Abstract
Background
Metaplastic breast carcinoma (MBC) is a rare histological type of breast cancer, which commonly shows resistance to standard therapies and is associated with poor prognosis. The immune microenvironment in MBC and its significance has not been well established due to its low incurrence rate and complex components. We aimed to investigate the diversity of immune parameters including subsets of TILs and PDL1/PD1 expression in MBC, as well as its correlation with prognosis.
Methods
A total of 60 patients diagnosed with MBC from January 2006 to December 2017 were included in our study. The percentage (%) and quantification (per mm2) of TILs and presence of tertiary lymphoid structures (TLS) were evaluated by hematoxylin and eosin staining (HE). The quantification of CD4+, CD8+ TILs (per mm2), and PD-1/PDL1 expression were evaluated through immunohistochemistry and analyzed in relation to clinicopathological characteristics. A ≥ 1% membranous or cytoplasmatic expression of PD1 and PDL1 was considered a positive expression.
Results
We found squamous cell carcinoma MBC (33/60, 55%) exhibiting most TILs of all the MBC subtypes (p = 0.043). Thirty-three of 60 (50%) of the patients had coexisting invasive ductal carcinoma of no special type (IDC-NST), and the average percentage of TILs in MBC components was lower compared with NST components (p < 0.001). Thirty (50%) patients exhibited positive (≥ 1%) PDL1 expression in their tumor cells, while 36 (60%) had positive (≥ 1%) PDL1 expression in their TILs. Twenty-seven (45%) of all the patients had positive (≥ 1%) PD1 expression in their tumor cells and 33 (55%) had PD1-positive (≥ 1%) stromal TILs. More CD8+ TILs were associated with positive PDL1 expression of tumor cells as well as positive PD1 expression in stromal cells. Greater number of stromal TILS (> 300/mm2, 20%), CD4+ TILs (> 250/mm2), and CD8+ TILs (> 70/mm2) in MBC were found associated with longer disease-free survival. Positive expression of PDL1 in tumor cells (≥ 1%) and PD1 in stromal cells (≥ 1%) were also associated with longer survival.
Conclusions
The immune characteristics differ in various subtypes as well as components of MBC. Immune parameters are key predictive factors of MBC and provide the clinical significance of applying immune checkpoint therapies in patients with MBC.
Keywords: Metaplastic breast cancer, Immune characteristics, Prognosis
Background
Metaplastic breast carcinoma (MBC) is a rare subtype of invasive breast carcinoma, which accounts for approximately 1% of all breast malignancies [1]. MBC displays various histological subtypes, exhibiting metaplastic change from neoplastic epithelium to squamous cells and/or mesenchymal elements. MBC usually lacks the expression of hormone receptor and HER2 and is considered as a subtype of triple-negative breast cancer (TNBC). Previous studies have shown that MBC is less sensitive to adjuvant therapy [2, 3] and has poorer prognosis compared with invasive ductal carcinoma in the same clinical stage [4, 5], including the TNBC [6].
Tumor-infiltrated lymphocytes (TILs) are mononuclear lymphocytes present in the tumoral tissue, reflect an immune response in the tumor microenvironment, and can be easily identified in formalin-fixed paraffin-embedded tissues. Increased TILs in tumors were found to be associated with better prognosis and an increase in systematic therapy sensitivity in TNBC [7–9]. The subsets of TILs were also shown to be a prognostic factor for TNBC [10, 11]. The dynamic expression of programmed cell death ligand 1 (PDL1) and PD1 on both the tumor and immune cells can either disrupt or sustain tumor growth, which is correlated with prognosis in breast cancer [12–14]. Furthermore, the TILs and PDL1/PD1 expression were both predictors of effectiveness of immune checkpoints therapy in breast cancer [15, 16] and TILs were shown to be correlating with PDL1/PD1 expression [17].
Data involving the immune microenvironment of MBC is limited and short of quantification. Given the heterogeneous components of MBC and the prognostic significance of TILs and its subsets as well as PDL1/PD1 expression in breast cancer, we investigated the expression of PD1/PDL1 and quantified TILs to determine their association with clinicopathological features and survival outcome in a cohort of MBC.
Methods
Patients and samples
This study was conducted using the data of patients diagnosed with MBC from January 2006 to December 2017, treated at the Sun Yat-sen University Cancer Center. All patients diagnosed with MBC were reviewed. Patients with recurrences at diagnosis, previous malignancies, and immune deficiencies were excluded. The clinical parameters investigated were age, pathological diagnosis, symptoms, present history, past history, image examination including ultrasound, and mammography results, operative records, and adjuvant therapy data were extracted from the original medical records. The follow-up information was gained from medical records and telephonic interviews. The primary endpoint of the study was disease-free survival. The protocol of this study was approved by the institutional Ethics Committee of Sun Yat-sen University Cancer Center, and consent for the use of data in research was obtained from each participant.
Pathological assessment
The pathological categories of MBC were considered based on the WHO classification [18]. The subtypes included low-grade fibromatosis-like carcinoma, squamous cell carcinoma, spindle cell carcinoma, and carcinoma with mesenchymal differentiation (chondroid, osseous, and other mesenchymal differentiation). A mixed type MBC was considered when 2 or more subtypes of MBC were present on the histological slides. All the original tumor slides of each patient were reviewed by 2 pathologists. The hormone receptor and HER2 receptor status were extracted from the original pathological reports.
