Abstract
Background
The dishevelled associated activator of morphogenesis (DAAM) family, consisting of DAAM1 and DAAM2, is an important component of the Wnt signal pathway. Previous studies have suggested that DAAM2 reduces Von Hippel-Lindau (VHL) expression by promoting its ubiquitination, but the correlation between DAAM and HIF-1α in hepatocellular carcinoma (HCC) has not been studied.
Material/Methods
In our study, expression of DAAM1 and DAAM2 in HCCs and tumor-adjacent liver tissues was assessed with qRT-PCR and immunohistochemistry. Correlations between DAAM1/2 and the clinicopathologic variables were evaluated with the Chi-square test. With univariate and multivariate analysis, we further evaluated the prognostic significance of DAAM1 and DAAM2. Using in vitro experiments, we assessed the functions of DAAM1 and DAAM2 in invasion and proliferation in different HCC cell lines and investigated their underlying mechanisms.
Results
DAAM1 and 2 overexpression were 18.8% and 48.7%, respectively, of the whole cohort. mRNAs of DAAM2 in HCCs were substantially higher than mRNAs in liver tissues, while DAAM1 mRNA had no marked difference. High DAAM2 expression was notably associated with advanced T stage (P=0.032), TNM stage (P=0.032), and overall survival (OS) rate (P=0.004). DAAM 2 knockdown promoted VHL accumulation and subsequent HIF-1α down-regulation in HCC cells. In HCC specimens, DAAM2 expression was also negatively correlated with VHL and positively associated with HIF-1α. Moreover, HIF-1α was required in DAAM2-induced invasion of HCC cells.
Conclusions
DAAM2, rather than DAAM1, was able to predict prognosis of HCC. DAAM2 decreased VHL expression and consequently upregulated HIF-1α, eventually facilitating invasion of HCC.
MeSH Keywords: Carcinoma, Hepatocellular; Cell Hypoxia; Neoplasm Invasiveness; Prognosis; Wnt Signaling Pathway
Background
Primary liver cancer is the fifth most common cancer and the second leading cause of cancer-related death globally, resulting in approximately 800,000 deaths each year [1]. Liver cancer is mainly divided into hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA), with different histologic features, treatment strategies, and outcomes [2]. Chronic infection with hepatitis B virus (HBV) is the main cause of HCC, especially China, which has the highest incidence of HCC due to the high prevalence of HBV infection [3]. HCC is a highly heterogeneous tumor, leading to its high resistance to therapy. The effects of systemic therapy on HCC are limited, and OS rates for HCC are still very low. The overall 5-year survival rate for HCC after liver resection is approximately 30% [4]. Predictive and prognostic biomarker are well known to be of benefit in stratifying patients based on risk, and in HCC, alpha fetoprotein (AFP) is used. However, more effective biomarkers and potential therapies for HCC are urgently needed.
Dishevelled associated activator of morphogenesis (DAAM) family, consisting of DAAM1 and DAAM2, are Wnt signaling effectors and function downstream of Wnt ligands and upstream of β-catenin [5]. DAAM1 and DAAM2 mediate coordination of formin-dependent actin remodeling by binding with Disheveled (DVL) and Wnt-dependent RhoA activation [6]. DAAMs interact with the actin cytoskeleton with its highly conserved Formin homology (FH) domains FH1 and FH2 [7]. Regulation of the actin cytoskeleton of DAAM regulates cell shape and polarized cell growth. DAAM 1 and 2 are recently identified proteins and their functions are gradually being elucidated. Reports also exist about the oncogenic role of DAAMs. For example, DAAM1 has been demonstrated to promote tumor progression in several cancers types, including breast and ovarian cancer [8,9]. Although several studies have suggested a tumor-promoting role for DAAM2 in some special tumors, such as glioma and renal cancer [10,11], less research has been done focusing on the oncogenic role of DAAM2 than on that of DAAM1. Moreover, expression and function of DAAM1 and DAAM2 in HCC are still obscure.
Von Hippel-Lindau (VHL) is a target recruitment subunit in the E3 ubiquitin ligase complex and recruits hydroxylated hypoxia-inducible factor (HIF) under normoxic conditions. HIF1 is a main intracellular effector to hypoxia, comprised of an unstable α-subunit (HIF-1α) and a stable β-subunit (HIF-1β). In normoxia, HIF-1α is hydroxylated by Prolyl hydroxylase domain proteins (PHDs) at prolines 402 and 564, then recognized by VHL and degraded in the E3 ubiquitin pathway [12–14]. Recent studies have shown that DAAM2 promotes degradation of VHL and facilitated gliomagenesis [10]. However, whether DAAM2 regulates VHL degradation and its downstream protein, HIF-1α, is unknown.
In our study, the mRNAs of DAAM1 and DAAM2 in 19 HCCs and tumor-adjacent liver tissues were assessed with quantified reverse transcription-PCR(qRT-PCR), and protein levels of DAAM1 and DAAM2 in 117 cases of HCC and paired normal liver tissues were investigated with immunohistochemistry (IHC). Correlations between DAAM1/2 and the clinicopathologic variables were evaluated with the Chi-square test. With univariate and multivariate analysis, we evaluated the prognostic significance of DAAM1 and DAAM2. Using in vitro experiments, we estimated the functions of DAAM1 and DAAM2 in invasion and proliferation in different HCC cell lines. Moreover, we investigated DAAM regulation of VHL and HIF-1α, and assessed the role of HIF-1α in DAAM-induced invasion of HCC.
