Abstract
Objective:
To date, little is known about the roles of FasL and TILs in cervical cancer. This study aims to determine the correlation between FasL expression and TILs presence in cervical cancer.
Methods:
In this study, we analysed the FasL and TIL presence in 32 squamous cell carcinoma or adenocarcinoma that were obtained from early stage (≤ IIA2) cervical cancer patients using immunohistochemistry. The level of FasL and TIL was assessed qualitatively, and then quantified with the H-Score system.
Results:
Most of the patients were between 30 to 50 years old (59,4%), and had never taken pap smear examination before (96,9%). Based on the Pearson analysis of FasL and TIL presence, we found that FasL was inversely correlated with CD45 or TIL number when the level of FasL is above 140 and the CD45 is below 160. Based on Chi-Square test of FasL and TIL classification, there was a nine-fold odds ratio (OR) of lower TILs classification in high expression of FasL classification (OR 9, p=0.01).
Conclusion:
An inverse correlation between FasL expression and TILs level, that might indicate FasL-induced TILs apoptosis in tumor tissue, was observed. The strong inverse correlation between FasL and TILs presence showed some insight about the interactions between cancer cells and its surroundings inside of the cervical cancer tissue. This might also be further developed to tailor a prognostic marker that can predict the outcome of therapy in patients, not only in cervical cancer, but generally in all cancer.
Key Words: Cervical cancer, FasL, TIL, apoptosis
Introduction
According to GLOBOCAN 2008, cervical cancer is the third most common cancer in the world and the fourth most common cause of mortality in women (Ferlay et al., 2013). The incidence of cervical cancer in 2008 was 471,000 cases, with 288,000 mortalities. High morbidity and mortality of cervical cancer is more commonly encountered in developing countries compared to developed countries. In Indonesia, cervical cancer is the most common cancer as consistently reported in 1990-1999 and 2005-2008. Cervical cancer is still a common problem and burden for the government due to high morbidity, high mortality, and high cost of treatment (Suzanna, 2009).
Cancer is a state of disease where tissue grows uncontrollably, and one of its hallmarks is the resistance of programmed cell death or apoptosis (Hanahan and Weinberg, 2000, 2011). Normally, if an unrepairable error were found in a cell or a group of cells, the system will drive the cells through apoptosis that may be induced intrinsically, such as through mitochondrial oxidative stress induction, and extrinsically, which mainly initiated by the contact of Fas receptor protein with its ligand, Fas ligand (FasL) (Lowin et al., 1994; Winter et al., 1999). FasL is a type II membrane protein and tumor necrosis factor (TNF) that could activate T-lymphocytes or Natural Killer (NK) cells leading to the apoptosis of Fas expressing cell (Walczak and Krammer, 2000).
However, in the state of cancer, FasL induction is not effective since most cancer cells are resistant to apoptosis (Peter et al., 2015). Furthermore, upregulation of FasL in cancer cells may worsen the prognosis by causing ‘counterattack’ against natural killer (NK) cells and tumor infiltrating lymphocytes (TILs) which are responsible as antitumor effector cells (Chappell and Restifo, 1998; Igney et al., 2000; O’connell et al., 1996).
The ‘counterattack’ effect can also be observed through the decline of tumor-infiltrating lymphocytes (TILs) in different kind of tumor tissues with FasL overexpression, such as colon (O’Connell et al., 2000), esophageal (Bennett et al., 1998), breast (Ioachim et al., 2005), and oral cancer (Fang et al., 2013). TILs are suspected to be a prognostic factor for cancer metastases, progression into advanced stages, and relapse incidence (de Jong et al., 2009). However, the association between FasL overexpression and T-lymphocytes is still controversial. A study on cervical carcinoma did not find any significant association between FasL expression and TILs levels (Munakata et al., 2005). In Indonesia, a previous study confirmed a significant association between FasL expression and lymph nodes metastases in patients with early stage cervical cancer who underwent primary surgery (Irwanto, 2016).