Evaluation of TILs
TILs were evaluated on the hematoxylin and eosin (H&E) sections of the tumor following the guidelines of the international TILs working group [19]. The TILs were evaluated within the invasive border and a percentage, as well as a quantification of TILs in square millimeter, was given. An average percentage and quantification of TILs were documented for each case. For mixed type or MBC with invasive ductal carcinoma of no-special type (IDC-NST), the TILs percentage was evaluated in different components, while the TILs quantification was counted as an average number. The stromal TILs were analyzed for the epithelial tumor component. For cases with mesenchymal compartment, the TILs in the epithelium were called intra-epithelium TILs, while the TILs analyzed within the mesenchymal element were called mesenchymal TILs. Tertiary lymphoid structure (TLS), aggregates that recapitulate the components and architecture of a lymph node, was also evaluated as previously described [19, 20]. Dual staining of CD3/CD20 was also performed to validate the TLS number according to Buisseret’s study [21]. Furthermore, the quantification of TILs was performed manually through the digital scan using the Aperio imagescope (Leica Biosystems). Two pathologists evaluated all the data above separately and blind to the clinical outcomes. Consensus was reached between the two authors if there was a discrepancy among the collected data.
Immunohistochemical evaluations
Formalin-fixed paraffin-embedded (FFPE) tissue sections were stained for PD-L1 (clone: antihuman PD-L1 rabbit monoclonal antibody E1L3N, Cell Signaling Technology), PD-1 (Clone UMAB199, ZSGB-Bio), CD4 (Clone EP204, ZSGB-Bio), CD8 (Clone SP16, ZSGB-Bio), and CD68 (Clone PG-M1, ZSGB-Bio). Dual CD3/CD20 immunohistochemical stain was performed as Buisseret et al. [21] (Supplemental Figure 1). A ≥ 1% membranous or cytoplasmatic expression of PD1 and PDL1 in tumor cells was considered positive expression. An immune cell was considered “PD-L1/PD-1 positive” if it featured any PD-L1 staining due to the small size of the lymphocytes. The percentage of PD-L1-positive tumor cells was proportionally evaluated in all tumor cells. PD-1 and PD-L1 immune cells were assessed relative to the whole tumor area, and as previously described [14, 22]. Quantification of CD4 and CD8 positive TILs were performed with digital imaging analysis (Halo imaging analysis software; Indica Labs, Corrales, NM) as well as manually. We manually annotated the system to indicate different components of MBC including epithelial area, mesenchymal area, and the stromal area. The software counted the number of positive immune cells in the tumor areas of the whole slides while the two pathologists counted the positive immune cells through the digital scan of the slides separately. Consensus was reached between the two authors if there was a discrepancy among the collected data.
Statistics
Categorical variables were grouped based on the clinical findings, and decisions on the groups were made before modeling. The results were compared using the χ2 test or Fisher’s exact test. Continuous variables were compared using the t test. Comparison of TILs parameters between different groups used Wilcoxon test due to the limited sample size. Spearman’s rank correlation tests were used to assess the associations among infiltrations of CD4+, CD8+TILs, and PD-L1+ tumor/immune cells. The median of the CD4+, CD8+, and overall TILs counts is used as a cut-off value. Cox regression models were used to examine the prognostic effect of each variable. Kaplan-Meier curves were used to compare subgroups defined by biomarkers. A p value < 0.05 was considered statistically significant. All statistical analyses were carried out using the SPSS software, version 25.0 (IBM Corp, 1987, Chicago, USA), and GraphPad Prism 8 (GraphPad software, Inc.).
Results
Clinicopathological characteristics
A total of 60 surgically resected FFPE MBC samples were assessed in our study. The median age at diagnosis was 50 years (range, 25–81 years). The clinicopathological characteristics are listed in Table 1. Of all the 60 MBC patients, 33 were diagnosed as squamous cell carcinoma, 4 as spindle cell carcinoma, 8 as mesenchymal differentiation, 2 as fibromatosis-like, and 13 as mixed type.
Table 1.
Clinicopathological characteristics
| Patients characteristics | n | % |
|---|---|---|
| Age (median/range) | 50/25–81 | |
| Histological subtype | ||
| Squamous cell carcinoma | 33 | 55.0 |
| Spindle cell carcinoma | 4 | 6.56 |
| Chondriod differentiation | 4 | 6.56 |
| Osseous differentiation | 4 | 6.56 |
| Fibromatosis-like | 2 | 3.28 |
| Mixed type | 13 | 21.67 |
| With invasive carcinoma (no special type) | 33 | 55.0 |
| Tumor size(mm) | ||
| 0–20 | 10 | 16.67 |
| 21–50 | 33 | 55 |
| > 50 | 17 | 28.33 |
| Lymph node metastasis | ||
| Negative | 41 | 68.33 |
| 1–3 | 17 | 28.33 |
| 4–10 | 2 | 3.33 |
| > 10 | 0 | – |
| Lymphovascular invasion | ||
| Present | 16 | 26.7 |
| Absent | 41 | 68.3 |
| Unknown | 3 | 5 |
| Surgery type | ||
| Breast-conserving surgery | 7 | 11.67 |
| Mastectomy | 53 | 88.67 |
| Systematic therapy | ||
| Chemotherapy* | 56 | 93.33 |
| Radiotherapy | 52 | 86.67 |
| Clinical outcome | ||
| Local recurrences | 5 | 8.02 |
| Distant metastasis | 7 | 11.48 |
| Total death | 6 | 9.81 |
| Cancer-specific death | 3 | 4.92 |
*4/56 patients had neoadjuvant chemotherapy
A mixed MBC was defined as two or more metaplastic components. For the 13 mixed MBCs, 4/13 were mixed with squamous cell carcinoma and chondroid/chondroid matrix production; 6/13 were mixed with squamous cell carcinoma, spindle cell type, and invasive carcinoma with no special type; and 3/13 were mixed with squamous cell carcinoma and spindle cell type.
Thirty-three of 60 of the patients had coexisting IDC-NST. Twenty-eight of 33 of these tumors were triple-negative while 5/33 were hormone receptor-positive. Additional details are listed in Supplemental Table 1.
