Abstract
While neutrophil production in emergency states has been extensively studied, regulation of neutrophil homeostasis in the steady-state remained incompletely understood. We have shown that innate immune receptor toll-like receptor (TLR)4 and downstream TIR-domain-containing adapter-inducing interferon-β (TRIF) are indispensable for the generation of a granulocyte-colony stimulating factor (G-CSF)-dependent regulatory feedback loop upon antibody-induced neutropenia. These findings demonstrated that steady-state granulopoiesis is a demand-driven process, which may rely on differential triggering of innate immune receptors by microbial cell wall constituents such as lipopolysaccharide. Herein, we present further evidence on underlying mechanisms: oral intake of highly endotoxic lipopolysaccharide, but not TLR-antagonistic lipopolysaccharide derived from Rhodobacter sphaeroides, induces hematopoietic stem and progenitor cell fate decisions toward the neutrophil lineage independent of G-CSF. TLR4 has been identified as the indispensable sensor for oral lipopolysaccharide-modulated steady-state granulopoiesis. These results have important implications: food lipopolysaccharide content or the composition of the gastrointestinal microbiome may be strongly underrated as determinants of peripheral blood neutrophil levels. Both neutrophilia and neutropenia are associated with drastically worse outcomes in epidemiological studies of the general population as well as in diseased states.
Impact statement
In our present study, we investigated the impact of LPS on neutrophil homeostasis and found that oral intake is sufficient to induce hematopoietic stem and progenitor cell fate decisions towards the neutrophil lineage independent of G-CSF. In addition, TLR4 has been identified as the indispensable sensor for oral LPS-modulated steady-state granulopoiesis. We provide evidence that the gastrointestinal microbiome is critical for neutrophil homeostasis, which has implications for patients being treated with chemotherapy or antimicrobial therapy, since both are significantly influencing the composition of the intestinal microbiome.
Keywords: Lipopolysaccharide, granulocytes, G-CSF, TLR4, granulopoiesis
Introduction
While neutrophil production in emergency states has been extensively studied, regulation of neutrophil homeostasis in the steady-state remained incompletely understood. We have shown that innate immune receptors TLR4/TLR2 are indispensable for the generation of a granulocyte colony-stimulating factor (G-CSF)-dependent regulatory feedback loop upon antibody-induced neutropenia.1 These findings demonstrated that steady-state granulopoiesis is a demand-driven process, which may rely on differential triggering of innate immune receptors by microbial cell wall constituents such as lipopolysaccharide (LPS). The fact that even germ-free kept mice, which are free from commensal microbiotas are able to generate feedback-granulopoiesis suggests that even small amounts of LPS as a contaminant in autoclaved chow may be sufficient to maintain permanent positive signals.2 Importantly, LPS derived from different bacterial strains is known to display different endotoxic potentials due to varying strength of interaction with innate immune receptors.3 Therefore, we hypothesized that oral intake of LPS-structures may modulate feedback granulopoiesis and added endotoxins to the drinking water of mice. Surprisingly, addition of endotoxins to the drinking water of mice is not only active as a modulator of feedback granulopoiesis upon antibody-induced neutropenia, but sufficient to induce hematopoietic stem/progenitor cell fate decisions in the marrow.
Materials and methods
Experimental animals and ethical statement
C57BL/6J, B6(Cg)-Tlr4tm1.1Karp/J (TLR4fl/fl), B6.Cg-Tg(Tek-cre)1Ywa/J (Tie2cre/+), B6.Cg-Tg(Nes-cre)1Kln/J (Nestincre/+) and B6.Cg-Tg(Vav1-icre)A2Kio/J (Vavcre/+) mice were obtained from The Jackson Laboratory. B6(Cg)-Tlr4tm1.2Karp/J (TLR4−/−) mice were provided by M. Radsak (Johannes Gutenberg University Medical Centre, Mainz, Germany). All mouse strains were bred and maintained under specific-pathogen-free conditions in the animal facility of the University of Tuebingen. For experimental studies, age and sex-matched littermates were used for the control and experimental groups following. Animal experiments were performed with the authorization of the Institutional Animal Care and Use Committee of the University of Tuebingen according to German federal and state regulations following the ARRIVE guidelines.4
In vivo administration of mAbs and LPS
For in vivo treatment with LPS, drinking water was supplemented with high endotoxic LPS (LPS-hi) (E. coli JM83, kindly provided by Dr Frick, Tuebingen) and TLR4 antagonistic LPS (LPS-RS) (Rhodobacter sphaeroides, InvivoGen) (both 5 mg/L) which was replaced twice a week. To induce neutropenia, mice were injected i.p. with 1.25 mg of an α-Ly6G mAb (clone 1A8, BioXCell) or vehicle control every 48 hours for a total of eight days. For depletion of G-CSF, mice were treated i.p. with 10 µg per day of an α-G-CSF mAb (clone MAB414, R&D Systems) or vehicle control for three days. PBS was used as vehicle control in all cases.