To date, little is known about the roles of FasL and TILs in cervical cancer. This study aims to determine the correlation between FasL expression and TILs presence, due to the described controversy, in early stage cervical cancer tissues. In the future, this study will lead to identification of high-risk cervical cancer with lymph node involvement and to improve the treatment options for cervical cancer management.
Materials and Methods
Study design and Sampling
This cross-sectional study was approved by the Ethical Committee at Faculty of Medicine Universitas Indonesia – Cipto Mangunkusumo Hospital (CMH), Jakarta. Forty three paraffin embedded squamous cell carcinoma or adenocarcinoma of cervical cancer samples were retrospectively and consecutively obtained from January 2007 to May 2011 in the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, and Department of Anatomical Pathology at CMH. Forty three samples were obtained from early stage (stage ≤ IIA2) cervical cancer patients based on the International Federation of Gynecology and Obstetrics (FIGO) classification, who underwent primary radical hysterectomy at the same period at CMH. Eleven tissue specimens were excluded due to lacking of tumor cells. Thirty-two samples were subjected to the examination of FasL and TIL levels by IHC.
Immunohistochemistry
For the IHC staining, 4 µm sections were made and processed according to the protocol of previous study. FasL and CD45+ cells (TIL) were detected using Thermo Fisher Scientific CD178/Fas Ligand Antibody (Thermo Fisher Scientific, QB1980882, USA) and Novocastra Lyophilized Mouse Monoclonal Antibody CD45 (Leica Biosystems, NCL-LLA, USA), respectively. Meanwhile, the primary antibodies were recognized by the secondary antibody Horseradish Peroxidase-labeled (HRP) septravidine/Trekavidine-HRP (Biocare Medical, STHRP700 L10, USA). Detection kit used in this study was Biocare Detection System (Biocare Medical, LLC, Concord CA, USA). IHC staining was performed according to the product manual (Irwanto, 2016).
Each slide was randomly observed on five visual fields; each visual field was divided onto nine smaller fields to be analyzed (see supplement 1). Analysis was performed independently using imageJ software. The level of FasL and TIL was assessed qualitatively and divided into four stages, a strong positive (given the value +3), moderate positive (+2), weak positive (+1), and negative (-). After qualitative assessment, quantitative calculations were performed using the formula H-Score (Białas et al., 2003; Ishibashi et al., 2003).
Statistical Analysis
All data were collected in a computerized database and statistically analyzed by SPSS 18.0 [SPSS Inc., Chicago, USA]. Bland-Altman analysis was performed to analyze the variance of the quantification between two observer. There was an agreed analysis guide between the two observers (pathologist as first observer and researcher as second observer) which was statistically proven by Bland-Altman analysis (see supplement 2). Correlation analysis was conducted using Pearson analysis to obtain cut-off-point of FasL (high and low expression) and TILs (high and low level). Data were analyzed by independent T-Test (Dahlan, 2011).
Results
Patients Characteristic and Demographic
Biopsy samples were obtained from a total of 32 patients with cervical cancer. The demographic and disease characteristics are shown in Table 1 below. Most of the patients were between 30 to 50 years old (59.4%), had married between the age of 16-24 years (56.3%), were married once (90.6%), had experienced childbirth mostly before reaching 25 years old (87.5%), and had delivered 1-2 children (59.4%). Contraceptive use was not a common practice among the patients since there was still 43.8% that never had contraception. Most of the patients (96.9%) had never taken pap smear examination before, and only 1 patient (3.1%) ever had pap smear examination.
Table 1.