Material and Methods
Patients and follow-up
A total of 323 patients underwent radical surgery for HCC in the YIDU Central Hospital and the Second Hospital affiliated with Shandong 1st Medical University from 2011 to 2016. Of these patients, 117 were enrolled in our study based on the following criteria: (1) more than 3-month follow-up; (2) available tumor tissues and adjacent liver tissues for IHC; and (3) no exposure to adjuvant therapy. Nineteen pairs of fresh tumor tissues were obtained during surgery without interfering with pathological diagnosis. Histopathologial grade of HCC was based on the Edmondson system [15]. TNM staging was performed according to the 8th American Joint Committee on Cancer/Union for International Cancer Control(AJCC/UICC) classification system. All specimens were obtained with informed consent from patients. The study was approved by the Ethics Committee of the Second Hospital affiliated to Shandong 1st Medical University and the YIDU Central Hospital.
Cells and agents
Human HCC cell lines HepG3B2, HepG2, HuH7, and SMMC7721 were all purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). These cells were cultured in DMEM (Thermo Fisher, Waltham, Massachusetts, United States), supplemented by 10% fetal bovine serum (FBS; Thermo Fisher), and 100 U/mL penicillin and streptomycin (Thermo Fisher). Primary antibodies were as follows: DAAM1 (Cat. 71499, Abcam, Cambridge, Massachusetts, United States), DAAM2 (Cat. NBP2–47496, Novus Biologicals, Centennial, Colorado, United States), VHL (Cat. 556347, BD Pharmingem), Hypoxia-inducible factor 1α (HIF-1α) (Cat. NB100-105, Novus), and β-actin (Cat. sc-8432, Santa Cruz Biotechnology, Santa Cruz, California, United States).
Tissue microarrays
Tissue microarrays (TMA) were generated with the formalin-fixed and paraffin-embedded tissue sections from all patients as described in a previous study [16]. Hematoxylin and eosin staining was carried out and evaluated by a pathologist before the IHC area was selected. The IHC area encompassed tumor and necrosis and normal tissues wree avoided. The samples were then arranged in the design of the array and 1-mm cylinders were collected for TMA slides. Two cylinders were created for each case in a TMA slide.
IHC and scoring
The streptavidin peroxidase complex method was carried out for IHC staining, as described in a previous study [17]. In brief, after deparaffinization and rehydration with xylene and graded alcohol, the TMA sections were immersed in 3% hydrogen peroxide to inactivate the endogenous peroxidase, following citrate buffer (pH=6.0) to get optimal antigen retrieval. Then 1% BSA in phosphate-buffered saline was used to incubate the TMA for 30 minutes to block the unspecific binding. Primary antibodies of DAAM1 (1: 200) and DAAM2 (1: 500) were used to incubate the TMA overnight and the secondary antibodies labelled with streptoavidin-biotin-peroxidase reagent. TMAs were finally incubated with 3,3′-diaminobenzidine solution for visualization.
Results of IHC staining were estimated and semi-quantified by IHC score after evaluation by two senior pathologists who were unaware of the clinical data. The IHC score took into consideration staining intensity and percentage of stained cells. Positive scores for tumor cells were defined as: 1 ifr <10% of cells were positive; 2 if 10% to 50% of cells were positive; and 3 if >50% of cells were positive. Staining intensity scores were 0 for negative staining, 1 for weak, 2 for moderate, and 3 for strong staining. The final IHC scores were the product of multiplication of these two scoresn. The cut-off was determined by the receiver operating characteristic(ROC) curve and used to divide the cohort into subgroups according to IHC scores [18].
Western blot
HCC cells were lysed on ice using RIPA lysis buffer supplemented with 1 mM sodium vanadate, 10 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatin, 1 μg/mL antipain, and 30 μg of phenylmethylsulfonyl fluoride [19]. Total lysates were centrifuged at 12000 g to remove pallets and preserve supernatants. After electrophoresis, proteins were then transferred to nitrocellulose membranes (BioTrace NT Nitrocellulose, PALL). The membranes were incubated in the primary antibody at 4°C overnight and the secondary antibody (Beyotime, Shanghai, China) in order. Protein bands were visualized by ECL (Merck Millipore, Burlington, USA) treatment.
Cell transfection
Knockdown of DAAM1 and DAAM2 was realized by transfection of siRNA purchased from the Santa Cruz Biotechnology. Target sequences of DAAM1 were GCCACTTTGTATCCTATCAGG and GCAGGATTTCTTTGTGAACAG; sequences of DAAM2 were GCAATAAACCGGCTAAA and GGACACAGCCTATGAGG. DAAM1 and DAAM2 overexpression was achieved by transfection of pcDNA-DAAM1/DAAM2. Plasmids were obtained from Sinobiological (Beijing, China). Lipofectamine 2000 was used for transfection of siRNA and plasmids. Results of transfection were verified by Western blot.
RNA extraction and qRT-PCR
Total RNAs were extracted using TRIzol reagent (Thermo Fisher) according to the manual. The qPCR RT kit (Qiagen, Venlo, Netherlands) and SYBR Green Master Mix (Thermo Fisher) were used for quantitative real-time PCR (qRT-PCR). GAPDH was used as an internal control for the 2−ΔΔCT calculation. Primers were as follows:
-
DAAM1 forward 5′-TCACCCAGAAATCACG-3′;
reverse 5′-GTCCAGTTCATCCACCA -3′.
-
DAAM2 forward 5′-CAAAGCCCAAAGTGGAAGC-3′;
reverse 5′-CATCTGTCTAAGACGCTTGCTG-3′.
