Abstract
Background
Correct subtyping of primary aldosteronism (PA) is critical for guiding clinical management. Adrenal imaging is less accurate than adrenal vein sampling (AVS); nonetheless, AVS is invasive, technically challenging, and scarcely available.
Objective
To identify predictors of concordance between cross-sectional imaging and lateralized AVS in patients with PA that could help circumvent AVS in a subset of patients.
Methods
We retrospectively studied all patients with PA who underwent AVS in a tertiary referral center from 2009 to 2019. AVS was performed before and after cosyntropin stimulation. Patients with lateralized AVS in at least one condition were included. Aldosterone synthase-guided next-generation sequencing was performed on available adrenal tissue. Logistic regression was implemented to identify predictors of imaging-AVS lateralization concordance.
Results
A total of 234 patients (62% men), age 20 to 79 years, 73% white, 23% black, and 2% Asian were included. AVS lateralization was found: 1) both pre- and post-cosyntropin (Uni/Uni) in 138 patients; 2) only at baseline (Uni/Bi) in 39 patients; 3) only after cosyntropin stimulation (Bi/Uni) in 29 patients. Catheterization partially failed in 28 patients. AVS-imaging agreement was higher in patients with KCNJ5 versus other aldosterone-driver somatic mutations (90.3% versus 64.6%; P < 0.001); in Asian and white versus black Americans (75%, 70%, and 36%, respectively); in younger patients; and those with left adrenal nodules and contralateral suppression. Conversely, AVS-imaging agreement was lowest in Uni/Bi patients (38% vs. 69% in Uni/Uni, and 62% in Bi/Uni; P = 0.007).
Conclusions
While AVS-imaging agreement is higher in young white and Asian patients, who have KCNJ5-mutated aldosterone producing adenomas, no predictor confers absolute imaging accuracy.
Keywords: adrenal, primary aldosteronism, adrenal vein sampling, imaging, aldosterone, computed tomography
Primary aldosteronism (PA) is the most common cause of endocrine hypertension, affecting up to 10% of all hypertensive patients (1-3). The 2 major subtypes of PA are bilateral hyperaldosteronism (BHA) and unilateral PA, which is frequently caused by an aldosterone-producing adenoma (APA) (3). Unilateral forms of PA are curable with ipsilateral adrenalectomy (3). PA is associated with higher risk of cardiovascular, renal, and metabolic complications than essential hypertension (4-8). Curative treatment with unilateral adrenalectomy abrogates the enhanced cardiovascular morbidity and mortality in APA patients (4, 5, 8). Conversely, patients with PA who do not undergo surgery require lifelong medical therapy, typically including a mineralocorticoid receptor antagonist (MRA) (9).
While adrenal imaging is commonly used to identify the source of most adrenal hormonal excess disorders, including ACTH-independent hypercortisolism and pheochromocytoma, cross-sectional imaging has modest accuracy for PA subtyping (10, 11). Consequently, expert guidelines endorse adrenal vein sampling (AVS) for localizing the source(s) of aldosterone excess in most candidates for surgical treatment (3, 12, 13). AVS, however, has several limitations, including its scarce availability, high cost, and reliance on highly skilled interventional radiologists. Furthermore, the procedure and data interpretation protocols vary widely among institutions (14, 15), lending ambiguity in up to a quarter of PA cases (16, 17). Some (3, 18-20), although not all, reports (21) suggest that cross-sectional adrenal imaging can substitute AVS in young patients with unequivocal PA who harbor a typical solitary adrenal adenoma. In addition, recent studies have revealed that APAs with various underlying aldosterone driver somatic mutations exhibit specific morphological characteristics, as well as sex- and race-dependent prevalence (22). Specifically, KCNJ5-mutated APA have been reported to be larger (23), and to account for the majority of APA cases in women and in Asians of both sexes (24-28). Apart from young age, however, the influence of other variables, such as race, sex, or APA size and underlying mutations, on the concordance between AVS and adrenal imaging has not yet been reported. The aim of our study was to identify possible predictors of agreement between lateralized AVS and cross-sectional imaging in patients with PA, which could help circumvent the need for AVS in a subset of patients.
Patients and Methods
We conducted a retrospective review of all patients with confirmed PA who underwent AVS at University of Michigan between January 2009 and December 2019. Data collected included patients’ demographics, imaging, biochemical, and pathological studies, as well as clinical management and follow-up data. In available cases, we performed aldosterone synthase (CYP11B2) immunohistochemistry (IHC)-guided next generation sequencing on formalin-fixed paraffin-embedded adrenal tissue sections, as previously reported (27, 28). Primary Aldosteronism Surgery Outcome (PASO) criteria were used to assess clinical and biochemical benefits after adrenalectomy (9).