Demographic Characteristics of the Subjects
| Variable | n (%) |
|---|---|
| Age (years) | |
| <35 years | 4 (12.5) |
| 35-50 years | 19 (59.4) |
| >50 years | 9 (28.1) |
| Marital age (years) | |
| <16 years | 11 (34.4) |
| 16-24 years | 18 (56.3) |
| >24 years | 3 (9.4) |
| Marital frequency | |
| Once | 29 (90.6) |
| Twice | 1 (3.1) |
| Thrice | 2 (6.3) |
| Parity | |
| 1-2 | 19 (59.4) |
| 3-5 | 9 (28.1) |
| ≥6 | 4 (12.5) |
| Age at first labor | |
| <25 years | 28 (87.5) |
| ≥25 years | 4 (12.5) |
| Contraceptive use | |
| Never | 14 (43.8) |
| Pills | 7 (21.9) |
| Injection | 8 (25.0) |
| IUD | 3 (9.4) |
| Pap smear history | |
| Never | 31 (96.9) |
| Yes | 1 (3.1) |
FasL Expression and TIL Levels in Cervical Cancer Biopsy
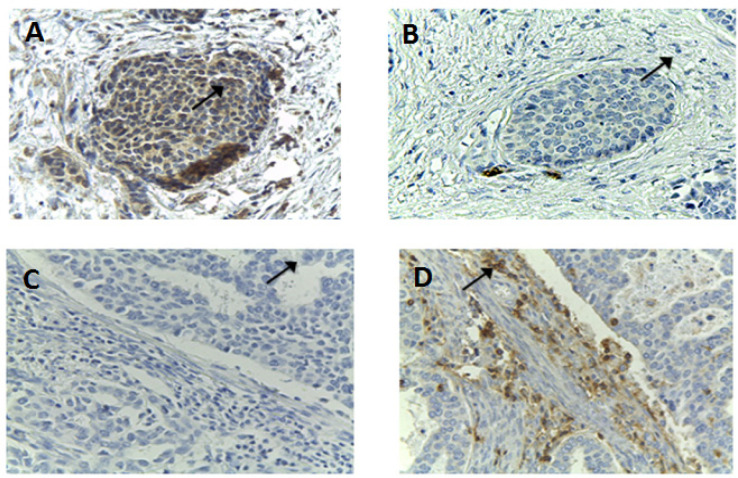
In order to elucidate the correlation between FasL expression and TIL presence in cervical cancer tissue, the levels of FasL and TIL were investigated using IHC. The levels of FasL and TILs are depicted in Figure 1. On every adjusted section, FasL high expression (Figure 1A) led to the decreased coloration of TILs-immunohistochemical staining cells (Figure 1B), and, accordingly, low expression of FasL (Figure 1C) was accompanied with high presence of TILs (Figure 1D).
Figure 1.
The Coloration of FasL and TIL Using IHC Staining. The sections were stained with antibody against fasL (A and C), and antibody against TIL (B and D). A and B were taken from the same section, so are C and D
Based on Pearson analysis, we found that FasL was inversely correlated with CD45 or TIL number when the level of FasL is above 140 and the CD45 is below 160 (as shown in Figure 2). Based on that observation, we categorized the expression of FasL as ‘high’ if the H-score was more than 140, otherwise it was classified as low. Similarly, the levels of TIL were classified as high when the H-score exceeded 160 and low if the H-score was less than 160.
Figure 2.
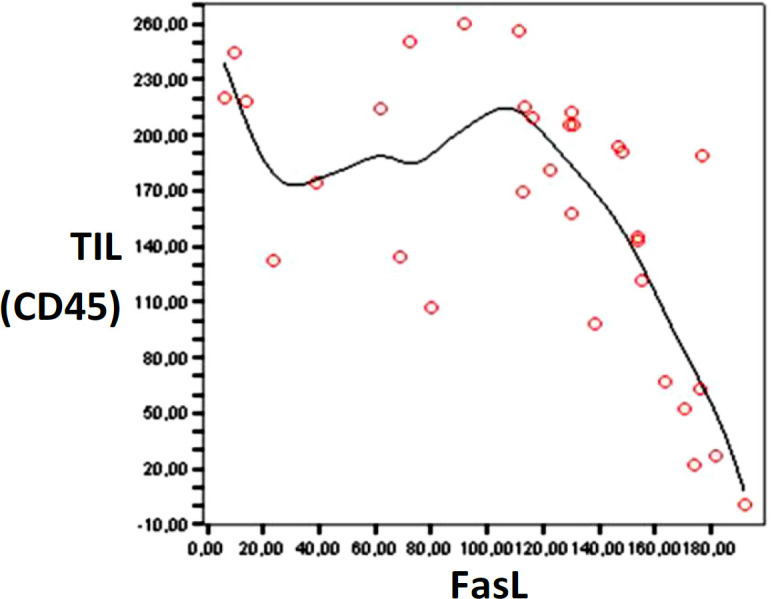
The Pearson Analysis between FasL and TILs Showed Inversed Correlation when the FasL is Above 140 and the TIL amount is below 160
The association between FasL and TIL classification was then analyzed using Chi-Square test (Table 2). Interestingly, there was a nine-fold odds ratio (OR) of lower TILs classification in high expression of FasL classification (OR 9, p=0.01) as presented in Table 2. The section, which showed high expression of FasL, tended to have lower level of TILs, and, reciprocally, sections with high level of TILs exhibited lower level of FasL expression. In other words, there was seemingly a negative correlations between the expression of FasL and the level of TILs in cervical cancer.