Immune characteristics of different subtypes of MBC
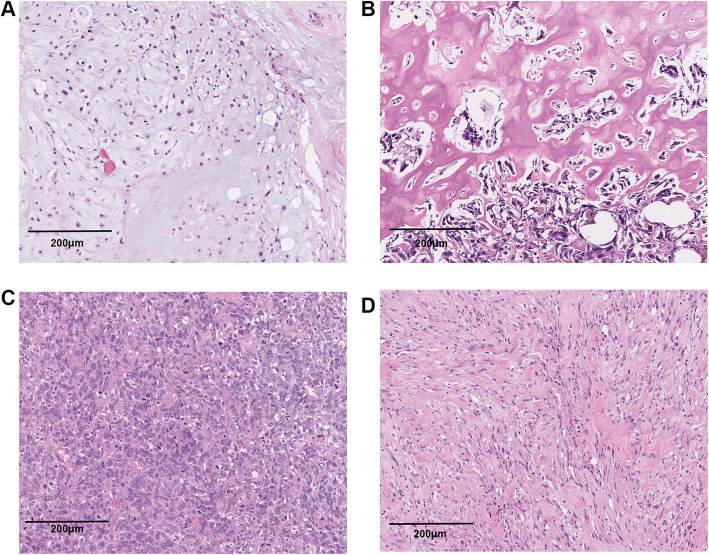
For pure carcinoma, the TILs mainly located in stroma around the carcinoma nests. While for MBC with mesenchymal components, the TILs infiltrated diffuse around the mesenchymal cells. MBC with different mesenchymal elements were displayed in Fig. 1.
Fig. 1.
Hematoxylin and eosin-stained section of MBC with mesenchymal elements and the distribution patterns of TILs. a Chondroid differentiation. b Osseous differentiation. c Spindle cell. d Fibromotosis-like
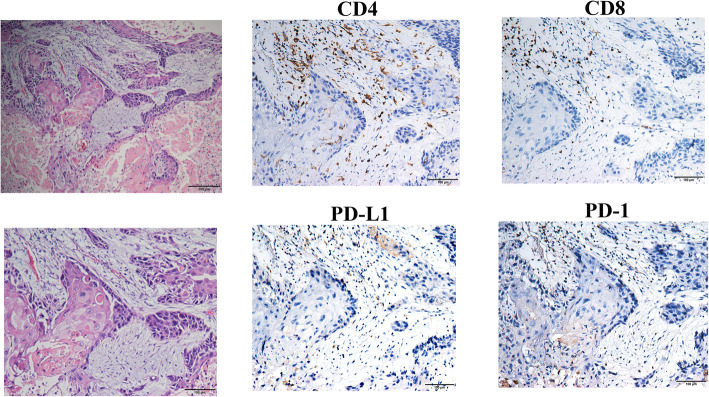
For each case, an average number of total TILs as well as CD4+ and CD8+ TILs, the percentage of TILs of each component, and the percentage of positive PD1 and PDL1 expression in both tumor cells and TIL was evaluated. Figure 2 showed a mixed type MBC composed of both squamous cell cancer and chondroid matrix and its expression of different immune parameters (CD4, CD8, PDL1, and PD1). The staining of CD68 in the same case was displayed in Supplemental Figure 2.
Fig. 2.
Representative hematoxylin and eosin-stained section of a mixed type MBC (squamous cell cancer component and chondroid-matrix component) with corresponding CD4, CD8, PDL1, and PD1 stains (original magnification × 200)
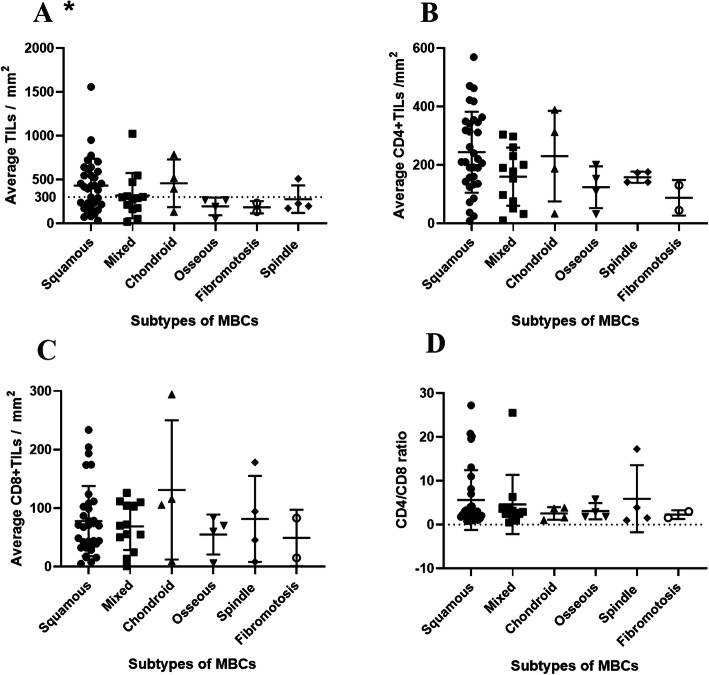
Of all subtypes of MBC, the squamous cell carcinoma MBC exhibited the greatest number of TILs. The squamous cell carcinoma MBCs were more likely to have > 300/mm2 (median) TILs compared to other subtypes (p = 0.043). None of this tendency was observed in CD4+ and CD8+ TILs. Neither does the CD4/CD8 ratio nor the CD68 positive cells displayed difference among MBC subtypes (Fig. 3 and Supplemental Figure 3).
Fig. 3.