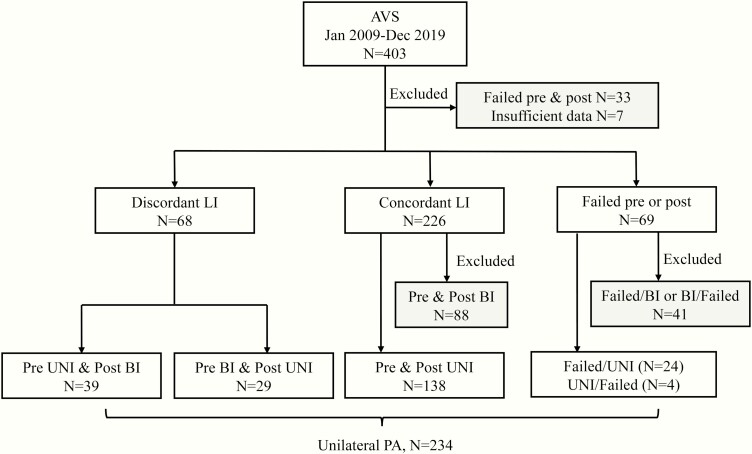
The diagnosis of PA was made in accordance with the Endocrine Society guidelines (3). Plasma aldosterone concentration, plasma renin activity, and serum cortisol concentration were measured by Clinical Laboratory Improvement Amendments (CLIA)-certified immunoassays, as previously reported (29). Baseline and cosyntropin-stimulated simultaneous AVS was performed by experienced interventional radiologists, as previously reported (29). A selectivity index (SI, defined by the cortisol concentrations in the adrenal vein/peripheral serum) greater than 2 at baseline and greater than 5 after cosyntropin stimulation was considered indicative of successful catheterization during AVS. A lateralization index (LI, determined by the ratio of aldosterone/cortisol between the dominant and contralateral adrenal veins) of minimum 4 pre- and/or post-cosyntropin stimulation was considered indicative of either unilateral or unilaterally dominant PA (Uni). Only patients with lateralized results in at least one AVS condition were included (Fig. 1). A contralateral index (CI, defined by the ratio of aldosterone/cortisol between the non-dominant adrenal vein and periphery) <1 was considered indicative of contralateral suppression.
Figure 1.
Study protocol and PA subtyping results. Abbreviations: AVS, adrenal vein sampling; PA, primary aldosteronism; pre, pre-cosyntropin stimulation; post, post-cosyntropin stimulation; Uni, unilateral; Bi, bilateral; LI, lateralization index; failed, failed catheterization.
Computed tomography (CT) scans were obtained in all but 9 patients, who had abdominal magnetic resonance imaging. In cases where multiple studies were available, the one closest to AVS and preceding surgery was used for analyses. An expert abdominal radiologist (E.C.), who was blinded to the AVS results, reviewed all cases where images were available to us. In 56 (24%) patients, outside images were not available for our review, and radiology reports were used. An adrenal nodule was defined by a contour-deforming mass within the adrenal gland and/or a mass with attenuation differences compared to the adrenal gland. As imaging studies were performed in different institutions and over more than a decade, a number of different protocols were performed (abdominal with and/or without contrast or adrenal protocol). In general, the slice thickness ranged from 0.625 mm to 5 mm.
Concordance of lateralization between cross-sectional imaging and AVS was established based on unilateral adrenal abnormalities on imaging studies along with ipsilateral AVS lateralization pre- and/or post-cosyntropin administration. Unilateral adrenalectomy was performed following shared clinical decision making between the treating team and the patient. These studies were conducted with the University of Michigan Institutional Review Boards approval (HUM00106809 and HUM00024461). A waiver of consent was granted for the retrospective data review; pathological studies, including IHC and genetic testing, were conducted with informed consent obtained from most patients. A waiver of consent was granted for the use of archival specimens for the experiments in this study (stored prior to 2/11/2011, HUM00083056).
Statistical differences in continuous parameters between 2 groups were evaluated using the Student t test (for normally distributed variables) and Mann-Whitney U-test (for variables with skewed distribution). Comparisons of continuous variables between 3 groups were assessed with the Kruskal-Wallis test. Chi-square and Fisher’s exact tests were used to compare proportions between groups. Univariate and multiple logistic regression with stepwise selection (forward, backward, and bidirectional) was performed to identify predictors of agreement between AVS lateralization cross-sectional and imaging. A P value < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of study participants
During the study period, 403 patients with confirmed PA underwent AVS in our institution. Of these, a total of 40 patients with unsuccessful AVS pre- and post-cosyntropin and/or incomplete clinical records available for our review were excluded (Fig. 1). Of the 69 cases with failed adrenal vein catheterization before or after cosyntropin stimulation, 28 had lateralized AVS results based on the available data (24 failed at baseline and 4 failed after cosyntropin stimulation) and were included in the study (Fig. 1). Of the remaining 294 patients, AVS subtyping was consistently lateralized both at baseline and post-cosyntropin stimulation (Uni/Uni) in 138 patients; only at baseline (Uni/Bi) in 39 patients; and only after cosyntropin stimulation (Bi/Uni) in 29 patients.
Of the 234 patients included, 146 (62%) were men, 170 (73%) were white, 53 (23%) were black, and 4 (2%) were Asian (Table 1). The median age was 53 years (range, 20-79 years), and 12 patients (6%) were younger than 35 years (9 Uni/Uni, 1 Bi/Uni, 1 failed/Uni, and 1 Uni/failed). Baseline and cosyntropin-stimulated LI were highest, while baseline CI was lowest in the Uni/Uni group compared to patients with inconsistent lateralized AVS results (Table 1). In addition, peripheral aldosterone was higher in the Uni/Uni group (P < 0.01) compared to those with discrepant AVS results, indicating more severe PA in the Uni/Uni patients.
Table 1.