Table 2.
The Classification and Correlation of FasL Expression and TIL Level
| TILs Classification |
P-value | OR | |||||
|---|---|---|---|---|---|---|---|
| High Level (>160) | Low Level (≤160) | ||||||
|
FasL
Classification |
n | (%) | n | (%) | Total | ||
| High expression (≥140) | 3 | 25 | 9 | 75 | 12 | 0.01 | 9 |
| Low expression (<140) | 15 | 75 | 5 | 25 | 20 | ||
| Total | 18 | 14 | 32 | ||||
Discussion
Based on a case-control study of cervical cancer patients in the USA, the number of pregnancies (parity) is positively correlated with higher relative risk of cervical cancer (Brinton et al., 1989). In addition, number of sexual partners is also positively correlated with higher odd ratios of cervical cancer in Thailand population (Chichareon et al., 1998). On the other hand, age at first intercourse is negatively correlated with the odds ratio, meaning that the older ones were at first intercourse, the smaller the odds ratio is for cervical cancer (Herrero et al., 2009; Plummer et al., 2014). Those findings were our basis in determining the demographic variables in this study, although they could not be coherently illustrated in our study, because we did not compare the data with controls. What can be consistently observed is of pap smear history, which showed that most of our patients (93,9%) had never had pap smear examination before, and pap smear examination has already been proven to be able to reduce the risk of cervical cancer (Chichareon et al., 1998; Vecchia et al., 1984).
In this study, we aimed to elucidate the association of FasL expression and the number of TILs in cervical cancer tissue since their correlation is quite controversial in a number of other cancers. Normally, immune cells employ FasL for defense mechanism to mediate the apoptosis of cancer cells and virus-infected cells. The binding of FasL on immune cells, such as cytotoxic-T and NK-cells, to the FasR of targeted cells stimulates the formation of death-inducing signaling complex which involves the adaptor molecule Fas-associated with a death domain (FADD), procaspase-8, procaspase-10, caspase-8/10 and regulator cellular FLICE inhibitory protein (c-FLIP). The recruitment of all these factors eventually leads to apoptosis of Fas-expressing cells (Walczak and Krammer, 2000).
Despite of Fas/FasL role in inducing apoptosis, most cancer cells are resistant to Fas-mediated apoptosis. There are several ways by which cancer cells avoid the Fas/FasL-induced apoptosis exerted by TIL. Firstly, cancer cells are capable of regulating the Fas trafficking to their cell surface by up-regulating Fas-associated phosphatase-1 (FAP-1). High expression of FAP-1 is associated with low expression of Fas on cell surface of melanoma cell line, whereas silencing FAP-1 expression results in restored Fas expression on the cell membrane (Ivanov et al., 2003). Secondly, tumor cells can sabotage apoptotic signal in many different levels by increasing cFLIP (Irmler et al., 1997), reducing FADD expression (Tourneur et al., 2003), and attenuating caspase-8 expression (Teitz et al., 2000).