The stromal TILs counts in different MBC subtypes (a). The squamous cell carcinoma MBCs were more likely to have > 300/mm2 TILs (p = 0.043); CD4+ TILs counts in different MBC subtypes (b); CD8+ TILs counts in different MBC subtypes (c) and CD4/CD8 ratio in different MBC subtypes. The asterisk in the figure refers to p < 0.05
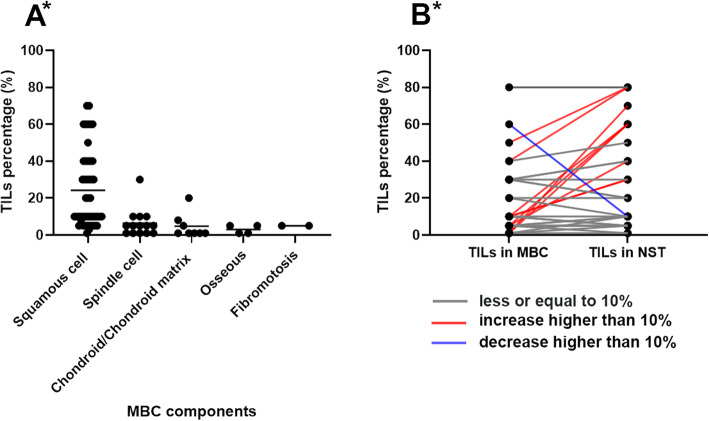
For different components of MBC, a comparison of TILs in percentage is shown in Fig. 4a. Of all the 5 components, squamous cells showed the most number of TILs (p < 0.001). For the 33 MBCs with IDC-NST, the average percentage of TILs in MBC components was lower in the IDC-NST components (Fig. 4b, p < 0.001). There is no difference in TILs percentage between simple MBC (27 cases) and MBC combined with IDC-NST (p = 0.25).
Fig. 4.
a The stromal TILs percentage in different MBC components. b Change of TILs between MBC components and accompanied invasive ductal carcinoma of no special type. Of all the 33 cases, increase in 9, no change in 23, and decrease in 1. TILs, tumor-infiltrating lymphocytes; MBC, metaplastic breast cancer; NST, no special type. The asterisk in the figure refers to p < 0.05
Thirty (50%) patients exhibited positive PDL1 expression in their tumor cells, while 36 (60%) had positive PDL1 expression in their TILs. Twenty-four (40%) patients had positive PDL1 expression in both tumor cells and TILs. Eighteen (30%) patients exhibited negative PDL1 in neither tumor cells nor TILs. Six (10%) patients had positive expression in tumor cells and negative expression in TILs. Twelve (20%) patients had positive expression in TILs and negative expression in tumor cells. Twenty-seven (45%) of all the patients had positive PD1 expression in their tumor cells and 24 (40%) had PD1 positive stromal TILs. The correlation of PDL1/PD1 expression in tumor cells and TILs were listed in Supplemental Table 2&3. There was no significant association of PDL1/PD1 expression between different MBC subtypes (Supplemental Table 4).
Among all the immune parameters, a higher level of CD8+ TILs was found to be correlated with positive PDL1 expression in both tumor cells and TILs (Table 2). Twenty-two patients had both greater number of CD8+ TILs (> 70/mm2) and positive PDL1 expression tumor cells.
Table 2.
Relationship of PDL1 PD1 expression in tumors and stromal TILs with other immune parameters by (non-parametric) Spearman’s rank correlation
| PDL1 in tumor | PDL1 in stromal | PD1 in tumor | PD1 in stromal | |
|---|---|---|---|---|
| TILs (> 300/mm2) | Rho 0.131 | Rho 0.193 | Rho 0.118 | Rho 0.12 |
| p | 0.33 | 0.17 | 0.38 | 0.38 |
| CD4 + TILs(> 250/mm2) | Rho 0.149 | Rho 0.094 | Rho 0.178 | Rho 0.125 |
| p | 0.26 | 0.51 | 0.18 | 0.36 |
| CD8+ TILs (> 70/mm2) | Rho 0.492 | Rho 0.367 | Rho −0.018 | Rho 0.128 |
| p | < 0.001 | 0.006 | 0.89 | 0.34 |
| CD68+ TILs (> 300/mm2) | Rho 0.1 | Rho − 0.053 | Rho 0.018 | Rho 0.015 |
| p | 0.45 | 0.71 | 0.89 | 0.91 |
| TILs percentage (> 20%) | Rho − 0.034 | Rho 0 | Rho − 0.016 | Rho − 0.165 |
| p | 0.80 | 1 | 0.42 | 0.22 |
Univariate and multivariate analysis
We further evaluated the prognostic values of these immune parameters. There is a median follow-up of 48 months (range, 22–163 months).