Characteristics of Study Participants
| Total N = 234 |
UNI/UNI N = 138 (59%) |
UNI/BI N = 39 (17%) |
BI/UNI N = 29 (12%) |
Partially failed (N = 28)* (12%) | P | |
|---|---|---|---|---|---|---|
| Age, years | 53 (20-79) | 53 (20-78) | 49 (36-79) | 53 (33-78) | 56 (31-73) | 0.92 |
| Men, N (%) | 146 (62%) | 86 (62%) | 20 (51%) | 23 (79%) | 17 (61%) | 0.13 |
| Race, N | ||||||
| White | 170 | 108 | 19 | 19 | 24 | 0.007 |
| Black | 53 | 24 | 17 | 8 | 4 | |
| Asian | 4 | 2 | 1 | 1 | 0 | |
| Unknown/Mixed | 7 | 4 | 2 | 1 | 0 | |
| BMI, kg/m 2 | 32.8 [29.0-36.6] | 32.1 [28.7-37.0] | 32.4 [29.5-35.3] | 35.2 [31.4-39.5] | 34.5 [28.4-35.9] | 0.24 |
| LI baseline | 9.8 [5.3-26.1] | 15.7 [7.6-37.4] | 6.8 [5.4-10.4] | 2.2 [1.7-3] | 24.1 [8.2-48.6]a | <0.0001 |
| LI post-ACTH | 11.1 [5-28.7] | 17 [8.4-40.4] | 2.7 [1.6-3.6] | 8.7 [4.4-17.6] | 18.1 [6.5-45.1]b | <0.0001 |
| CI baseline | 0.5 [0.3-1.1] | 0.4 [0.2-0.7] | 0.9 [0.5-1.5] | 0.9 [0.5-2.7] | 0.3 [0.1-0.6]a | <0.0001 |
| CI post-ACTH | 0.2 [0.1-0.6] | 0.2 [0.1-0.4] | 1.0 [0.4-1.6] | 0.3 [0.1-0.6] | 0.2 [0.1-0.6]b | <0.0001 |
| Peripheral aldosterone, ng/dL | 24 [14-41] | 29 [15-43] | 17 [10-27] | 20 [12-31] | 30 [18-54] | 0.001 |
| Mass size, mm | 14 (4-56) | 14 (4-56) | 12 (6-34) | 15 (9-36) | 15 (6-22) | 0.45 |
| Ipsilateral nodule, N (%) | 169 (72%) | 111 (80%) | 17 (44%) | 18 (62%) | 23 (82%) | <0.0001 |
| Dominant side, Left, % | 57% | 59% | 36% | 69% | 64% | 0.02 |
| Mutation, N | ||||||
| KCNJ5 | 31 | 25 | 2 | 1 | 3 | 0.54 |
| CACNA1D | 26 | 15 | 3 | 4 | 4 | |
| ATP1A1 | 12 | 7 | 1 | 3 | 1 | |
| ATP2B3 | 4 | 4 | 0 | 0 | 0 | |
| CACNA1H | 1 | 1 | 0 | 0 | 0 | |
| CTNNB1 | 1 | 0 | 0 | 1 | 0 | |
| AVS-Imaging agreement | 146 (62%) | 95 (69%) | 15 (38%) | 18 (62%) | 18 (64%) | 0.007 |
Data are expressed as median [interquartile range] or (range), numbers (N) and %.
Abbreviations: AVS, adrenal vein sampling; Bi, bilateral; BMI, body mass index; CI, contralateral suppression index; LI, lateralization index; Uni, unilateral.
*includes 24 patients with Failed/Uni and 4 patients with Uni/Failed AVS data; aonly Uni/Failed; bonly Failed/Uni
Genetic studies
To date, 153 patients underwent unilateral adrenalectomy: 105 Uni/Uni, 12 Uni/Bi, 19 Bi/Uni, 15 failed/Uni, and 2 Uni/failed patients. CYP11B2 IHC-guided next-generation sequencing has so far been performed in 77 patients. Of these, 31 (41%) patients were found to have KCNJ5 somatic mutations, 26 (35%) CACNA1D mutations, 12 (16%) ATP1A1 mutations, 4 (5%) ATP2B3, 1 (1.5%) CTNNB1, 1 (1.5%) CACNA1H mutations (Table 1), and no known aldosterone-driver mutations were identified in 2 CYP11B2-positive APAs (2.6%). Multiple aldosterone-producing cell clusters (APCCs) were found in 3 patients, and 5 other patients had CYP11B2-negative cortical adenomas; two to six additional blocks per case were obtained from pathology and all were negative for CYP11B2 staining.
Postoperative outcomes
Of the 153 patients who underwent unilateral adrenalectomy, clinical and biochemical follow-up were available in 133 (87%) and 104 (68%) patients, respectively. Based on the PASO criteria, clinical benefit was achieved in 89% of patients: complete clinical success in 28 (21%), and partial clinical success in 91 (68%) patients; clinical benefit was absent in 14 (11%) patients (Table 2). Biochemical cure was achieved in 86 (83%) patients, partial biochemical success in 13 (12%) patients, and 5 patients had no biochemical benefit after adrenalectomy (Table 2). The AVS-imaging agreement was higher in patients with biochemical cure (73%) than in all other patients together (28%), while no difference was observed between clinical outcome groups (Table 2). There was no significant difference in biochemical (P = 0.47) or clinical outcomes (P = 0.58) between patients in the Uni/Bi and Bi/Uni groups.