Flow cytometric analysis of peripheral blood and bone marrow cells
Retro-orbital blood was collected and differential blood counts were obtained using an automated Bayer Advia 120 MultiSpecies Analyzer (Bayer HealthCare). Bone marrow cells were harvested from femora and tibiae. For flow cytometric analysis (FACS-Canto II, BD Bioscience), erythrocytes were lysed with red blood cell lysis buffer (0.150 mM NH4Cl, 0.1 mM EDTA, 0.150 mM KHCO3) for 10 min on ice. Cells were stained for flow cytometric analysis to identify myeloid cells, hematopoietic stem, and progenitor cells. Antibodies were purchased from eBioscience (Thermo): CD34 (clone RAM34), Sca-1 (clone D7), Ly6G/Gr-1 (clone 1A8), CD3 (clone eBio500A2), CD45 (clone 30-F11), Ter119 (clone Ter-119), CD11b (clone M1/70), CD31 (clone 390), c-kit (clone 2B8), Ly6C/G (clone RB6-8C5), CD16/32 (clone 2.4G2), and CD127 (clone A7R34). CD3 (clone 145-2C11), CD11b (clone M1/70), B220 (clone RA3-6B2), and Ter119 (clone Ter-119) were used as mouse lineage panel (BD Pharmingen). Data analysis was performed using FlowJo software (FlowJo LCC).
Cytokine ELISA
Protein plasma levels of G-CSF were measured using Duo Set ELISA kits (R&D Systems) according to the manufacturer’s recommendations.
Statistics
For statistical analysis, GraphPad Prism 8.1.0 (GraphPad Software) was used. Mean values and standard error of the mean (SEM) are shown. Normal distribution was calculated with the Shapiro–Wilk test. The 95% confidence level was used and P-values were calculated with a two-tailed Student´s t-test in the case of normally distributed data. Significance of not normally distributed data was calculated with a two-tailed Mann–Whitney test. An unpaired analysis of variance (ANOVA) was used to analyze the differences among group means. P-value of <0.05 (*) was used as cut-off for significance.
Results and discussion
The regulation of neutrophil homeostasis under steady-state conditions as well as emergency granulopoiesis are both a precisely tuned demand-driven process to provide immediate and effective innate immune responses to pathogens and danger signals. For example, bloodstream lipopolysaccharides from Gram negative bacteria are potent drivers of emergency granulopoiesis being an adequate response to control disseminated infection and sepsis. Boettcher et al.5 have shown previously that LPS promotes G-CSF release from endothelial cells resulting in a robust expansion of granulocyte-macrophage progenitors (GMPs). In contrast, regulation of steady-state granulopoiesis is poorly understood. In particular, the role of commensal bacteria and their endotoxins demands further investigation. Balmer et al.6 established a TLR-dependent cross-talk between the intestinal microbiome and efficient granulopoiesis using serum transfer experiments and MyD88−/− TICAM1−/− mice. Furthermore, the observation that a feed-back signal can be induced in germ-free mice upon depletion of neutrophils led to the hypothesis that low levels of LPS, e.g., from autoclaved chow could be a sufficient stimulus.1 Consequently, steady release and fluctuations of LPS from intestinal bacteria influence steady-state granulopoiesis having implications for clinical practice. Recent data suggest that gut decontamination by antibiotics is associated with prolonged aplasia after stem cell transplantation.7 This observation is supported by preclinical models where intestinal bacteria promote granulocyte recovery and improve pathogen clearance after chemotherapy.8 Moreover, recent data from Weaver et al.9 indicate that chronic stimulation through commensal bacteria seems to be required to induce efficient and sustained emergency granulopoiesis after repeated TLR signaling. In the present study, we administered oral LPS to investigate the impact of intestinal endotoxin fluctuations on steady-state granulopoiesis and studied the role of pattern-recognition receptor TLR4 in various bone marrow compartments.