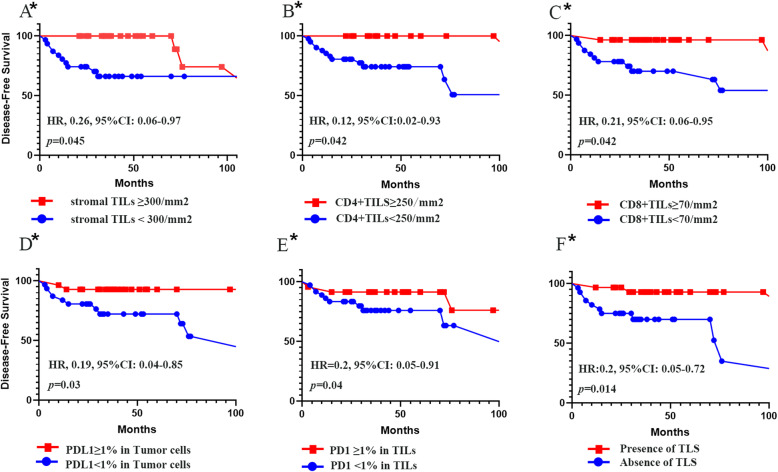
More TILs (cut-off ≥ 300/mm2) in patients with MBC showed a trend for better prognosis (HR, 0.26; 95%CI, 0.07–0.97; p = 0.045), and this trend become stronger in MBC with squamous cell cancer (HR, 0.07; 95%CI, 0.008–0.64; p = 0.018). Also, higher expression of CD4+ (cut-off ≥ 250/mm2) and CD8+ (cut-off ≥ 70/mm2) TILs keep this trend (CD4: HR, 0.12; 95%CI, 0.02–0.93; p = 0.042; CD8: HR, 0.21; 95%CI, 0.05–0.95; p = 0.042). Higher number of CD68-positive cells (> 300/m2) did not correlate with the patient’s outcome (HR 0.74, 95%CI 0.25–2.21). All the cut-off above was median of the data. The CD4/CD8 ratio had no correlation with DFS (HR, 0.95; 95%CI, 0.86–1.04; p = 27). Positive PDL1 expression in tumor cells (HR, 0.19; 95%CI, 0.04–0.85; p = 0.03) and PD1 expression in stromal TILs (HR, 0.20; 95%CI, 0.05–0.91; p = 0.04) also predicted a longer DFS. The presence of TLS in tumor predicted better prognoses as well (HR, 0.2; 95%CI, 0.05–0.75; p = 0.014) (Fig. 5).
Fig. 5.
The prognostic values of immune parameters in MBC. a Stromal TILs. b CD4+ Stromal TILs. c CD8+ Stromal TILs. d PDL1 expression in tumor cells. e PD1 expression in stromal TILs. f Present of TLS in tumor. TILs, tumor-infiltrating lymphocytes; MBC, metaplastic breast cancer; TLS, tertiary lymphoid structure. The asterisk in the figure refers to p < 0.05
In addition to the immune parameters described above, the lymphovascular invasion was also found to be significantly associated with shorter DFS (HR, 3.97; 95%CI, 1.19–13.23; p = 0.03). MBC histological subtypes, age, tumor size, lymph node status, and surgical type were not associated with DFS.
In multivariate analyses, only stromal TIL was identified as an independent indicator for DFS (HR, 0.17; 95%CI, 0.04–0.79; p = 0.02) (Table 3).
Table 3.
Univariate and multivariate cox regression disease-free survival
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Age < 50 vs ≥ 50 | 0.81 (0.27–2.41) | 0.70 | – | – |
| Tumor size | ||||
| 0–20 | 1 | – | – | |
| 21–50 | 1.03 (0.12–8.99) | 0.98 | ||
| > 50 | 3.73 (0.74–32.06) | 0.23 | ||
| Lymph node status | ||||
| Positive vs negative | 0.92 (0.28–2.96) | 0.88 | – | – |
| Lymphvascular invasion | ||||
| Present vs absent | 3.97 (1.19–13.23) | 0.03 | 3.52 (0.89–13.89) | 0.07 |
| With invasive ductal carcinoma (NST) | 0.98 (0.33–2.98) | 0.97 | – | – |
| Surgery type | ||||
| BCS vs mastecomy | 1.07 (0.13–8.71) | 0.95 | – | – |
| Histological subtype | ||||
| Squamous cell | 1 | 0.58 | ||
| Spindle cell | 2.20 (0.25–19.50) | |||
| Chondriod | 1.36 (0.16–11.68) | |||
| Osseous | 4.44 (0.84–23.49) | |||
| Fibromatosis-like | 5.34 (0.61–47.10) | |||
| Mixed type | 1.31 (0.31–5.49) | |||
| Without/with NST | 1.05 (0.35–3.14) | 0.93 | ||
| Stromal TILs(/mm2) | ||||
| ≥ 300 vs < 300 | 0.26 (0.07–0.97) | 0.045 | 0.17 (0.04–0.79) | 0.024 |
| CD4+ TILs(/mm2) | ||||
| ≥ 250 vs < 250 | 0.12 (0.02–0.93) | 0.042 | 0.99 (0.04–22.43) | 0.99 |
| CD8+ TILs(/mm2) | ||||
| ≥ 70 vs < 70 | 0.21 (0.05–0.95) | 0.042 | 0.56 (0.11–2.94) | 0.49 |
| Tumoral PDL1 | ||||
| Positive vs negative | 0.19 (0.04–0.85) | 0.03 | 0.39 (0.08–2.03) | 0.26 |
| Stromal PD1 | ||||
| Positive vs negative | 0.20 (0.05–0.91) | 0.04 | 1.32 (0.26–6.87) | 0.74 |
| TLS | ||||
| Present vs absent | 0.2(0.05–0.75) | 0.014 | 0.61 (0.15–2.59) | 0.51 |
| TMIT* vs others | 0.02 (0.01–2.53) | 0.116 | ||
NST, with invasive carcinoma (no special type)
*TMIT tumor microenviroment type refers to the higher CD8+ TILs and positive PDL1 expression of tumor cells
Discussion
MBC is a rare subtype of TNBC with poor prognosis and usually not sensitive to conventional adjuvant therapy. Thus immune therapy may be a promising way to improve the outcome of MBC. In this study, we investigated 60 MBC samples for immune parameters associated with survival, searching for possible prognostic factors and treatment strategies.
MBC exhibited various histological features from epithelia to mesenchyme. Previous studies using whole-exome sequencing found that different components in MBC share identical somatic alterations [23]. Though different components of MBC shares the same origin, the immune characteristics vary a lot. To our knowledge, this is the first large study focusing on the different components of MBC to assess their immune microenvironment. We have observed that squamous cell MBCs were more likely to have > 300/mm2 TILs compared to other subtypes (p = 0.043). Also, squamous cell component displays the most TILs compared with other components (p < 0.001). For the 33 MBCs with IDC-NST, the average percentage of TILs in MBC components was lower in the MBC components compared with NST components. These results indicate that more epithelial differentiation, more TILs.