Table 2.
Comparison of Postoperative Outcomes Between AVS Lateralization Groups and Between Patients with AVS-Imaging Agreement vs Disagreement
| Clinical benefit (N = 133) |
P | Biochemical benefit (N = 104) |
P | |||||
|---|---|---|---|---|---|---|---|---|
| Complete | Partial | Absent | Complete | Partial | Absent | |||
| Pre- and Post- ACTH AVS lateralization | 0.78 | 0.19 | ||||||
| Uni/Uni | 20 | 59 | 12 | 60 | 9 | 2a | ||
| Uni/Bi | 2 | 6 | 0 | 5 | 0 | 1 | ||
| Bi/Uni | 3 | 13 | 2 | 9 | 3 | 2a | ||
| Partially failedb | 3 | 13 | 0 | 12 | 1 | 0 | ||
| AVS-imaging agreement | 0.33 | 0.0003 | ||||||
| Yes | 22 | 59 | 11 | 63 | 3 | 2a | ||
| No | 6 | 32 | 3 | 23 | 10 | 3a |
Abbreviations: AVS, adrenal vein sampling; Uni, unilateral; Bi, bilateral;
a1 patient had partial adrenalectomy; b Includes patients with Failed/Uni (N = 15) and Uni/Failed (N = 1).
In total, 18 patients with lateralized AVS and negative ipsilateral imaging findings underwent adrenalectomy: 9 Uni/Uni, 4 Bi/Uni, 3 Uni/Bi, and 2 Failed/Uni. Clinical pathology showed subcentimeter nodules in 10 cases, a central 1.2 cm nodule in 1 case, nodular hyperplasia in 4 cases, and apparently normal adrenal tissue in 1 case. Pathological reports were not available in 2 patients who underwent adrenalectomy in an outside hospital. CYP11B2 IHC has been performed in 9 cases, and it showed APAs in 7 patients (all with CACNA1D mutations), a CYP11B2 negative tumor in 1 patient, and multiple APCCs in 1 patient. Following adrenalectomy, clinical success was achieved in 15/16 patients with available data (complete in 3, and partial in 12 patients). Biochemical cure was achieved in 8/14 patients, improvement in 4 patients, and 2 patients had no biochemical benefit.
Of the 5 total patients without biochemical benefit, imaging findings were concordant with the AVS lateralization in 2 patients. Of the latter, 1 patient underwent partial adrenalectomy, and in both patients no CYP11B2-positive areas were identified. Of the remaining 3 patients, imaging showed no abnormalities in 1 patient, bilateral nodules (1.3 cm on the dominant side and 1 cm on the opposite side) in 1 patient, and a 2.2 cm contralateral nodule and nodular thickening of both adrenal glands in the third patient. One of these patients had a CACNA1D mutation, 1 had an ATP1A1 mutation, and 1 had no CYP11B2 positive areas in the available blocks. Of the 5 patients with CYP11B2-negative IHC, AVS results were Uni/Uni in 3 patients, Uni/Bi in 1 patient, and the Bi/Uni in 1 patient. All patients had partial clinical benefit; biochemical remission was achieved in 2 patients, 2 patients had no biochemical benefit after surgery (1 with partial adrenalectomy), and no postoperative data were available in the remaining patient.
Factors associated with imaging-AVS lateralization agreement
Overall, the concordance rate between lateralized AVS and cross-sectional imaging was only 62% (146/234), and it was highest in the Uni/Uni group (69% vs 49% in the discordant group; P = 0.005, Table 3). In contrast, patients in the Uni/Bi group had the lowest rate of AVS-cross-sectional imaging agreement (38%), and 22/39 (56%) patients in the Uni/Bi group had no ipsilateral adrenal mass detected by cross-sectional imaging (Table 1). While, overall, patients with imaging-AVS agreement were younger (mean age 50.5 vs 56.5 years, P < 0.0001), 2 patients under age 35 (25 and 31 years) had bilateral adrenal abnormalities on imaging, but lateralized AVS (1 harboring a KCNJ5 and 1 a CACNA1D somatic mutation). The proportion of imaging-AVS agreement was higher in Asian and white than in black PA patients (75%, 70%, and 36%, respectively, P < 0.0001) and among patients with APAs harboring KCNJ5 mutations versus other aldosterone-driver somatic mutations (90.3% vs 61.4%; P < 0.0001, Table 3). Compared with patients with discrepant imaging-AVS results, patients with imaging-AVS agreement harbored more often a left adrenal mass (68.5% vs 37.5%; P < 0.0001, Table 3) On average, patients with imaging-AVS agreement also had higher peripheral aldosterone levels (26 vs 21 ng/dL; P = 0.03), and more profound basal and post-cosyntropin stimulation contralateral suppression (P < 0.0001, Table 3).
Table 3.
