qi Y, Katovich MJ, Raizada MK. ACE2, a promising therapeutic target for pulmonary hypertension. Curr Opin Pharmacol. 2011;11(2):150–155. doi: 10.1016/j.coph.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS: Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. 2020:2020.2002.2011.944462. [DOI] [PMC free article] [PubMed]
- 12.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020. [DOI] [PMC free article] [PubMed]
- 15.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, Navalesi P, Simioni P. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed]
- 16.https://www.webmd.com/lung/news/20200424/blood-clots-are-another-dangerous-covid-19-mystery. 2020. Accessed 27 Apr 2020.
- 17.Hu HH, Zhang RF, Dong LL, Chen EG, Ying KJ. Overexpression of ACE2 prevents hypoxia-induced pulmonary hypertension in rats by inhibiting proliferation and immigration of PASMCs. Eur Rev Med Pharmacol Sci. 2020;24(7):3968–3980. doi: 10.26355/eurrev_202004_20867. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Wu Y, Zhao M, Liu C, Zhou L, Shen S, Liao S, Yang K, Li Q, Wan H. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 19.Shved N, Warsow G, Eichinger F, Hoogewijs D, Brandt S, Wild P, Kretzler M, Cohen CD, Lindenmeyer MT. Transcriptome-based network analysis reveals renal cell type-specific dysregulation of hypoxia-associated transcripts. Sci Rep. 2017;7(1):8576. doi: 10.1038/s41598-017-08492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23(14):1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 22.Smyth GK. limma: linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and computational biology solutions using R and bioconductor. New York: Springer New York; 2005. pp. 397–420. [Google Scholar]
- 23.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92(1):367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015;122(4):932–946. doi: 10.1097/ALN.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 25.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gries RE, Brooks LJ. Normal oxyhemoglobin saturation during sleep. How low does it go? Chest. 1996;110(6):1489–1492. doi: 10.1378/chest.110.6.1489. [DOI] [PubMed] [Google Scholar]
- 27.Rafanan AL, Golish JA, Dinner DS, Hague LK, Arroliga AC. Nocturnal hypoxemia is common in primary pulmonary hypertension. Chest. 2001;120(3):894–899. doi: 10.1378/chest.120.3.894. [DOI] [PubMed] [Google Scholar]
- 28.von Eiff M, Grone E, Herbort C, Zuhlsdorf M, Van de Loo J. Nocturnal hypoxemia and oxygen desaturation events in neutropenic patients with sepsis or pneumonia. Intensive Care Med. 1996;22(2):174. doi: 10.1007/BF01720726. [DOI] [PubMed] [Google Scholar]
- 29.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkerson RG, Adler JD, Shah NG, Brown R: Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med 2020:S0735–6757(0720)30390–30399. [DOI] [PMC free article] [PubMed]
- 31.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jouffroy R, Jost D, Prunet B. Prehospital pulse oximetry: a red flag for early detection of silent hypoxemia in COVID-19 patients. Crit Care. 2020;24(1):313. doi: 10.1186/s13054-020-03036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Consortium atNC-R. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 34.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E et al: Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv 2020:2020.2006.2022.20137273.
- 35.Mitra AR, Fergusson NA, Lloyd-Smith E, Wormsbecker A, Foster D, Karpov A, Crowe S, Haljan G, Chittock DR, Kanji HD, et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. Can Med Assoc J. 2020;192(26):E694–E701. doi: 10.1503/cmaj.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumdar SR, Eurich DT, Gamble J-M, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis. 2011;52(3):325–331. doi: 10.1093/cid/ciq076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable