Abstract
Lipotoxicity, defined as the cell death and/or cellular dysfunction induced by ectopic lipid deposition, plays a pathological role in the development of many metabolic diseases. Although endoplasmic reticulum stress is a well-documented mechanism behind, how endoplasmic reticulum stress is initiated during lipotoxicity remains obscure. In this study, using palmitate exposure (a 16-C saturated fatty acid) of AML12 hepatocytes, a non-transformed murine hepatocyte cell line, as an experimental model, we identified mammalian target of rapamycin complex 1 (mTORC1) to be a critical contributor to palmitate-elicited lipotoxicity, manifested by incremental triglycerides secretion and cell death. Unlike oleate (an 18-C monounsaturated fatty acid), palmitate strongly induced mTORC1 activation in hepatocytes. Importantly, mTOR inhibitors, torin-1, and rapamycin prevented hepatocytes from palmitate-induced triglyceride overproduction and cell death. We further showed that the intracellular metabolism of palmitate is required for its stimulatory effect on mTORC1. Whereas the inhibition of long-chain acyl-CoA synthetase, which converts palmitate to palmitoyl-CoA, attenuated mTORC1 activation and protected against cell death, the inhibition of stearoyl-CoA desaturase-1, the enzyme desaturating palmitate to palmitoleate, strengthened mTORC1 activation and aggravated triglyceride overproduction and cell death. Our further investigations revealed that the palmitate-induced mTORC1 activation was required for its endoplasmic reticulum stress-inducing action as mTORC1 inhibition ablated palmitate-induced activation of IRE1α, one of the three canonical pathways activated during unfolded protein response. Finally, our data demonstrated that IRE1α inhibition ameliorated triglyceride overproduction and cell death in response to palmitate exposure. Collectively, our data identify that mTORC1-IRE1α pathway is coordinately implicated in the development of lipotoxicity in hepatocyte.
Impact statement
Lipotoxicity induced by saturated fatty acids (SFA) plays a pivotal role in the pathogenesis of a variety of obesity-related metabolic disorders; however, the exact mechanism(s) underlying lipotoxicity development remains elusive. The liver plays a central role in regulating intrahepatic and circulatory lipid homeostasis. In the current study, we identified that mammalian target of rapamycin complex 1 (mTORC1) activation plays an important role in regulating the detrimental effects of SFA palmitate in hepatocytes, in specific cell death, and TG overproduction. Furthermore, our data confirmed that palmitate-induced mTORC1 activation is attributable to its stimulatory effect on IRE1α, one of three canonical pathways activated during ER stress. Importantly, IRE1α inhibition prevented palmitate-triggered cell death and TG overproduction, suggesting mTORC1-IRE1α pathway is mechanistically implicated in palmitate lipotoxicity. The data obtained in the current investigation support future study to explore the therapeutic potential of targeting the mTORC1-IRE1α pathway as a novel clinical strategy for the treatment of metabolic disorders involving lipotoxicity.
Keywords: Lipotoxicity, palmitate, mTORC1, IRE1α, ER stress, SCD-1
Introduction
Lipotoxicity, the term developed to describe the detrimental impact of lipids accumulation in non-adipose tissues (ectopic lipid deposition), is intimately associated with many obesity-related metabolic disorders, including non-alcoholic fatty liver disease (NAFLD), cardiovascular disease, among others.1–3 Free fatty acids (FFAs) play a pivotal role in the elicitation of lipotoxicity. In comparison to most unsaturated fatty acids, saturated fatty acids (SFA) are more detrimental to cells, giving rise to not only cell death but also functional aberrations in a broad range of mammalian cell types, including hepatocytes.4–6 For instance, NAFLD is associated with increased plasma levels of palmitate (a 16-carbon SFA), one of the most abundant FFAs detected in human circulation.7 Accumulated evidence supports that increased plasma levels of FFAs, especially SFA, contribute to the liver pathologies in NAFLD.8
Through very low-density lipoprotein (VLDL) production and secretion, the liver plays a central role in the regulation of intrahepatic and circulatory lipid homeostasis.9 Obesity is closely related with elevated levels of circulatory FFAs, which is primarily derived from uncontrolled adipose tissue lipolysis due to peripheral insulin resistance. Under this circumstance, liver triglycerides (TG) production and secretion in the form of VLDL are enhanced, leading to fatty liver development and hypertriglyceridemia, which is closely associated with an increased risk of cardiovascular disease.10 Although it has been long appreciated that the long-term saturated fat consumption is closely associated with the development of hypertriglyceridemia,11,12 the mechanism behind this phenomenon is not completely understood.
Mammalian target of rapamycin (mTOR) is an evolutionarily conserved protein serine/threonine kinase and a master regulator in promoting growth and cellular anabolic processes in response to growth factors and nutrients excess. It forms two structurally and functionally distinct kinase complexes, mTOR complex 1 (mTORC1) and mTORC2, with distinct components and substrates. The mTORC1 is sensitive to rapamycin and regulates anabolic processes via directly phosphorylating ribosomal p70S6 kinase (p70S6K) and eIF4E-binding protein (4E-BP).13,14 Dependent on growth factors stimulation and nutrients availability, mTORC1 activation integrates a wide range of extracellular and intracellular signals to regulate cell growth, anabolism, and protein synthesis.