Comparison of Patients with Agreement vs Disagreement Between AVS and Cross-Sectional Imaging
| AVS-imaging agreement N = 146 |
AVS-imaging disagreement N = 88 |
P | |
|---|---|---|---|
| Age, years | 50.5 (20-78) | 56.5 (25-79) | <0.0001 |
| Race, N | |||
| White | 120 | 50 | <0.0001 |
| Black | 19 | 34 | |
| Asian | 3 | 1 | |
| Unknown/Mixed | 4 | 3 | |
| Men, N (%) | 84 (58%) | 62 (70%) | 0.048 |
| BMI, kg/m 2 | 33 [28-37] | 32 [29-36] | 0.26 |
| Mass size, mm | 15 (4-36) | 13.5 (6-56) | 0.79 |
| Ipsilateral nodule, N (%) | 135 (92%) | 34 (38%) | <0.0001 |
| Pre- and post-ACTH AVS lateralization, N | |||
| Concordant | 95 | 43 | 0.0047 |
| Discordant | 33 | 35 | |
| LI baseline | 11.8 [5.3-35.5] | 8.4 [5.1-19.6] | 0.11 |
| LI post-ACTH | 14.8 [6.7-39] | 6.9 [3.3-17.9] | <0.0001 |
| CI baseline | 0.4 [0.2-0.8] | 0.8 [0.4-1.5] | <0.0001 |
| CI post-ACTH | 0.2 [0.1-0.4] | 0.4 [0.2-1.1] | <0.0001 |
| Peripheral aldosterone, ng/dL | 26 [17-44] | 21[11-40] | 0.03 |
| Dominant side, N | |||
| Left | 100 | 33 | <0.0001 |
| Right | 46 | 55 | |
| Mutation, N | |||
| KCNJ5 | 28 | 3 | <0.0001 |
| CACNA1D | 14 | 12 | |
| ATP1A1 | 10 | 2 | |
| ATP2B3 | 1 | 3 | |
| CACNA1H | 1 | 0 | |
| CTNNB1 | 1 | 0 |
Data are expressed as median [interquartile range] or (range), numbers (N) and %.
Abbreviations: AVS, adrenal vein sampling; BMI, body mass index; CI, contralateral suppression index; LI, lateralization index.
After adjusting for other variables, age, race, adrenal mass side, and post-cosyntropin stimulation CI was a consistent independent predictor of imaging-AVS agreement. Overall, younger age, left adrenal lesions, and lower post-cosyntropin stimulation CI were associated with higher likelihood of agreement between imaging and lateralized AVS, while black Americans had higher odds of discrepant imaging-AVS results as compared with white Americans (Table 4). Female sex, consistent AVS results, post-cosyntropin stimulated LI, and KCNJ5-mutated APAs were also associated with higher likelihood of imaging-AVS agreement in univariate analyses. In contrast, BMI, baseline LI, aldosterone concentrations, and the lesion size did not predict the overall likelihood of imaging-AVS agreement in lateralized PA. Stepwise regression analysis restricted to patients with known biochemical benefit following adrenalectomy found that younger age and lower CI after cosyntropin stimulation were the best predictors of imaging-AVS lateralization agreement (Table 4).
Table 4.
Predictors of Agreement Between Lateralized AVS and Cross-Sectional Imaging
| A. All Patients (N = 234) | |||
|---|---|---|---|
| Variable | Odds ratio | 95% Confidence interval | P |
| A.1. Univariate Logistic regression | |||
| Age | 0.95 | 0.92-0.97 | <0.0001 |
| Race (vs White) | |||
| Black | 0.23 | 0.12-0.45 | <0.0001 |
| Asian | 1.25 | 0.13-12.31 | 0.85 |
| Sex (F vs M) | 1.76 | 1.002-3.09 | 0.049 |
| Uni/Uni (vs discordant AVS) | 2.34 | 1.25-4.11 | 0.005 |
| LI baseline | 1.003 | 1.00-1.01 | 0.317 |
| LI post-ACTH | 1.02 | 1.005-1.02 | 0.004 |
| CI baseline | 0.82 | 0.69-0.99 | 0.038 |
| CI post-ACTH | 0.26 | 0.14-0.48 | <0.0001 |
| Mutation (vs CACNA1D) | |||
| KCNJ5 | 7.47 | 1.82-30.65 | 0.01 |
| ATP1A1 | 4.00 | 0.73-21.84 | 0.20 |
| ATP2B3 | 0.27 | 0.03-2.9 | 0.04 |
| Dominant side, Left | 3.62 | 2.08-6.31 | <0.0001 |
| A.2. Multivariate Logistic regression | |||
| Age | 0.94 | 0.92-0.98 | 0.0004 |
| Dominant side, Left | 3.53 | 1.72-7.22 | <0.0001 |
| CI post-ACTH | 0.24 | 0.1-0.54 | <0.0001 |
| Race | 0.0107 | ||
| Black | 0.28 | 0.12-0.66 | |
| Asian | 0.56 | 0.05-6.19 | |
| B. Patients with biochemical benefit (N = 99) | |||
| Multivariate Logistic regression | |||
| Age | 0.94 | 0.90-0.99 | 0.012 |
| CI post-ACTH | 0.14 | 0.03-0.66 | 0.014 |
Abbreviations: AVS, adrenal vein sampling; CI, contralateral suppression index; F, female; LI, lateralization index; M, male; Uni, unilateral.
Discussion
In this study, we have conducted a comprehensive analysis to identify predictors of concordance between cross-sectional imaging and lateralized AVS in patients with PA. While several variables were associated with higher odds of imaging-AVS data agreement, no parameter, including young age, was able to fully predict this association.