Cell proliferation assay
HCC proliferation was detected with MTT assay after regulating DAAM1 and DAAM2 expression. In brief, HCC cells were transfected with siRNAs of DAAM1/2 and then cultured for another 48 hr. After that, cells were seeded into 96-well plates and incubated for the indicated time until the addition of 10 μl MTT per well. After 4 hr of MTT incubation, the crystals at the bottom of wells were dissolved by 100-μL dimethyl sulfoxide and absorbance at 570 nm was measured in a spectrophotometer (Molecular Devices Company, United States). OD570 of the control group was set as the base for data standardization. All data were normalized to control to calculate the proliferation index.
Matrigel transwell assay
The 8-μm-pore matrigel-coated transwells (BD Biosciences, United States) were used to detect tumor invasion of HCC. After transfection of siRNA or plasmid carrying DAAM2, cells were seeded into chambers and then cultured for another 24 hr with 10% FBS at bottom as chemotactic factor. At the end of incubation, cells in the upper chamber were removed and the cells in the bottom chamber were fixed and stained. Cell numbers were counted from six random fields. The invasion ratio was calculated by normalizing the invading number of testing groups to the control group.
Statistical analysis
SPSS 22.0 software was used for data analysis and GraphPadPrism 5.0 was for graph display. The relationships between DAAM1/2 expression and clinicopathologic factors were calculated by the Chi-square method. OS curves were shown with the Kaplan-Meier method, and statistical differences between different groups were calculated by the log-rank test. Statistical significances in the in vitro experiments were analyzed by Student’s t-test. P<0.05 indicated statistical significance.
Results
Expression of DAAM family in HCC
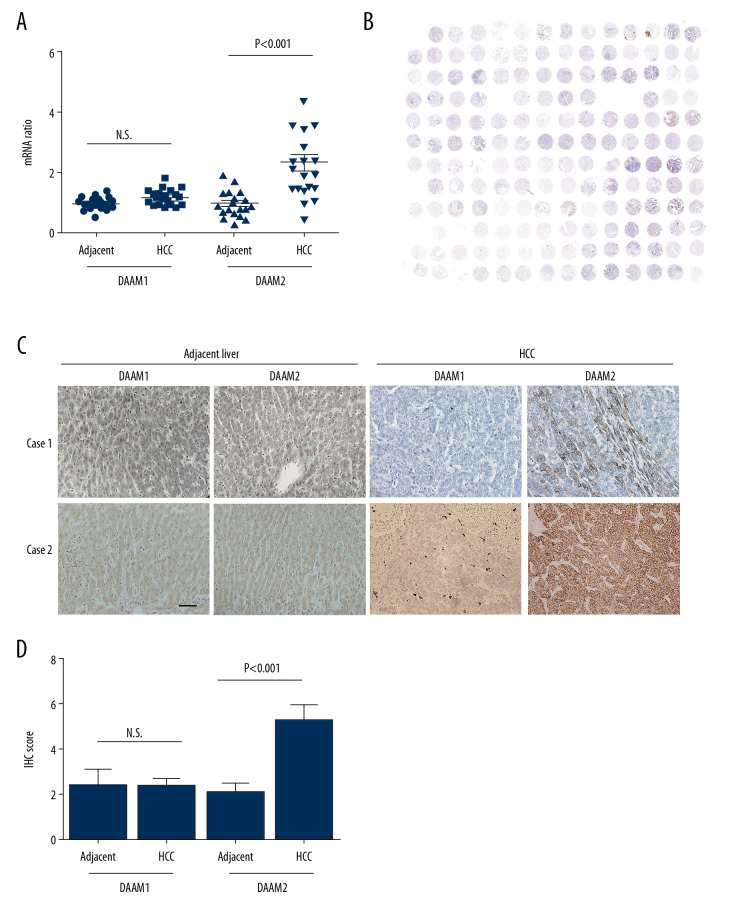
Expressions of DAAM1 and DAAM2 in 19 pairs of HCC and their adjacent liver tissues were investigated with qRT-PCR to analyze their expression patterns in HCC (Figure 1A). The mRNA levels in normal liver tissues were set as baseline and mRNAs in HCC were standardized to the baseline. Consequently, DAAM2 mRNAs in HCCs were substantially higher than mRNAs in adjacent liver tissues while there were no marked differences in DAAM1 mRNA between tumors and liver tissue (Figure 1A), which indicated that DAAM2, instead of DAAM1, has an important role in HCC oncogenesis. Moreover, expression of DAAM1 and DAAM2 in a tissue microarray consisting of 117 HCCs was detected with IHC (Figure 1B). According to the cut-off IHC score, the cohort was categorized into patients with low and high DAAM expression. Rates of DAAM1 and DAAM2 overexpression of 18.8% and 48.7%, respectively, were seen in the cohort (Table 1). Figure 1C shows images of DAAM1 and DAAM2 expression in HCC samples and corresponding tumor-adjacent liver tissues in two representative patients. In addition, IHC scores of DAAM2 in HCC was substantially higher than those in liver tissues, but there was no difference in DAAM1 between HCCs and normal tissues (Figure 1D). These results suggest that DAAM2 rather than DAAM1 plays an essential role in tumorigenesis of HCC.
Figure 1.