In this study, we found the inverse correlation between FasL expression on the cell surface of cancer cells and TIL counts. It has been reported that FasL upregulation in cancer cells can actually induce apoptosis in TILs as most TILs in cancer cells also express Fas and TILs themselves are prone to Fas-induced apoptosis. Another reason why we observed declined number of TILs is because not only cancer cells, but also endothelial cells in tumor tissue express FasL. It was demonstrated that FasL-expressing endothelial cells form a barrier for TIL resulting in the declined number of TIL that can actually infiltrate the tumor. Such upregulation of FasL in tumor endothelium is stimulated by the secretion of some factors by cancer cells including interleukin-10 (IL-10), prostaglandin E2, and vascular endothelial growth factor-2 (VEGF-2). It is noteworthy that the increased expression of FasL induces apoptosis only in effector T-cells, but not in regulatory T (T-reg) cells, indicating that FasL also contributes in attenuating the anti-tumor immunity. Syngeneic in vivo mouse model for ovarian cancer showed high expression of FasL on endothelial cells which led to decreased number of CD8+ T cells in tumor tissue. Accordingly, the inhibition of FasL using antibody resulted in increased infiltration of adoptively transferred tumor vaccine-primed CD8+ T cells (Motz et al., 2014; Zhu et al., 2018).
In addition to membrane FasL in tumor, FasL is also found in sera of patients suffering from various types of cancer such as ovarian, pancreatic and head and neck cancer (Walczak and Krammer, 2000). The presence of soluble FasL and, in some cases, also Fas in patients’ sera is associated with tumor growth, metastases and eventually with poor prognosis. FasL ability to promote tumor growth was confirmed by a study showing that FasL stimulation on apoptosis-resistant tumor cells activates downstream signaling cascade involving urokinase plasminogen activator, which ultimately induces motility and invasiveness. This finding is highly relevant with the fact that increased level of soluble FasL in patients’ sera was detected after the exposure of chemotherapy and might play an important role in treatment-resistance of the tumor (Barnhart et al., 2004).
This study demonstrated semi-quantitative FasL expressions in cervical cancer tissue, which could ilustrate the present state of the tissue compared to cell culture. Yet, this study did not take Fas expression and apoptosis into account that may describe the interaction within the whole extrinsic apoptosis system.
In conclusion, there is an inverse correlation between FasL expression and TILs levels that might indicate FasL-induced TILs apoptosis in tumor tissue. This association is shown between FasL expression (overexpression and non-overexpression) and TILs (levels and classification) in early stage cervical cancer (stage ≤ IIA2) that underwent radical hysterectomy.
The strong inverse correlation between FasL and TILs presence showed some insight about the interactions between cancer cells and its surroundings inside of the cervical cancer tissue. This might also be further developed to tailor a prognostic marker that can predict the outcome of therapy in patients, not only in cervical cancer, but generally in all cancer.
Acknowledgements
The study was greatly supported by the Division of Gynecologic Oncology - Department of Obstetrics and Gynecology, and the Department of Anatomic Pathology, Faculty of Medicine Universitas Indonesia - Cipto Mangunkusumo Hospital (CMH).
Ethical Clearance
This study was approved by the Ethics Committee of Faculty of Medicine Universitas Indonesia - Cipto Mangunkusumo Hospital (CMH), Jakarta.
Conflicts of Interest
The authors declared no conflict of interest relevant to this article.