TILs are basic parameters reflecting the immune status of the tumor. The higher the expression of TILs has been associated with better survival in TNBC and HER-2 positive breast cancer, in both research and clinical practice. In MBC, more TILs were also proved to be correlated with longer DFS (HR, 0.26; 95%CI, 0.07–0.97; p = 0.045). The most of TILs are cytotoxic CD8+ T cells and CD4+ helper T cells. Both of these two groups play major parts in antitumor immunity [24]. CD8+ T cells can directly kill tumor cells. We found that more CD8+ TILs in MBC predicts better prognosis in MBC (HR, 0.21; 95%CI, 0.05–0.95; p = 0.042), which resonates with the findings of a previous study involving 1334 patients of all subtypes of breast cancer [25]. While another study found that the CD4+ TILs were independent prognostic factors in hormone negative breast cancer, no correlation was found between CD8+ TILs and clinical outcome [26]. Findings from the present study showed that CD4+ TILs correlated with better survival (HR, 0.12; 95%CI, 0.02–0.93; p = 0.042) as well as CD8+ TILs in MBC. TLSs are ectopic lymph node-like structures characterized by lymphoid aggregation with high endothelial venules. TLSs are induced in a chronic inflammatory environment and their presence is associated with the exacerbation of local immune responses [27]. Here, we found that the presence of TLS was associated with longer survival in MBC (HR, 0.2; 95%CI, 0.05–0.75; p = 0.014), as well as which were found in TNBC [28].
The expression of PDL1 in breast cancer has a controversial role in predicting prognosis of breast cancer. In the present study, the PDL1 expression in the tumor of MBC appeared in 50% (36/60) of all the patients, which is similar to the previously reported rate of 20%–58.5% in TNBC [14, 29, 30]. Our data suggested that PDL1 expression correlated with better survival (HR, 0.19; 95%CI, 0.04–0.85; p = 0.03) in MBC, which was consistent with some previous studies [31, 32] but contradicted with that of a meta-analysis involving 9 studies [33]. Different PDL1 immunohistochemistry assays, various scoring systems, and evaluation of different tumor compartments may be the reasons for this observed diversity [34]. Thus, a prospective study correlated with the treatment effect of PDL1/PD1 blockade is awaited to define the proper PDL1 expression assay.
The performance of different clones in PDL1 detection differs in breast cancer. PDL1 (E1L3N) identified more PD-L1-positive cases (14.7%, cut-off 1%) compared with SP142 (11.5%) and 28–8 (13.3%) in triple-negative breast cancer [35]. Adams et.al used PDL1 (E1L3N) detecting 62(48.75%) positive expression on tumor cells (cut-off 10%) in triple-negative breast cancer [36]. A meta-analysis of 38 studies revealed clone 28-8 yielding the highest positive rate (39%) on tumor cells in all breast cancer with different thresholds in all studies [37]. Data from IMpassion130 study using SP142 identified 369 (40.9%) PD-L1-positive expression on immune cells [38]. Our study used PDL1(E1L3N) identified 30 (50%) positive expression on tumor cells and 36 (60%) positive expression on immune cells, which may identify more PD-L1-positive tumors.
TILs infiltration pattern in MBC with mesenchymal elements differs from carcinoma due to the histological characteristics. The TILs infiltrated diffusely in the mesenchymal elements. The distribution of CD4+ and CD8+ TILs also follows this trend. Previous studies involved TILs and its subtypes also correlated with survival in soft tissue sarcoma [39–41]. A study involved 47 leiomyosarcomas revealed an average number of 10.5 CD4+ TILs and 16.1 CD8+ TILs per high power filed (CD4 33.87/mm2, CD8 51.93/mm2) [42], which is lower than the average number in MBC with mesenchymal elements (CD4 209.99 ± 140.10/mm2, CD8 83.34 ± 78.09/mm2). Further, no significant difference in TILs was found among different mesenchymal subtypes (p = 0.30). Another study revealed different PDL1 expression in various sarcoma [43]. Overall, 6/14 MBC with mesenchymal elements had positive PDL1 expression, no significant difference was observed among mesenchymal subtypes (p = 0.83).
MBC displays aggressive biological behavior, which is not sensitive to adjuvant therapy. Thus, immune checkpoints therapy may be a promising treatment for MBC. Previous clinical trials demonstrated that patients with PDL1-positive cells and increased TILs have better response to immune therapy [15, 44]. The selection of proper patients for immune therapy is largely based on the expression of PDL1/PD1 and density of TILs. We also found that both of the PDL1 expression in tumor cells (rho 0.492, p < 0.001) and stromal TILs (rho 0.367, p = 0.006) had a strong correlation with CD8+ TILs number. And these results were as expected that CD8+ T cells may be more sensitive to modulation of PDL1/PD1 pathway [45]. Tumors with a greater number of CD8+ TILs and positive PDL1 expression tumor cells were identified as tumor microenvironment type (TMIT) 1 according to the previous study [46]. Twenty-two patients were identified as TMIT 1 in our study, who can benefit from anti-PDL1-PD1 therapy. Our results suggest that the combination of these immune parameters may help to improve MBC patient selections for immune therapy. Further, a study indicated the relationship between PDL1 expression and macrophages (CD68 positive) [47], while we did not observe the tendency in MBC.