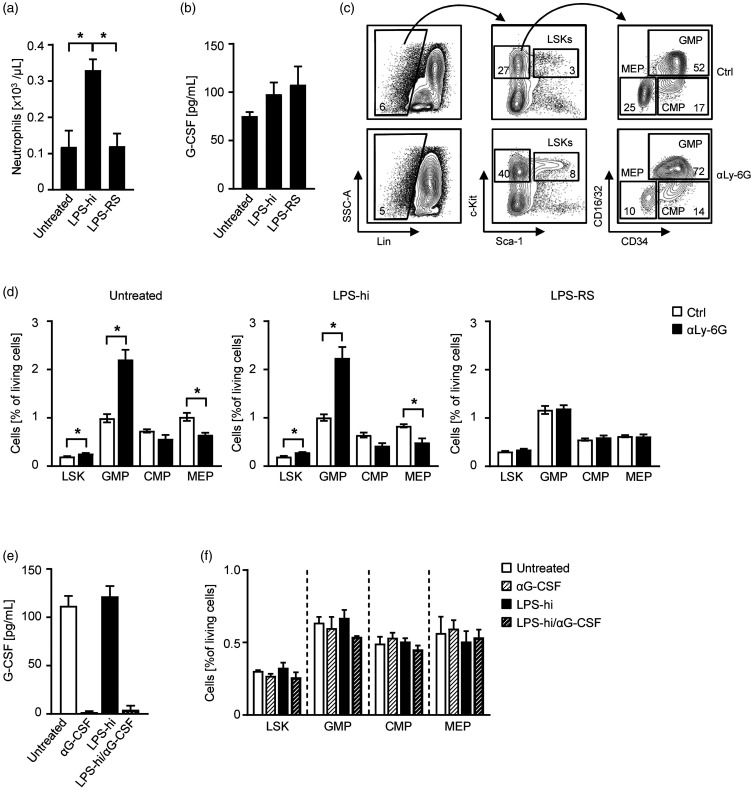
To avoid possible internal bleedings and infections and therefore to specifically simulate steady-state conditions, we supplemented the drinking water of C57BL/6 mice with TLR4 agonistic LPS of Enterobacteriaceae (LPS high endotoxic, LPS-hi)10 or TLR4 antagonistic LPS of Rhodobacteraceae (LPS-RS)10,11 and analyzed the peripheral neutrophil counts as well as G-CSF levels in the sera. While mice treated with LPS-hi showed a significant increase of neutrophils compared to untreated mice, the cohort treated with LPS-RS was comparable to the controls (Figure 1(a)). G-CSF levels showed no relevant changes upon stimulation with both LPS structures (Figure 1(b)). Since regulation of granulopoiesis was shown to be G-CSF-dependent, we further induced neutropenia in LPS-challenged mice by injecting a neutrophil-depleting αLy-6G antibody as previously described and analyzed the bone marrow for hematopoietic stem cells (HCS) and progenitor cell populations (HPC).1 Interestingly, the administration of LPS-RS is sufficient to inhibit the neutrophil homeostatic feedback loop induced by antibody-induced neutropenia, while LPS-hi treated mice showed increased Lin–Sca-1+c-kit+ hematopoietic stem cells (LSK) and granulocyte-monocyte progenitors (GMP) populations and decreased megakaryocyte-erythroid progenitors (MEP) and common myeloid progenitors (CMP) as untreated controls (Figure 1(c) and (d)). These results suggest a LPS-dependent feedback granulopoiesis.
Figure 1.
Oral intake of LPS induces feedback-granulopoiesis independent of G-CSF. (a–d) C57BL/6 mice were treated for 11 days with LPS-hi or LPS-RS in the drinking water (both 5 mg/L). (a) Blood was obtained at d11 and analyzed by flow cytometry. Neutrophil numbers were calculated according to the white blood count obtained with an Advia blood analyzer (n = 3 per group). (b) G-CSF levels in the sera of LPS-hi (n = 3), LPS-RS (n = 7) or untreated mice (n = 6) were analyzed at d11 by ELISA. (c–d) On d3 of the experiment, mice were additionally treated with an αLy-6G mAb (1A8, 1.25 mg/mouse i.p.) or vehicle control every 48 h and the bone marrow was analyzed at d11 by flow cytometry. (c) Exemplary hierarchical gating scheme of GMPs (CD16/32+CD34+), CMPs (CD16/32loCD34+), and MEPs (CD16/32−CD34−) within the Lin−c-kit+Sca-1− population and LSKs (Lin−c-kit+Sca-1+) of untreated mice receiving vehicle control or αLy-6G mAb. (d) On d11, bone marrow progenitor cells were analyzed in mice treated with LPS-hi (n = 3), LPS-RS (n = 4) or untreated controls (n = 3) by flow cytometry. (e–f) C57BL/6 mice were treated for three days with LPS-hi (5 mg/L) in the drinking water or left untreated. In addition, αG-CSF mAb (10 µg/mouse every 24 h i.p.) was injected to deplete G-CSF and mice were analyzed at d3. (e) G-CSF levels in the sera were analyzed by ELISA (n = 4 per group). (f) Bone marrow (n = 3 per group) was analyzed for LSK, GMP, CMP and MEP populations by flow cytometry.