Expression of DAAM1 and DAAM2 in HCCs and tumor-adjacent liver tissues. (A) mRNA levels of DAAM1 and DAAM2 in 19 pairs of HCCs and tumor-adjacent liver tissues were detected with qRT-PCR. (B) Overall view of the tissue microarray stained with DAAM2 antibody. (C) Expression and location of DAAM1 and DAAM2 in 117 pairs of HCCs and tumor-adjacent liver tissues were detected with IHC, and two cases of DAAM1/2 expression were displayed. Scale bar: 50 μm. (D) IHC scores of DAAM2 in 117 HCCs were substantially higher than those in adjacent tissues.
Table 1.
Basic information about patients with HCC.
| Factor | Number | Percentage |
|---|---|---|
| Sex | ||
| Female | 37 | 31.62% |
| Male | 80 | 68.38% |
| Age | ||
| <50 | 46 | 39.32% |
| ≥50 | 71 | 60.68% |
| Tumor size (cm) | ||
| ≤5 | 45 | 38.46% |
| >5 | 72 | 61.54% |
| Tumor number | ||
| Single | 107 | 91.45% |
| Multiple | 10 | 8.55% |
| Histopathological grade | ||
| I+II | 84 | 71.79% |
| III | 33 | 28.21% |
| HBsAg | ||
| Negative | 36 | 30.77% |
| Positive | 81 | 69.23% |
| HCV | ||
| Negative | 107 | 91.45% |
| Positive | 10 | 8.55% |
| Microvascular invasion | ||
| Negative | 54 | 46.15% |
| Positive | 63 | 53.85% |
| Cirrhosis | ||
| Negative | 63 | 53.85% |
| Positive | 54 | 46.15% |
| T stage | ||
| I+II | 57 | 48.72% |
| III+IV | 60 | 51.28% |
| N stage | ||
| N0 | 115 | 98.29% |
| N1 | 2 | 1.71% |
| TNM stage | ||
| I+II | 57 | 48.72% |
| III+IV | 60 | 51.28% |
| DAAM1 | ||
| Low | 95 | 81.20% |
| High | 22 | 18.80% |
| DAAM2 | ||
| Low | 60 | 51.28% |
| High | 57 | 48.72% |
HCV – hepatitis C virus.
DAAM2, instead of DAAM1, indicated poor prognosis of HCC
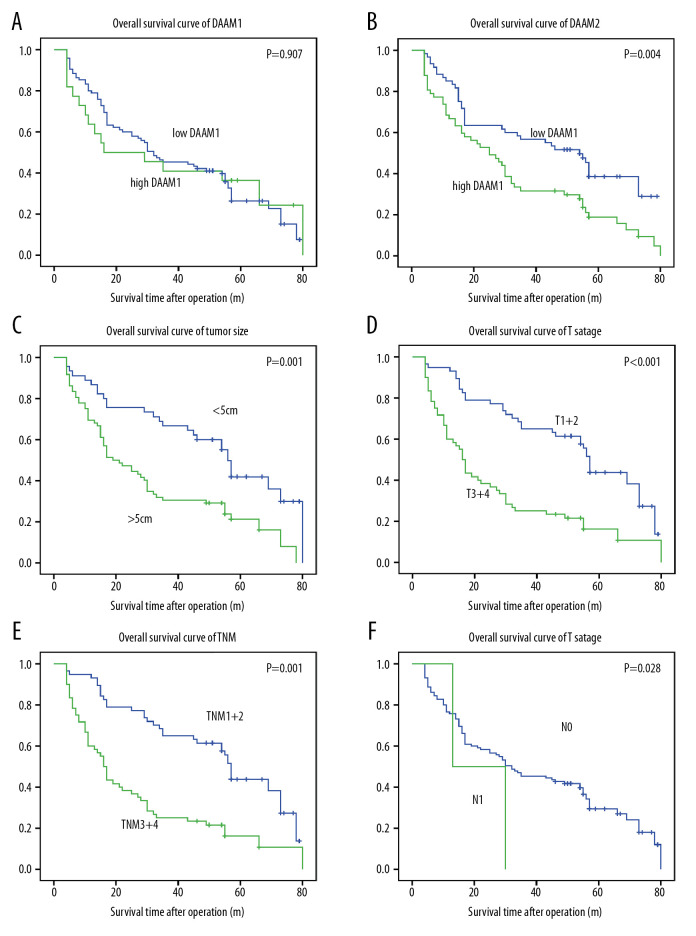
DAAM1 and DAAM2 expression, as well as other clinical factors, were included in the univariate analysis of prognostic value of HCC. The Kaplan-Meier method was used to display OS curves, and the log-rank test was performed to calculate statistical significance. In our study, DAAM1 expression had no dominant effect on the OS rate (P=0.907), while high DAAM2 expression was significantly associated with lower OS rate (P=0.004) (Figure 2A, 2B). The 5-year OS rates for low and high DAAM2 expression were 38.5% and 18.8%, respectively (Table 2). In addition, tumor size (>5 cm), T stage (III+IV), TNM stage (III+IV) all indicated poor prognosis of HCC (Figure 2C–2E). T stage is the dominant determining factor for TNM stage for HCC, so the survival curves of TNM and T stage were very similar in our study. N stage seemed to be related to OS, but the tendency was not that significant because the number of patients with lymphatic invasion was too small (n=2) (Figure 2F).
Figure 2.
DAAM2 instead of DAAM1 correlated with the poor prognosis of HCC. Correlations between (A) DAAM1; (B) DAAM2; (C) tumor size; (D) T stage; (E) TNM stage; and (F) N stage and overall survival rates were analyzed with log-rank test.
Table 2.