In this study, we have evaluated the TILs and immune checkpoints parameters expression in different components of MBC. The percentage of TILs as well as counts of TILs was both studied. These immune parameters have also been proved to be correlated with survival in MBC. However, we still have some limitations in this study. The sample size was limited due to the low incidence of MBC. Although the immune characteristics of MBC are similar with those observed in triple-negative breast cancer, it needs to be further validated in other data sets. Also, the detection of PDL1 expression was not correlated with treatment effect. The antibody (E1L3N) we used for PDL1 detection has been used and proved to have the highest positive rate in triple-negative breast cancer [35, 48]; it was not correlated with any PDL1/PD1 blockade drugs now and maybe overestimating PD-L1-positive tumors compared with FDA approved PDL1(SP142).
Conclusions
We have demonstrated the immune characteristics of different subtypes in MBC. TILs, CD4+ TILs, CD8+ TILs, and the presence of TLS were found to be correlated with better prognosis in MBC. The expression of PDL1 in tumor cells was also found to be correlated with CD8+ TILs and associated with longer survival. These data suggested that immune checkpoint therapy may be a promising treatment in a certain type of MBC.
Supplementary information
Additional file 1: Supplemental Figure 1. Representative images of the tertiary lymphoid structures stained with CD3/CD20 (CD3 = T cells, brown; CD20 = B cells, red).
Additional file 2: Supplemental Figure 2. Representative tissue section of a mixed type MBC (squamous cell cancer component and chondroid-matrix component, the same case in Fig. 2) with CD68 stains (original magnification × 200).
Additional file 3: Supplemental Figure 3. CD68+ TILs counts in different subtypes of MBC.
Additional file 4: Supplemental Table 1. Pathological characteristics of 33 cases with invasive carcinoma of no special type. Supplemental Table 2. The correlation of PDL1 expression in both tumor and stromal cells. Supplemental Table 3. The correlation of PD1 expression in both tumor and stromal cells. Supplemental Table 4. The PDL1 and PD1 expression in both tumor and stromal cells shares no difference in all subtypes of the MBCs.
Acknowledgments
We thank all the patients who participated in this study and for their willingness to contribute their data.
Authors’ contributions
CX participated in the interpretation and analysis of data and drafted the manuscript. LLL and SP participated in the interpretation of the data. YX carried out the experiments. LM, LRZ, HYH, and HJH participated in the acquisition and interpretation of pathologic data. YJP conceived of the study, participated in its design, and edited the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of China (81702759).
Availability of data and materials
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2020001424.
Ethics approval and consent to participate
The study was approved by the ethics committee of the Sun Yat-sen University Cancer Center. All patients were systemically asked for consent to use their data anonymously for analysis and publication.
Consent for publication
Not applicable.
Competing interests
There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue Chao and Lili Liu contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13058-020-01330-6.
References
- 1.Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007;14(1):166–173. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 2.Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol. 1999;10(4):413–419. doi: 10.1023/a:1008329910362. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hilli Z, Choong G, Keeney MG, Visscher DW, Ingle JN, Goetz MP, et al. Metaplastic breast cancer has a poor response to neoadjuvant systemic therapy. Breast Cancer Res Treat. 2019;176(3):709–716. doi: 10.1007/s10549-019-05264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai HW, Tseng LM, Chang TW, Kuo YL, Hsieh CM, Chen ST, et al. The prognostic significance of metaplastic carcinoma of the breast (MCB)--a case controlled comparison study with infiltrating ductal carcinoma. Breast. 2013;22(5):968–973. doi: 10.1016/j.breast.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Nelson RA, Guye ML, Luu T, Lai LL. Survival outcomes of metaplastic breast cancer patients: results from a US population-based analysis. Ann Surg Oncol. 2015;22(1):24–31. doi: 10.1245/s10434-014-3890-4. [DOI] [PubMed] [Google Scholar]
- 6.El Zein D, Hughes M, Kumar S, Peng X, Oyasiji T, Jabbour H, et al. Metaplastic carcinoma of the breast is more aggressive than triple-negative breast cancer: a study from a single institution and review of literature. Clin Breast Cancer. 2017;17(5):382–391. doi: 10.1016/j.clbc.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 9.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto H, Thike AA, Li H, Yeong J, Koo SL, Dent RA, et al. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat. 2016;156(2):237–247. doi: 10.1007/s10549-016-3743-x. [DOI] [PubMed] [Google Scholar]
- 11.Wang K, Shen T, Siegal GP, Wei S. The CD4/CD8 ratio of tumor-infiltrating lymphocytes at the tumor-host interface has prognostic value in triple-negative breast cancer. Hum Pathol. 2017;69:110–117. doi: 10.1016/j.humpath.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47(1):78–84. doi: 10.1016/j.humpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Yan L, Zhang W. Precision medicine becomes reality-tumor type-agnostic therapy. Cancer Commun (Lond) 2018;38(1):6. doi: 10.1186/s40880-018-0274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146(1):15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 16.Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noske A, Mobus V, Weber K, Schmatloch S, Weichert W, Kohne CH, et al. Relevance of tumour-infiltrating lymphocytes, PD-1 and PD-L1 in patients with high-risk, nodal-metastasised breast cancer of the German Adjuvant Intergroup Node-positive study. Eur J Cancer. 2019;114:76–88. doi: 10.1016/j.ejca.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Lakhani SR, Ellis IO, Schnitt, SJ, Tan PH, van de Vijver MJ. World Health Organization classification of tumours of the breast. World Health Organization Classification of Tumours. 2012;4:48–52.