Similar to previous reports (10, 11, 18, 19, 30, 31), the rate of agreement between cross-sectional imaging and AVS was overall modest (62%) in this study, despite including only lateralized cases. An important caveat to previously published studies is that PA subtyping based on AVS data might vary depending on the protocol used. As most centers perform AVS either with or without cosyntropin, the proportion of lateralization concordance with adrenal imaging studies can be impacted in a considerable number of patients. In agreement with previous data (16), including from our group (17), AVS subtyping was conflicting based on pre- versus post-cosyntropin results in more than a quarter of cases. Interestingly, more than half of patients with lateralization only at baseline had no detectable masses by imaging, leading to the highest rate of imaging-AVS disagreement in this group. In contrast, patients with consistent lateralization regardless of the AVS protocol had the highest rate of concordance with adrenal imaging. As we have previously reported (17, 32), such cases (Uni/Uni) tend to have more severe forms of PA, with higher peripheral aldosterone, higher LI, and more profound contralateral suppression than cases with discordant AVS results. These findings suggest distinct features between patients with consistent versus discrepant AVS results, that might reflect the complex spectrum of PA pathologies, which expands beyond the traditionally acknowledged single APAs and bilateral adrenal hyperplasia (32-35).
Another important finding of our study is that APAs harboring KCNJ5 mutations have higher rates of concordance between imaging and AVS (90%) compared to those with other aldosterone-driver somatic mutations. The prevalence of KCNJ5-mutated APAs varies geographically. Specifically, such mutations are found in over 80% of East Asian populations with APAs, but only in 40% of white American, European, Australian, and Brazilian APA patients (25, 28, 36-39). Nevertheless, the rate of concordance between CT and AVS (50.5%) was not above average in a large Chinese PA population (40). In addition, although APAs harboring KCNJ5 mutations tend to be larger (22, 39) and more common in women, regardless of race (24, 26-28), adrenal nodule size and sex were not good predictors of imaging-AVS concordance. Both findings might be explained by the high prevalence of sizable adrenal incidentalomas that are not APAs, and which occur with similar rates across sexes (41, 42). Using CYP11B2 IHC, we and others have shown that even ipsilateral dominant adrenal nodules are not always the sources of aldosterone excess (29, 35, 43, 44).
In contrast with APAs harboring KCNJ5 mutations, those with CACNA1D mutations had a low rate (54%) of CT-AVS concordance. This finding is not surprising, given that CACNA1D mutations were identified in 65% of micronodules not detected by imaging in patients with unilateral PA (45), as well as in 58% of aldosterone-producing cell clusters isolated from patients with BHA (46). Furthermore, we found that compared with white and Asian patients, black patients have lower imaging-AVS data agreement, and this population also has the highest prevalence of CACNA1D mutations (27).
In agreement with previous reports (18, 19, 40), we found that young age was a predictor of concordant imaging-AVS results. Considering such reports, along with the low prevalence of adrenal incidentalomas in young individuals (41, 42), the Endocrine Society guidelines suggest that AVS is not necessary in patients younger than 35 years with convincing PA and solitary adrenal nodules on imaging (3). In a Japanese cohort, 3 patients younger than 35 years had bilateral AVS results, but all had favorable outcomes after unilateral CT-guided adrenalectomy (18). AVS, however, was conducted only with cosyntropin stimulation in this study, possibly masking baseline lateralization. Conversely, in our study, 2 patients younger than 35 with bilateral adrenal abnormalities on CT had lateralized AVS, and both had documented aldosterone-driver somatic mutations in the removed adrenals.
In addition to age, profound contralateral suppression of aldosterone production following cosyntropin stimulation was strongly associated with agreement between AVS and cross-sectional imaging. Patients with lateralized AVS results might have asymmetrical PA, which likely explains the imperfect cure rates after AVS-guided adrenalectomy. The predictive value of contralateral suppression in regard to postoperative outcomes in PA has been variable (47-49). Profound contralateral suppression following cosyntropin stimulation appears to at least predict the absence of sizable aldosterone-producing nodules within the nondominant adrenal gland.
A left adrenal nodule was overall predictive of concordant imaging-AVS data when including all patients. While APAs were previously reported to be more common on the left (30, 50), the agreement between AVS and CT was similar between the 2 sides in a large Japanese cohort (51). Considering that not all imaging studies performed in our patients were dedicated to the adrenal glands, it is conceivable that the rate of adrenal nodules detection is less accurate on the right, possibly due to proximity to the liver. Indeed, besides APAs, other adrenal cortical adenomas have also been reported to be more common on the left (52).
Overall, clinical outcomes were similar between patients with and without imaging-AVS concordance. While biochemical cure was significantly higher in patients with imaging-AVS agreement, 27% of patients with biochemical cure had discordant AVS versus imaging results. The higher rate of AVS-imaging discordance in patients with partial biochemical benefit could be due to asymmetrical BHA. Both clinical and biochemical outcomes were comparable between AVS lateralization subgroups. In total, only 5 patients had absence of biochemical cure, and 2 of these had partial adrenalectomy. In the remaining patients, CT demonstrated bilateral nodules (1 case), or mild thickening (2 cases). In a randomized controlled trial, Dekkers et al reported no difference in clinical benefit between patients who were offered unilateral adrenalectomy based on either CT or AVS results, despite the fact that the rate of concordance between CT and AVS was only 50% (50). The relevance of this outcome is, however, questionable, as treatment with mineralocorticoid receptor antagonists, the most effective medical therapy for PA, was permitted. Because AVS was performed only under continuous cosyntropin infusion, postoperative outcomes of patients with bilateral baseline AVS results could not be assessed in this study. Furthermore, clinical benefit has been reported both in patients with BHA (53), as well as in those with bilateral lesions on CT but lateralized PA on AVS (54). Conversely, the proportion of biochemical failure was higher in the CT group both in the study by Dekkers and colleagues (20% vs 10% in the AVS group), as well as in an international study of 761 patients from 18 centers (20). Taken together, these studies suggest that the accuracy of cross-sectional imaging for PA lateralization remains inferior to AVS.