G-CSF is a main driver of emergency granulopoiesis in infectious diseases and inflammatory conditions.5,12 G-CSF is also routinely administered to shorten neutropenia after chemotherapy or to mobilize hematopoietic stem cells for apheresis. In contrast, G-CSF-deficient mice are also capable of mobilizing granulocytes and combat infections indicating redundant signaling pathways ensuring reliable and adequate immune responses towards pathogens.13,14 However, the role of G-CSF for steady-state granulopoiesis is rather poorly defined. To investigate whether the regulation of oral LPS is G-CSF-dependent, we first depleted G-CSF by administration of an αG-CSF mAb and progenitor cell populations in the bone marrow were assessed. Successful G-CSF depletion in the sera was confirmed by ELISA (Figure 1(e)); however, no alterations were observed in any progenitor cell population of the experimental groups (Figure 1(f)) suggesting a G-CSF-independent feedback loop. This is supported by our observation that G-CSF levels are not affected upon oral LPS challenge or use of oral LPS antagonists.
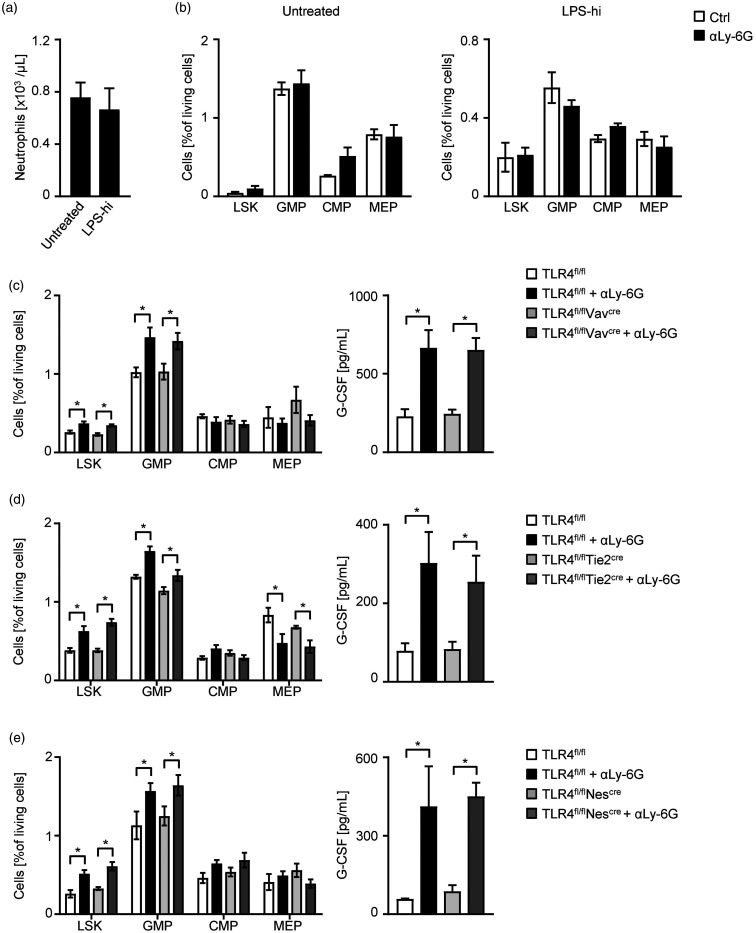
The inhibition of feedback-granulopoiesis by TLR4 antagonistic LPS-RS suggests a TLR4-dependent sensor for neutrophil regulation through oral LPS. To proof this hypothesis, we performed the antibody-induced neutropenia model in TLR4−/− mice. We could not detect a neutrophil increase or changes in HSC or HPC populations upon LPS-hi treatment, indicating a TLR4-dependent mechanism for oral intake of LPS (Figure 2(a) and (b)). Takizawa et al.15 demonstrated that dormant hematopoietic stem cells directly sense LPS through TLR4 signaling which induces proliferation and differentiation in a murine model of infection. To further elucidate the location of a TLR4-dependent sensor to regulate feedback-granulopoiesis, we used different conditional TLR4 knockout strains. First, we used the Vav-cre strain for specific deletion of TLR4 in the hematopoietic linage. Comparing these results with littermate controls harboring an intact TLR4 expression, the feedback loop stimulated by neutrophil depletion with αLy-6G was induced with respect to increased LSK and GMP in the bone marrow as well as elevated G-CSF levels in the sera (Figure 2(c)). Endothelial cell deletion of TLR4 with Tie2-cre mice (Figure 2(d)) and deletion of TLR4 in mesenchymal stromal cells with Nestin-cre mice (Figure 2(e)) showed the same results compared to the hematopoietic Vav-cre mice. These results clearly demonstrate the independency of hematopoietic, endothelial, and mesenchymal stromal cells for oral LPS-driven feedback granulopoiesis. This suggests that the TLR4 sensor for intestinal LPS is not located in the bone marrow. We can only speculate that other tissues might be part of this feed-back loop. For instance, oral LPS is transported to the liver by the portal vein and encounters hepatocytes that are known to respond directly through TLR4 and MyD88 signaling.16 Hepatocytes might produce IL-6 known to stimulate granulopoiesis.14,17