- 19.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311–335. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M, Heo SH, Song IH, Rajayi H, Park HS, Park IA, et al. Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod Pathol. 2019;32(1):70–80. doi: 10.1038/s41379-018-0113-8. [DOI] [PubMed] [Google Scholar]
- 21.Buisseret L, Desmedt C, Garaud S, Fornili M, Wang X, Van den Eyden G, et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol. 2017;30(9):1204–1212. doi: 10.1038/modpathol.2017.43. [DOI] [PubMed] [Google Scholar]
- 22.Qin T, Zeng YD, Qin G, Xu F, Lu JB, Fang WF, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6(32):33972–33981. doi: 10.18632/oncotarget.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avigdor BE, Beierl K, Gocke CD, Zabransky DJ, Cravero K, Kyker-Snowman K, et al. Whole-exome sequencing of metaplastic breast carcinoma indicates monoclonality with associated ductal carcinoma component. Clin Cancer Res. 2017;23(16):4875–4884. doi: 10.1158/1078-0432.CCR-17-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bense RD, Sotiriou C, Piccart-Gebhart MJ, Haanen J, van Vugt M, de Vries EGE, et al. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst. 2017;109(1):djw192. 10.1093/jnci/djw192. Print 2017 Jan. [DOI] [PMC free article] [PubMed]
- 25.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 26.Chung YR, Kim HJ, Jang MH, Park SY. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res Treat. 2017;161(3):409–420. doi: 10.1007/s10549-016-4072-9. [DOI] [PubMed] [Google Scholar]
- 27.Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology. 2013;2(12):e26836. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, Yu JH, et al. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2016;69(5):422–430. doi: 10.1136/jclinpath-2015-203089. [DOI] [PubMed] [Google Scholar]
- 29.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69(1):25–34. doi: 10.1111/his.12904. [DOI] [PubMed] [Google Scholar]
- 32.Uhercik M, Sanders AJ, Owen S, Davies EL, Sharma AK, Jiang WG, et al. Clinical significance of PD1 and PDL1 in human breast cancer. Anticancer Res. 2017;37(8):4249–4254. doi: 10.21873/anticanres.11817. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Zhu H, Zhou Y, Mao F, Lin Y, Pan B, et al. Prognostic value of PD-L1 in breast cancer: a meta-analysis. Breast J. 2017;23(4):436–443. doi: 10.1111/tbj.12753. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Xi J, Zhang H, Li J, Xia Y, Xi R, et al. Somatic mutations in renal cell carcinomas from Chinese patients revealed by targeted gene panel sequencing and their associations with prognosis and PD-L1 expression. Cancer Commun (Lond). 2019;39(1):37. doi: 10.1186/s40880-019-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun WY, Lee YK, Koo JS. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med. 2016;14(1):173. doi: 10.1186/s12967-016-0925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams TA, Vail PJ, Ruiz A, Mollaee M, McCue PA, Knudsen ES, et al. Composite analysis of immunological and metabolic markers defines novel subtypes of triple negative breast cancer. Mod Pathol. 2018;31(2):288–298. doi: 10.1038/modpathol.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matikas A, Zerdes I, Lovrot J, Richard F, Sotiriou C, Bergh J, et al. Prognostic implications of PD-L1 expression in breast cancer: systematic review and meta-analysis of immunohistochemistry and pooled analysis of transcriptomic data. Clin Cancer Res. 2019;25(18):5717–5726. doi: 10.1158/1078-0432.CCR-19-1131. [DOI] [PubMed] [Google Scholar]
- 38.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 39.Fujii H, Arakawa A, Utsumi D, Sumiyoshi S, Yamamoto Y, Kitoh A, et al. CD8(+) tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer. 2014;134(10):2393–2402. doi: 10.1002/ijc.28581. [DOI] [PubMed] [Google Scholar]
- 40.Liu P, Xiao Q, Zhou B, Dai Z, Kang Y. Prognostic significance of programmed death ligand 1 expression and tumor-infiltrating lymphocytes in axial osteosarcoma. World Neurosurg. 2019;129:e240–ee54. doi: 10.1016/j.wneu.2019.05.121. [DOI] [PubMed] [Google Scholar]
- 41.Feng Y, Shen J, Gao Y, Liao Y, Cote G, Choy E, et al. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget. 2015;6(13):11139–11149. doi: 10.18632/oncotarget.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee ATJ, Chew W, Wilding CP, Guljar N, Smith MJ, Strauss DC, et al. The adequacy of tissue microarrays in the assessment of inter- and intra-tumoural heterogeneity of infiltrating lymphocyte burden in leiomyosarcoma. Sci Rep. 2019;9(1):14602. doi: 10.1038/s41598-019-50888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017;123(17):3291–3304. doi: 10.1002/cncr.30726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort a of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 45.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32(3):634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Ock CY, Keam B, Kim S, Lee JS, Kim M, Kim TM, et al. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clin Cancer Res. 2016;22(9):2261–2270. doi: 10.1158/1078-0432.CCR-15-2834. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger SN, Herbst RS, et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26(4):970–977. doi: 10.1158/1078-0432.CCR-19-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3(4):326–332. doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Figure 1. Representative images of the tertiary lymphoid structures stained with CD3/CD20 (CD3 = T cells, brown; CD20 = B cells, red).
Additional file 2: Supplemental Figure 2. Representative tissue section of a mixed type MBC (squamous cell cancer component and chondroid-matrix component, the same case in Fig. 2) with CD68 stains (original magnification × 200).
Additional file 3: Supplemental Figure 3. CD68+ TILs counts in different subtypes of MBC.
Additional file 4: Supplemental Table 1. Pathological characteristics of 33 cases with invasive carcinoma of no special type. Supplemental Table 2. The correlation of PDL1 expression in both tumor and stromal cells. Supplemental Table 3. The correlation of PD1 expression in both tumor and stromal cells. Supplemental Table 4. The PDL1 and PD1 expression in both tumor and stromal cells shares no difference in all subtypes of the MBCs.
Data Availability Statement
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2020001424.