Relationship between 5-year OS and clinical factors.
| Factor | 5-year OS (%) | P* |
|---|---|---|
| Sex | ||
| Female | 26.5 | 0.585 |
| Male | 29.0 | |
| Age | ||
| <50 | 29.6 | 0.754 |
| ≥50 | 28.4 | |
| Tumor size (cm) | ||
| ≤5 | 41.9 | 0.001 |
| >5 | 21.3 | |
| Tumor number | ||
| Single | 27.7 | 0.732 |
| Multiple | 40.0 | |
| Histopathological grade | ||
| I+II | 28.4 | 0.951 |
| III | 33.3 | |
| HBsAg | ||
| Negative | 40.7 | 0.280 |
| Positive | 23.1 | |
| HCV | ||
| Negative | 28.9 | 0.302 |
| Positive | 30.0 | |
| Cirrhosis | ||
| Negative | 34.0 | 0.798 |
| Positive | 25.6 | |
| Microvascular invasion | ||
| Negative | 34.3 | 0.531 |
| Positive | 28.2 | |
| T stage | ||
| I+II | 43.7 | <0.001 |
| III+IV | 16.2 | |
| N stage | ||
| N0 | 29.4 | 0.280 |
| N1 | 0.0 | |
| TNM stage | ||
| I+II | 43.7 | <0.001 |
| III+IV | 16.2 | |
| DAAM1 | ||
| Low | 26.3 | 0.907 |
| High | 36.4 | |
| DAAM2 | ||
| Low | 38.5 | 0.004 |
| High | 18.8 | |
HCV – hepatitis C virus.
Variables with P<0.300 in univariate analysis used in the Cox regression model for multivariate analysis included HBV infection, T stage, tumor size, N stage and DAAM2 status (Table 3). T stage was identified as an independent prognostic factor for HCC, with a hazard ratio (HR) as high as 2.16. DAAM2 tended to be an independent prognostic factor, but the statistical significance was more than 0.05 (P=0.074). The independent prognostic significance of DAAM2 needs further verification in larger cohorts and multi center studies.
Table 3.
Independent prognostic factors for HCC.
| Factor | HR | 95%CI | P* |
|---|---|---|---|
| Tumor size (cm) | |||
| ≤5 | 1.00 | ||
| >5 | 1.35 | 0.77–2.34 | 0.294 |
| HBsAg | |||
| Negative | 1.00 | ||
| Positive | 1.26 | 0.76–2.11 | 0.375 |
| T stage | |||
| I+II | 1.00 | ||
| III+IV | 2.16 | 1.27–3.68 | 0.004 |
| N stage | |||
| N0 | 1.00 | ||
| N1 | 1.40 | 0.33–6.33 | 0.630 |
| DAAM2 | |||
| Low | 1.00 | ||
| High | 1.50 | 0.96–2.24 | 0.074 |
Calculated with Cox-regression model.
Relations between DAAM2 and clinicopathologic factors in HCC
In the clinical analysis, we demonstrated that DAAM2 expression was a prognostic biomarker for HCC, and therefore, we carried out the Chi-Square test to estimate its correlations with clinicopathologic factors in HCC (Table 4). In our analysis, high DAAM2 expression was substantially relevant to advanced T stage (P=0.032) and TNM stage (P=0.032), which suggested that DAAM2 was a promoter of HCC proliferation or invasion. Considering that TNM stage was a consequent product of T stage, we suspected that DAAM2 mainly influenced tumor infiltration and invasion of HCC.
Table 4.
Relationship between DAAM2 and clinical factors.
| Factors | DAAM2 | P* | |
|---|---|---|---|
| Low | High | ||
| Sex | |||
| Female | 14 | 23 | 0.047 |
| Male | 46 | 34 | |
| Age | |||
| <50 | 25 | 21 | 0.705 |
| ≥50 | 35 | 36 | |
| Tumor size (cm) | |||
| ≤5 | 28 | 17 | 0.087 |
| >5 | 32 | 40 | |
| Tumor number | |||
| Single | 55 | 52 | 0.932 |
| Multiple | 5 | 5 | |
| Histopathological grade | |||
| I+II | 44 | 40 | 0.837 |
| III | 16 | 17 | |
| HBsAg | |||
| Negative | 19 | 17 | 0.844 |
| Positive | 41 | 40 | |
| HCV | |||
| Negative | 57 | 50 | 0.197 |
| Positive | 3 | 7 | |
| Microvascular invasion | |||
| Negative | 24 | 30 | 0.392 |
| Positive | 33 | 30 | |
| Cirrhosis | |||
| Negative | 35 | 28 | 0.357 |
| Positive | 25 | 29 | |
| T stage | |||
| I+II | 35 | 22 | 0.032 |
| III+IV | 25 | 35 | |
| N stage | |||
| N0 | 59 | 56 | 0.971 |
| N1 | 1 | 1 | |
| TNM stage | |||
| I+II | 35 | 22 | 0.032 |
| III+IV | 25 | 35 | |
| DAAM1 | |||
| Low | 48 | 47 | 0.815 |
| High | 12 | 10 | |
Calculated by the Chi-square test.
HBsAg – hepatitis B surface antien; HCV – hepatitis C virus.