Our study has several limitations, including its retrospective design and heterogeneity in cross-sectional imaging protocols. Considering that the agreement between AVS and cross-sectional imaging was not associated with the size of detectable masses, it remains, however, uncertain if studies with higher resolution could add value in PA subtyping. While the stratification of AVS results based on pre- and post-cosyntropin data is an asset that few institutions have access to, the limited number of patients with discrepant AVS results who typically undergo surgery in our center remains small, primarily due to the lack of clarity regarding clinical benefits that surgery might have in such patients. Moreover, as in other tertiary-referral centers, surgical specimens and/or postoperative follow-up data were not available in patients who had only partial care in our institution. The lack of standardized postoperative follow-up further limits the conclusions regarding the accuracy of AVS-based PA subtyping.
In summary, we have found that the agreement between cross-sectional imaging and lateralized AVS is higher in young white and Asian PA patients, with APAs harboring KCNJ5 mutations, and imaging abnormalities in the left adrenal gland. Nonetheless, all predictors remain relatively poor, rendering AVS necessary in most PA cases. Large-scale multicenter prospective studies with standardized imaging and AVS protocols, and long-term postsurgical follow-up are essential to evaluate the accuracy of noninvasive PA subtyping modalities.
Acknowledgments
We thank the University of Michigan research and clinical adrenal team members; Dr. Thomas J. Giordano and Ms. Michelle Vinco, for assistance in tissue slides preparation; Ms. Amy Blinder, for assistance with adrenal tissue studies; and the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation, management, and distribution services in support of the research reported in this publication (55).
Financial Support: A.F.T. was supported by grants 1K08DK109116 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and 2019087 from the Doris Duke Charitable Foundation. W.E.R. was supported by grant R01DK106618 from the NIDDK. K.N. and A.T.N. were supported by grants 17SDG33660447 and 18POST33990227, respectively, from the American Heart Association.
Glossary
Abbreviations
- APA
aldosterone-producing adenoma
- APCC
aldosterone-producing cell cluster
- AVS
adrenal vein sampling
- BHA
bilateral hyperaldosteronism
- CI
contralateral index
- CT
computed tomography
- CYP11B2
aldosterone synthase
- IHC
immunohistochemistry
- LI
lateralization index
- PA
primary aldosteronism
- PASO
Primary Aldosteronism Surgery Outcome
- SI
selectivity index
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies–a review of the current literature. Horm Metab Res. 2012;44(3):157-162. [DOI] [PubMed] [Google Scholar]
- 2. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811-1820. [DOI] [PubMed] [Google Scholar]
- 3. Funder JW, Carey RM, Mantero F, et al. The Management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 4. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3(8):768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulatero P, Monticone S, Bertello C, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826-4833. [DOI] [PubMed] [Google Scholar]
- 7. Fallo F, Veglio F, Bertello C, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454-459. [DOI] [PubMed] [Google Scholar]
- 8. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72(3):658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams TA, Lenders JWM, Mulatero P, et al. ; Primary Aldosteronism Surgery Outcome (PASO) investigators . Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nwariaku FE, Miller BS, Auchus R, et al. Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg. 2006;141(5):497-502; discussion 502. [DOI] [PubMed] [Google Scholar]
- 11. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136(6):1227-1235. [DOI] [PubMed] [Google Scholar]
- 12. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285(2):126-148. [DOI] [PubMed] [Google Scholar]
- 13. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151-160. [DOI] [PubMed] [Google Scholar]
- 14. Monticone S, Viola A, Rossato D, et al. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. Lancet Diabetes Endocrinol. 2015;3(4):296-303. [DOI] [PubMed] [Google Scholar]
- 15. Laurent I, Astere M, Zheng F, et al. Adrenal venous sampling with or without adrenocorticotropic hormone stimulation: a meta-analysis. J Clin Endocrinol Metab. 2019;104(4):1060-1068. [DOI] [PubMed] [Google Scholar]
- 16. El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and post-ACTH aldosterone and its ratios are useful during adrenal vein sampling in primary aldosteronism. J Clin Endocrinol Metab. 2016;101(4):1826-1835. [DOI] [PubMed] [Google Scholar]
- 17. Wannachalee T, Zhao L, Nanba K, et al. Three discrete patterns of primary aldosteronism lateralization in response to cosyntropin during adrenal vein sampling. J Clin Endocrinol Metab. 2019;104(12):5867-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Umakoshi H, Ogasawara T, Takeda Y, et al. Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin Endocrinol (Oxf). 2018;88(5):645-651. [DOI] [PubMed] [Google Scholar]
- 19. Lim V, Guo Q, Grant CS, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J Clin Endocrinol Metab. 2014;99(8):2712-2719. [DOI] [PubMed] [Google Scholar]
- 20. Williams TA, Burrello J, Sechi LA, et al. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension. 2018;72(3):641-649. [DOI] [PubMed] [Google Scholar]