Figure 2.
Oral LPS is sensitized by TLR4 signalling. (a–b) TLR4−/− mice were treated for 11 days with LPS-hi (5 mg/L) in the drinking water or left untreated. On d3 of the experiment, mice were additionally treated with an αLy-6G mAb (1A8, 1.25 mg/mouse i.p.) or vehicle control every 48 h and the bone marrow was analyzed at d11 by flow cytometry. (a) Blood was obtained at d11 and analyzed by flow cytometry. Neutrophil numbers were calculated according to the white blood count obtained with an Advia blood analyzer (n = 7 per group). (b) On d11, bone marrow progenitor cells were analyzed in mice treated with LPS-hi (n = 3) and untreated controls (n = 5) by flow cytometry. (c–e) Mice of different conditional TLR4 knockout strains were treated with an αLy-6G mAb (1A8, 1.25 mg/mouse i.p.) or vehicle control every 48 h and analyzed at d8. (c) Bone marrow of TLR4fl/fl and TLR4fl/flVavcre mice was analyzed for progenitor cell populations (n = 8 per group) by flow cytometry (left) and G-CSF levels in the sera were measured by ELISA (n = 10 per group) (right). (d) Bone marrow of TLR4fl/fl and TLR4fl/flTie2cre mice was analyzed for progenitor cell populations (n = 4 per group) by flow cytometry (left) and G-CSF levels in the sera were measured by ELISA (n = 4 per group) (right). (e) Bone marrow of TLR4fl/fl (Ctrl. n = 5; αLy-6G n = 4) and TLR4fl/flNescre (n = 5 per group) mice was analyzed for progenitor cell populations by flow cytometry (left) and G-CSF levels in the sera of TLR4fl/fl (Ctrl. n = 4; αLy-6G n = 3) and TLR4fl/flNescre (n = 5 per group) mice were measured by ELISA (right).
In summary, we provide evidence that oral endotoxins are important regulators of steady-state granulopoiesis independent of G-CSF. Hematopoiesis is skewed towards GMPs and TLR4 sensing is required to maintain an adequate neutrophil homeostasis. Our results provide new mechanistic insights into the crosstalk between commensal bacteria and the immune system and highlight the importance of an intact intestinal microbiome for granulopoiesis and pathogen defense.
ACKNOWLEDGMENTS
The authors thank Natalia Rehband, Elke Malenke and Hildegard Keppeler for expert technical assistance and the Flow Cytometry Core Facility Berg of the University Hospital Tuebingen for their excellent technical support.
Authors’ contributions
MM and SB designed, performed, and interpreted experiments. SW, JSF, and MRM provided analysis and interpretation of data. MM, SW, and DS were involved in drafting the manuscript or revising it critically for important intellectual content. HGK and DS designed the study. All authors contributed to writing of the paper and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants of the Deutsche Forschungsgemeinschaft [BU 3154/1–1 to SB] and Deutsche Krebshilfe [70112548 to DS and 70113496 to MM].