DAAM2 decreased VHL expression and upregulated HIF-1α expression
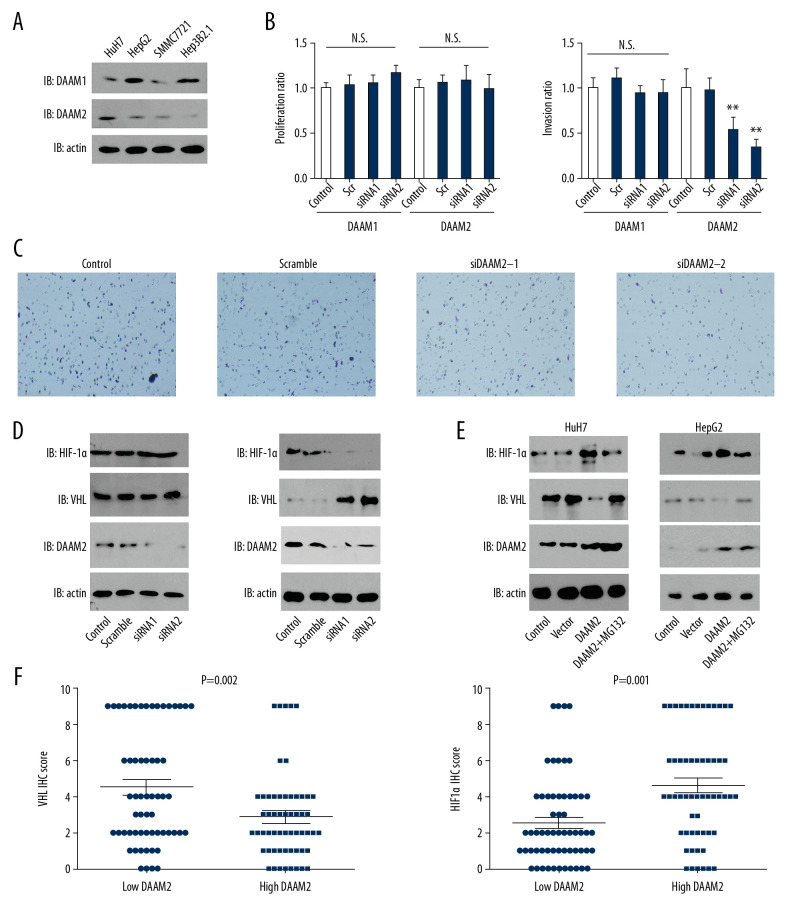
In our clinical analysis, we demonstrated that high DAAM2 expression was related with advanced T stage, so we further investigated the function of DAAM2 in HCC progression, including proliferation and invasion. Expression of DAAM1 and DAAM2 in four different HCC cell lines including HepG2, HuH7, SMMC7721 and Hep3B2.1 were first detected with Western blot. DAAM1 and DAAM2 expression were detectable and variable in these cells (Figure 3A). Based on their expression pattern, we silenced DAAM1 in HepG2 and knocked down DAAM2 in HuH7 by independent siRNAs in case of off-target effect, and detected their influence on proliferation and invasion. The knockdown of both DAAM1 and DAAM2 had blunting effects on proliferation, while the knockdown of DAAM2, instead of DAAM1, substantially decreased the invasion of HuH7 (Figure 3B, 3C). A previous study suggested that DAAM2 could reduce VHL expression by promoting its ubiquitination [10]. Moreover, HIF-1α is a well-accepted downstream protein regulated by VHL, and it is widely involved in the progression of numerous tumors [20]. So, we further investigated the influence of DAAM2 on VHL and its downstream HIF-1α.
Figure 3.
DAAM2 decreased VHL and up-regulated HIF-1α in a ubiquitin-dependent pathway. (A) Expressions of DAAM1 and DAAM2 in different HCC cell lines. (B) Cell proliferation (left) and invasion (right) were detected with MTT assay and transwell assay, respectively, after silencing DAAM1 or DAAM2 expression by siRNA. DAAM1 was silenced in HepG2 while DAAM2 was knocked down in HuH7. N.s. represented not significant and ** meant P<0.01 compared with the control group. (C) Representative images of HuH7 cells transfected with scrambled siRNA, siDAAM2-1 and siDAAM2-2 in transwell assay. (D) Expression of VHL and HIF-1α was detected with Western blot 48 hr after knocking down DAAM1 or DAAM2. DAAM1 was silenced in HepG2 while DAAM2 was knocked down in HuH7 (E). In HuH7 and HepG2, expression of VHL and HIF-1α was detected 48 hr after transfection of empty vector or plasmid carrying DAAM2, in the presence or absence of 10 μM G132 for 6 hr. (F) In the 117 patients with HCC, patients with high DAAM2 expression had lower VHL IHC score and higher HIF-1α IHC score.
The effect of DAAM1 knockdown was not notable on VHL and HIF-1α expression, whereas DAAM2 knockdown substantially increased VHL expression and consequently decreased HIF-1α expression (Figure 3D). This was inconsistent with previous results in glioma, which showed DAAM2-driven degradation of VHL [10]. To confirm the result, we overexpressed DAAM2 in HuH7 and HepG2, and verified the effects of DAAM2 overexpression on VHL and HIF-1α after treatment with 10 μM MG132 for 6 hr. MG132 is an inhibitor of proteasome and widely used to suppress ubiquitination. DAAM2 overexpression decreased VHL expression and upregulated HIF-1α in both SMMC7721 and HepG2, whereas MG132 treatment notably reversed this effect (Figure 3E). In addition, we analyzed the correlation between DAAM2 expression and VHL/HIF-1α. Patients with higher DAAM2 had downregulated VHL expression and upregulated HIF-1α expression, which was consistent with the results in the cell lines (Figure 3F).