- 21. Riester A, Fischer E, Degenhart C, et al. Age below 40 or a recently proposed clinical prediction score cannot bypass adrenal venous sampling in primary aldosteronism. J Clin Endocrinol Metab. 2014;99(6):E1035-E1039. [DOI] [PubMed] [Google Scholar]
- 22. Ono Y, Yamazaki Y, Omata K, et al. Histological characterization of aldosterone-producing adrenocortical adenomas with different somatic mutations. J Clin Endocrinol Metab. 2020;105(3):E282-E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089-E1095. [DOI] [PubMed] [Google Scholar]
- 24. Okamura T, Nakajima Y, Katano-Toki A, et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr J. 2017;64(1):39-47. [DOI] [PubMed] [Google Scholar]
- 25. Zheng FF, Zhu LM, Nie AF, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65(3):622-628. [DOI] [PubMed] [Google Scholar]
- 26. Wang B, Li X, Zhang X, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore). 2015;94(16):e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73(4):885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nanba K, Omata K, Else T, et al. Targeted molecular characterization of aldosterone-producing adenomas in White Americans. J Clin Endocrinol Metab. 2018;103(10):3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nanba AT, Nanba K, Byrd JB, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87(6):665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sam D, Kline GA, So B, Leung AA. Discordance between imaging and adrenal vein sampling in primary aldosteronism irrespective of interpretation criteria. J Clin Endocrinol Metab. 2019;104(6):1900-1906. [DOI] [PubMed] [Google Scholar]
- 31. Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151(5):329-337. [DOI] [PubMed] [Google Scholar]
- 32. Turcu AF, Wannachalee T, Tsodikov A, et al. Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension. 2020;75(1):183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Sousa K, Boulkroun S, Baron S, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75(4):1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ono Y, Yamazaki Y, Omata K, et al. Histological characterization of aldosterone-producing adrenocortical adenomas with different somatic mutations. J Clin Endocrinol Metab. 2020;105(3):e282-e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nanba K, Chen AX, Omata K, et al. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101(3):999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azizan EA, Lam BY, Newhouse SJ, et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97(5):E819-E829. [DOI] [PubMed] [Google Scholar]
- 37. Boulkroun S, Beuschlein F, Rossi GP, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59(3):592-598. [DOI] [PubMed] [Google Scholar]
- 38. Azizan EA, Murthy M, Stowasser M, et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension. 2012;59(3):587-591. [DOI] [PubMed] [Google Scholar]
- 39. Vilela LAP, Rassi-Cruz M, Guimaraes AG, et al. KCNJ5 somatic mutation is a predictor of hypertension remission after adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2019;104(10):4695-4702. [DOI] [PubMed] [Google Scholar]
- 40. Zhu L, Zhang Y, Zhang H, et al. Comparison between adrenal venous sampling and computed tomography in the diagnosis of primary aldosteronism and in the guidance of adrenalectomy. Medicine (Baltimore). 2016;95(39):e4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2016;175(2):G1-G34. [DOI] [PubMed] [Google Scholar]
- 42. Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637-644. [DOI] [PubMed] [Google Scholar]
- 43. Dekkers T, ter Meer M, Lenders JW, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99(7):E1341-E1351. [DOI] [PubMed] [Google Scholar]
- 44. Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamazaki Y, Nakamura Y, Omata K, et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017;102(4):1182-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Omata K, Satoh F, Morimoto R, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72(4):874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolley MJ, Gordon RD, Ahmed AH, Stowasser M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. J Clin Endocrinol Metab. 2015;100(4):1477-1484. [DOI] [PubMed] [Google Scholar]
- 48. Tagawa M, Ghosn M, Wachtel H, et al. Lateralization index but not contralateral suppression at adrenal vein sampling predicts improvement in blood pressure after adrenalectomy for primary aldosteronism. J Hum Hypertens. 2017;31(7):444-449. [DOI] [PubMed] [Google Scholar]
- 49. Shariq OA, Bancos I, Cronin PA, et al. Contralateral suppression of aldosterone at adrenal venous sampling predicts hyperkalemia following adrenalectomy for primary aldosteronism. Surgery. 2018;163(1):183-190. [DOI] [PubMed] [Google Scholar]
- 50. Dekkers T, Prejbisz A, Kool LJS, et al. ; SPARTACUS Investigators . Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4(9):739-746. [DOI] [PubMed] [Google Scholar]
- 51. Wada N, Shibayama Y, Yoneda T, et al. ; JPAS/JRAS Study Group . Lateralizing asymmetry of adrenal imaging and adrenal vein sampling in patients with primary aldosteronism. J Endocr Soc. 2019;3(7):1393-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hao M, Lopez D, Luque-Fernandez MA, et al. The lateralizing asymmetry of adrenal adenomas. J Endocr Soc. 2018;2(4):374-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab. 2009;94(7):2437-2445. [DOI] [PubMed] [Google Scholar]
- 54. Aono D, Kometani M, Karashima S, et al. Primary aldosteronism subtype discordance between computed tomography and adrenal venous sampling. Hypertens Res. 2019;42(12):1942-1950. [DOI] [PubMed] [Google Scholar]
- 55. Kheterpal S Rdw/Datadirect: A Self-Serve Tool for Data Retrieval. Ann Arbor, MI: University of Michigan; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.