ORCID iD
Melanie Märklin https://orcid.org/0000-0002-2920-3894
References
- 1.Bugl S, Wirths S, Radsak MP, Schild H, Stein P, Andre MC, Muller MR, Malenke E, Wiesner T, Marklin M, Frick JS, Handgretinger R, Rammensee HG, Kanz L, Kopp HG. Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood 2013; 121:723–33 [DOI] [PubMed] [Google Scholar]
- 2.Hrncir T, Stepankova R, Kozakova H, Hudcovic T, Tlaskalova-Hogenova H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: studies in germ-free mice. BMC Immunol 2008; 9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erridge C, Moncayo-Nieto OL, Morgan R, Young M, Poxton IR. Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J Med Microbiol 2007; 56:165–71 [DOI] [PubMed] [Google Scholar]
- 4.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boettcher S, Gerosa RC, Radpour R, Bauer J, Ampenberger F, Heikenwalder M, Kopf M, Manz MG. Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood 2014; 124:1393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balmer ML, Schurch CM, Saito Y, Geuking MB, Li H, Cuenca M, Kovtonyuk LV, McCoy KD, Hapfelmeier S, Ochsenbein AF, Manz MG, Slack E, Macpherson AJ. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol 2014; 193:5273–83 [DOI] [PubMed] [Google Scholar]
- 7.Routy B, Letendre C, Enot D, Chenard-Poirier M, Mehraj V, Seguin NC, Guenda K, Gagnon K, Woerther PL, Ghez D, Lachance S. The influence of gut-decontamination prophylactic antibiotics on acute graft-versus-host disease and survival following allogeneic hematopoietic stem cell transplantation. Oncoimmunology 2017; 6:e1258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salva S, Marranzino G, Villena J, Aguero G, Alvarez S. Probiotic Lactobacillus strains protect against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int Immunopharmacol 2014; 22:209–21 [DOI] [PubMed] [Google Scholar]
- 9.Weaver LK, Minichino D, Biswas C, Chu N, Lee JJ, Bittinger K, Albeituni S, Nichols KE, Behrens EM. Microbiota-dependent signals are required to sustain TLR-mediated immune responses. JCI Insight 2019; 4:e124370 [ 10.1172/jci.insight.124370] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gronbach K, Flade I, Holst O, Lindner B, Ruscheweyh HJ, Wittmann A, Menz S, Schwiertz A, Adam P, Stecher B, Josenhans C, Suerbaum S, Gruber AD, Kulik A, Huson D, Autenrieth IB, Frick JS. Endotoxicity of lipopolysaccharide as a determinant of T-cell-mediated colitis induction in mice. Gastroenterology 2014; 146:765–75 [DOI] [PubMed] [Google Scholar]
- 11.Aida Y, Kusumoto K, Nakatomi K, Takada H, Pabst MJ, Maeda K. An analogue of lipid A and LPS from Rhodobacter sphaeroides inhibits neutrophil responses to LPS by blocking receptor recognition of LPS and by depleting LPS-binding protein in plasma. J Leukoc Biol 1995; 58:675–82 [DOI] [PubMed] [Google Scholar]
- 12.Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, Brechlin AM, Schambach A, Hinrichsen L, Meyer G, Gaestel M, Stanulla M, Tong Q, Welte K. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med 2009; 15:151–8 [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood 2000; 95:3725–33 [PubMed] [Google Scholar]
- 14.Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood 1997; 90:2583–90 [PubMed] [Google Scholar]
- 15.Takizawa H, Fritsch K, Kovtonyuk LV, Saito Y, Yakkala C, Jacobs K, Ahuja AK, Lopes M, Hausmann A, Hardt WD, Gomariz A, Nombela-Arrieta C, Manz MG. Pathogen-induced TLR4-TRIF innate immune signaling in hematopoietic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell 2017; 21:225–40. e5 [DOI] [PubMed] [Google Scholar]
- 16.Lee YS, Kim YH, Jung YS, Kim KS, Kim DK, Na SY, Lee JM, Lee CH, Choi HS. Hepatocyte toll-like receptor 4 mediates lipopolysaccharide-induced hepcidin expression. Exp Mol Med 2017; 49:e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norris CA, He M, Kang LI, Ding MQ, Radder JE, Haynes MM, Yang Y, Paranjpe S, Bowen WC, Orr A, Michalopoulos GK, Stolz DB, Mars WM. Synthesis of IL-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS One 2014; 9:e96053. [DOI] [PMC free article] [PubMed] [Google Scholar]