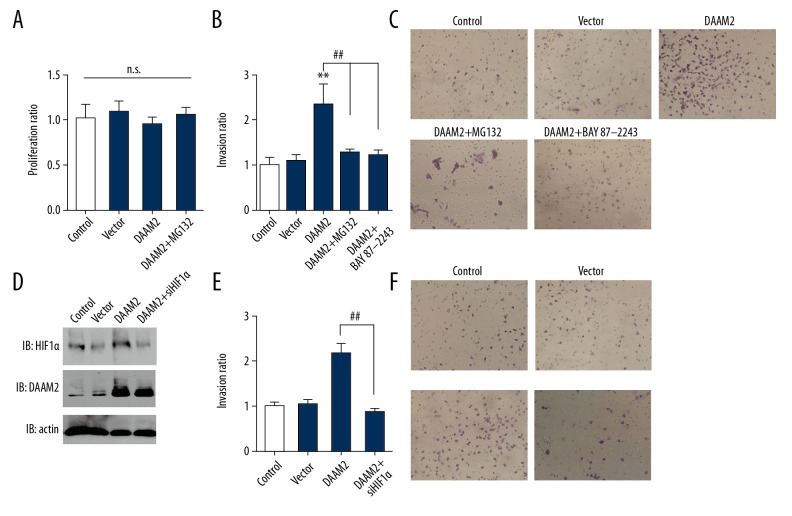
HIF-1α was critically required in DAAM2-induced invasion of HCC
To investigate the role of HIF-1α in DAAM2-induced progression of HCC, we treated HuH7 cells with 10 μM MG132 for 6 hr and/or 10 μM HIF-1α inhibitor BAY87-2243 for 12 hr. MG132 had no significant influence on proliferation (Figure 4A) but attenuated invasion induced by DAAM2 overexpression (Figure 4B). BAY87-2243 had a similar suppressive effect on DAAM2-induced invasion (Figure 4C). We silenced HIF-1α in HuH7 cells to further confirm the role of HIF-1α in DAAM2-induced invasion (Figure 4D). As with BAY87-2243, HIF-1α knockdown significantly impaired DAAM2-induced invasion of HuH7 (Figure 4E, 4F). All these results suggest HIF-1α plays a critical role in DAAM2-induced invasion in HCC.
Figure 4.
HIF-1α was required in DAAM2-induced invasion of HCC. (A) MG132 (10μM) incubation for 6 hr had no influence on proliferation of HuH7. Proliferation was assessed by MTT assay. (B) Invasion of HuH7 was detected after overexpressing DAAM2. 10 μM MG132 or 10 μM HIF-1α inhibitor BAY87-2243 was used to incubate HuH7. ** Represented P<0.01 compared with control group, and ## represented P<0.01 between indicated groups. Analyzed data were from three independent experiments. (C) Representative images in transwell assay of B. (D) Western blot showed that HIF-1α was knocked down while DAAM2 was overexpressed in HuH7. (E) HIF-1α knockdown extensively decreased the DAAM2-induced invasion. The invasion was detected with transwell assay. ## Represented P<0.01 between indicated groups. (F) Representative images in transwell assay of (E).
Discussion
The Wnt signaling pathway is the essential signaling involved in numerous physical and pathological processes [21]. It can be divided into canonical and non-canonical pathways according to participation of β-catenin. Both the canonical and the non-canonical pathways require the involvement of the DAAM family [22]. Dysregulation of the Wnt/β-catenin pathway has been implicated in progression or tumorigenesis of HCC in numerous reports [ 23]. However, as an important component of Wnt singling, direct evidence previously has been lacking ofg DAAM’s role in progression of HCC. In this study, our results underscore the important function of DAAM2, not DAAM1, in HCC progression and also show that it is a prognostic biomarker, which indicates that DAAM2 could be a promising drug target for HCC treatment.
In our study, DAAM2 was associated with the T and TNM stage in Chi-square test. However, it was difficult determine whether the relationship between them is causal. Based on in vitro experiments, we are inclined to believe that DAAM2 led to tumor invasion, and therefore, resulted in advanced T and TNM stage. Moreover, DAAM2 had reached statistical significance as a prognostic indicator(P=0.004), but its significance as an independent prognostic biomarker was not so remarkable (P=0.074). There are two possibilities: (1) the sample size may not have been large enough, because the independent prognostic significance is close to statistical significance; and (2) DAAM2 is not an independent prognostic biomarker but correlates with prognosis because of its interaction with T and TNM stage. We hope that our findings trigger more multicenter studies that can verify the independent prognostic value of DAAM2.
In this study, for the first time, we reported on the influence of DAAM on HCC progression and showed that DAAM2, but not DAAM1, resulted in invasion and poor prognosis of HCC. This is interesting because the functions of DAAM1 and DAAM2 are almost the same although their expression is tissue-specific. Here we proved that DAAM1 and 2 are both expressed in HCC, but their functions have remarkable different impact on VHL expression and HCC invasion. This is an intriguing result, expanding the understanding of the DAAM family and how they function in different tissues and tumors.
As a key Wnt effector, DAAM2 has been suggested to interact with several proteins and influence Wnt downstream signaling. For example, DAAM2-PIP5K interaction was a component of a novel pathway regulating Wnt signaling and myelination [22]. Besides interacting with DVL in Wnt signal transducing, several other proteins reportedly interact with DAAM. For example, DAAM acts downstream of PITX2 in the developing gut and is required for left/right asymmetry within the dorsal mesentery [22]. In addition, there are other functions of DAAM independent of the Wnt signal. A previous study reported that DAAM2 could promote tumorigenesis of glioma by driving the degradation of VHL [10]. Here we showed that DAAM2 decreased VHL expression and subsequently increased HIF-1α, which was inconsistent with the previous study to some extent. We expanded the understanding of DAAM2 as an oncogene and revealed its role in HCC invasion. However, several important and interesting issues were not resolved in our study. For example, we do not know what differences between DAAM1 and DAAM2 result in their different functions in regulating VHL or how DAAM2 decreased VHL expression as a Wnt effector but not an E3 ligase or transcription. We hope our study will trigger more interest in these concerns and in resolving more issues related to DAAM2.