References
- Barnhart BC, Legembre P, Pietras E, et al. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004;23:3175–85. doi: 10.1038/sj.emboj.7600325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett , O’Connell J, O’Sullivan GC, et al. 1998) The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 160:5669–75. [PubMed] [Google Scholar]
- Białas M, Okoń K, Czopek J. 2003) Assessing microvessel density in gastric carcinoma: a comparison of three markers. Pol J Pathol. 54:249–52. [PubMed] [Google Scholar]
- Brinton LA, Reeves WC, Brenes MM, et al. Parity as a risk factor for cervical cancer. Am J Epidemiol. 1989;130:486–96. doi: 10.1093/oxfordjournals.aje.a115362. [DOI] [PubMed] [Google Scholar]
- Chappell DB, Restifo NP. T cell–tumor cell: a fatal interaction? Cancer Immunol Immunother. 1998;47:65–71. doi: 10.1007/s002620050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichareon S, Herrero R, Munoz N, et al. Risk factors for cervical cancer in Thailand: a case-control study. J Natl Cancer Inst. 1998;90:50–7. doi: 10.1093/jnci/90.1.50. [DOI] [PubMed] [Google Scholar]
- Dahlan MS. Penelitian prognostik dan sistem skoring: disertai praktik dengan SPSS dan Stata. Seri Evidence-Based Med. 2011;8:118–25. [Google Scholar]
- de Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–10. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Fang L, Sun L, Hu F-F, Chen Q-E. Effects of FasL expression in oral squamous cell cancer. Asian Pac J Cancer Prev. 2013;14:281–5. doi: 10.7314/apjcp.2013.14.1.281. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Herrero R, Meijer CJ, Shah K, et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br J Cancer. 2009;100:1191–7. doi: 10.1038/sj.bjc.6604974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igney FH, Behrens CK, Krammer PH. Tumor counterattack–concept and reality. Eur J Immunol. 2000;30:725–31. doi: 10.1002/1521-4141(200003)30:3<725::AID-IMMU725>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ioachim HL, Decuseara R, Giancotti F, Dorsett BH. FAS and FAS-L expression by tumor cells and lymphocytes in breast carcinomas and their lymph node metastases. Pathol Res Pract. 2005;200:743–51. doi: 10.1016/j.prp.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388 doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Irwanto Y. Expression of Fas ligand is higher in early stage cervical cancer with lymph nodes metastasis. Indones J Obstet Gynecol. 2016:194. [Google Scholar]
- Ishibashi H, Suzuki T, Suzuki S, et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88:2309–17. doi: 10.1210/jc.2002-021353. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Bergami PL, Maulit G, et al. FAP-1 Association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–35. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370 doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Moskowitz CS, Gonen M. Bland-Altman Plots for evaluating agreement between solid tumor measurements. 2011:pp 1–18. [Google Scholar]
- Motz GT, Santoro SP, Wang L, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;8:1–11. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata S, Watanabe O, Ohashi K, Morino H. Expression of Fas ligand and bcl-2 in cervical carcinoma and their prognostic significance. Am J Clin Pathol. 2005;123:879–85. doi: 10.1309/0773-N4Q3-GFP3-4J5V. [DOI] [PubMed] [Google Scholar]
- O’Connell J, Bennett M, Nally K, et al. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci U S A. 2000;910:175–8. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- O’connell J, O’sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–82. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Hadji A, Murmann AE, et al. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015;22:549–59. doi: 10.1038/cdd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M, Peto J, Franceschi S. Time since first sexual intercourse and the risk of cervical cancer. Int J Cancer. 2014;130:2638–44. doi: 10.1002/ijc.26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzanna E. Progress Report National Cancer Registry in Indonesia. Indones J Cancer. 2009:3. [Google Scholar]
- Teitz T, Wei T, Valentine MB, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- Tourneur L, Mistou S, Michiels F-M, et al. Loss of FADD protein expression results in a biased Fas-signaling pathway and correlates with the development of tumoral status in thyroid follicular cells. Oncogene. 2003;22:2795. doi: 10.1038/sj.onc.1206399. [DOI] [PubMed] [Google Scholar]
- Vecchia C La, Decarli A, Gentile A, et al. “PAP” Smear and the risk of cervical neoplasia: Quantitative estimates from a case-control ctudy. Lancet. 1984;2:779–82. doi: 10.1016/s0140-6736(84)90705-0. [DOI] [PubMed] [Google Scholar]
- Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- Winter H, Hu H-M, Urba WJ, Fox BA. Tumor regression after adoptive transfer of effector T cells is independent of perforin or Fas ligand (APO-1L/CD95L) J Immunol. 1999;163:4462–72. [PubMed] [Google Scholar]
- Zhu J, Petit PF, Eynde BJ Van Den. Apoptosis of tumorinfiltrating T lymphocytes: a new immune checkpoint mechanism. Cancer Immunol Immunother. 2018 doi: 10.1007/s00262-018-2269-y. DOI:10.1007/s00262-018-2269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]