Until 2007, and the discovery of sorafenic, no targeted therapy was available for HCC Sorafenib is a multi-target tyrosine kinase inhibitor with anti-angiogenic and anti-proliferative effects that prolongs median survival time for advanced-stage HCC from 8 to 11 months. Recently, additional drugs such as lenvatinib, regorafenib, cabozantinib, and ramucirumab have been proven effective for treatment of HCC [24–26]. However, these drugs have a limited effect onextending survival and the prognosis for HCC is still very poor. In our study, we concluded that DAAM2, rather than DAAM1, promoted HCC invasion and led to unfavorable prognosis by reducing VHL and increasing HIF-1α expression. This finding points the way to new horizons in targeted therapy for HCC. Though a specific inhibitor of DAAM2 has not been developed, use of a VHL and HIF-1α antagonist may be a promising approach to treatment of HCC in the future.
Conclusions
For the first time, we demonstrated that DAAM2 was upregulated in HCC compared with normal liver tissues. DAAM2, rather than DAAM1, was shown to be a prognostic biomarker of HCC predicting poor prognosis. DAAM2 decreased VHL expression and consequently upregulated HIF-1α in HCC cells. HIF-1α was essentially required for DAAM2-induced invasion of HCC. These results suggest that DAAM2 has an important role in HCC progression and indicate that DAAM2 may be a potential target for HCC treatment.
Footnotes
Source of support: Departmental sources
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Sun R, Liu Z, Qiu B, et al. Annexin10 promotes extrahepatic cholangiocarcinoma metastasis by facilitating EMT via PLA2G4A/PGE2/STAT3 pathway. EBioMedicine. 2019;47:142–55. doi: 10.1016/j.ebiom.2019.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YM, Cao L, Li B, et al. Clinicopathological significance of ZEB1 protein in patients with hepatocellular carcinoma. Annals Surg Onc. 2012;19(5):1700–6. doi: 10.1245/s10434-011-1772-6. [DOI] [PubMed] [Google Scholar]
- 5.Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 2008;27(4):606–17. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 7.Lee HK, Deneen B. Daam2 is required for dorsal patterning via modulation of canonical Wnt signaling in the developing spinal cord. Develop Cell. 2012;22(1):183–96. doi: 10.1016/j.devcel.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei J, Huang Y, Hao L, et al. DAAM1-mediated migration and invasion of ovarian cancer cells are suppressed by miR-208a-5p. Path Res Prac. 2019;215(7):152452. doi: 10.1016/j.prp.2019.152452. [DOI] [PubMed] [Google Scholar]
- 9.Xiong H, Yan T, Zhang W, et al. miR-613 inhibits cell migration and invasion by downregulating Daam1 in triple-negative breast cancer. Cell Sign. 2018;44:33–42. doi: 10.1016/j.cellsig.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, Krishna S, Garcia C, et al. Daam2 driven degradation of VHL promotes gliomagenesis. eLife. 2017;6:e31926. doi: 10.7554/eLife.31926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata H, Hinoda Y, Nakajima K, et al. Wnt antagonist gene polymorphisms and renal cancer. Cancer. 2009;115(19):4488–503. doi: 10.1002/cncr.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Cockman ME, Masson N, Mole DR, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275(33):25733–41. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 14.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 15.Kenmochi K, Sugihara S, Kojiro M. Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver. 1987;7(1):18–26. doi: 10.1111/j.1600-0676.1987.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu YF, Yang XQ, Lu XF, et al. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Research Commun. 2014;446(1):54–60. doi: 10.1016/j.bbrc.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 17.Qiu B, Chen T, Sun R, et al. Sprouty4 correlates with favorable prognosis in perihilar cholangiocarcinoma by blocking the FGFR-ERK signaling pathway and arresting the cell cycle. EBioMedicine. 2019;50:166–77. doi: 10.1016/j.ebiom.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu YF, Liu ZL, Pan C, et al. HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene. 2019;38(6):868–80. doi: 10.1038/s41388-018-0485-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang HM, Xu YF, Ning SL, et al. The catalytic region and PEST domain of PTPN18 distinctly regulate the HER2 phosphorylation and ubiquitination barcodes. Cell Res. 2014;24(9):1067–90. doi: 10.1038/cr.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123(9):3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Sun R, Zhang X, et al. Transcription factor 7 promotes the progression of perihilar cholangiocarcinoma by inducing the transcription of c-Myc and FOS-like antigen 1. EBioMedicine. 2019;45:181–91. doi: 10.1016/j.ebiom.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HK, Chaboub LS, Zhu W, et al. Daam2-PIP5K is a regulatory pathway for Wnt signaling and therapeutic target for remyelination in the CNS. Neuron. 2015;85(6):1227–43. doi: 10.1016/j.neuron.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Sem Cancer Biol. 2011;21(1):44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 25.Personeni N, Pressiani T, Rimassa L. Cabozantinib in patients with hepatocellular carcinoma failing previous treatment with sorafenib. Future Oncol. 2019;15(21):2449–62. doi: 10.2217/fon-2019-0026. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]