Endoplasmic reticulum (ER) stress represents a well-established mechanism implicating in palmitate‐induced lipotoxicity in hepatocytes. ER stress occurs when there is an imbalance between the unfolded proteins and the capacity of ER folding machinery inside the ER lumen. This leads to the unfolded protein response (UPR), which represents a physiological response functioning to facilitate the restoration of ER homeostasis. UPR consists of three canonical signaling pathways: PKR‐like ER kinase (PERK), inositol‐requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6), with IRE1α being the most conserved one, possessing both endoribonuclease activity and kinase activity, leading to generation of X-box binding protein 1 (XBP1s) and c-Jun N-terminal kinase (JNK) activation, respectively.15,16 ER stress induction in the presence of excessive palmitate exposure has been reported in a variety of cell types including hepatocytes.17–19 The evidence that ER stress alleviation using chemical ER chaperons or blockade of UPR pathways via either pharmacological or genetic approach protects against palmitate-induced lipotoxicity20–22 confirms the mechanistic role of ER stress in palmitate-induced lipotoxicity. However, it is currently unknown as to how palmitate triggers ER stress in hepatocytes and whether/how ER stress contributes to SFA-triggered incremental TG secretion from hepatocytes.
Emerging evidence suggests that mTORC1-mediated signaling events and UPR pathways are intertwined.23,24 ATF6 (one of three canonical UPR pathways) was reported to activate mTORC1 through RHEB (Ras homolog enriched in brain) upregulation.23 Furthermore, both tunicamycin and thapsigargin (two well-established ER stress inducers) strongly activate mTORC1, while mTORC1 inhibition prevents ER stress-induced cell death, suggesting that the UPR and mTORC1 conspire to regulate cell death.24 In this study, using AML12 cells, a non-transformed mouse hepatocyte cell line, we demonstrate that palmitate is a nutrient activator of mTORC1 in hepatocytes and identify the mTORC1-IRE1α pathway activation to be a critical contributor to palmitate-induced incremental TG secretion and cell death.
Materials and methods
Reagents
Palmitate and oleate were purchased from Sigma (St. Louis, USA). SC79, torin-1, and rapamycin were purchased from Apexbio Technology LLC (Houston, USA). Triacsin C was purchased from ENZO (Broomfield, USA). For Western blotting, antibodies for p-S6 (S235/236), S6, p-P70S6(T389), P70S6, p-AKT(S473), AKT, β-actin, and FITC-conjugated secondary antibodies were purchased from Cell Signaling Technology (Beverly, USA).
Cell culture
AML12 murine hepatocytes, purchased from the American Type Culture Collection (Manassas, USA), were cultured as monolayers using Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 supplemented with 2% or 10% fetal bovine serum, 100 units/mL penicillin, 0.1 mg/mL streptomycin, 40 ng/mL dexamethasone and ITS (containing 5 mg/L insulin, 5 mg/L transferrin and 5 μg/L selenium). Cells were grown in 75 cm2 flasks and kept at 37°C in a humidified atmosphere of air and 5% CO2. Cells were grown at 80% of confluence prior to the exposure to treatments in the various experiments.
LDH release assay
AML12 cells at 1 × 105 cells/mL were seeded in 24-well plates and cultured overnight. After indicated treatments, culture medium was collected and detected using a Pierce LDH Cytotoxicity Assay kit (Thermo Scientific Inc., Rockford, IL, USA) according to the manufacturer’s instructions. The absorption at OD510 was measured using a microplate spectrophotometer (SPECTRAmax® 340PC; Molecular Devices Corp., Sunnyvale, CA, USA). The relative LDH release levels were scaled as folds of the controlled untreated group.
MTT assay
(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method was used to determine the effects of torin-1 and rapamycin protection against palmitate-induced cell death in hepatocytes. Briefly, 5 × 103 cells/well were evenly distributed and incubated on 96-well plates overnight. After indicated treatments, the medium in each well was replaced with 20 µl MTT (5 mg/mL in PBS) and incubated at 37°C for 4 h. The purple-blue formazan precipitate was dissolved in 100 µl dimethyl sulfoxide and the optical density was measured at a wavelength of 490 nm on a microplate spectrophotometer reader (SPECTRAmax® 340PC; Molecular Devices Corp., Sunnyvale, CA, USA).
Cell lysates and Western blotting
Total protein from AML12 hepatocyte was obtained using lysis buffer from Thermo Scientific Inc. (Rockford, USA). Samples were incubated on ice with frequent vortex for 15 min and centrifuged for 10 min at 12,000g. The protein concentration was determined using an Enhanced BCA Protein Assay Kit which was from Thermo Scientific Inc. (Rockford, USA) in accordance with the manufacturer’s instructions. Equal amounts of protein (30 µg) were subjected to 10% or 8% SDS-PAGE depending on the molecular weight of the desired proteins and were transferred to a nitrocellulose transfer membrane (Pall Corporation, New Port Richey, FL). After transfer, membranes were blocked in 5% (wt./vol.) nonfat dry milk in PBS-0.1% Tween 20 and probed with specific antibodies. After incubation with primary antibodies, membranes were washed with PBST, then incubated in fluorescein-conjugated secondary antibodies (1:10,000 dilution in blocking buffer at room temperature for 1 h, and then washed with PBST again. Immunoreactive bands of predicted molecular mass were visualized using an LI-COR Odyssey CLx system and quantified with Image Studio Ver 4.0.
Quantitative real-time RT-PCR
Total RNA from AML12 hepatocytes was isolated with a phenol-chloroform extraction. For each sample, 1.0 μg total RNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Vilnius, LT). The cDNA was amplified in MicroAmp Optical 96-well reaction plates with an SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) on a Life Technologies ABI 7500 FAST sequence detection system. Relative gene expression was calculated after normalization by a house-keeping gene (mouse GAPDH mRNA).
Primers:
GAPDH: 5′-ATGCCAGTGAGCTTCCCGTTCAG-3′ and 5′-CATCACTGCCACCCAGAAGACTG-3′;
XBP1: 5′-TAGACCTCTGGGAGTTCCTCCA -3’ and 5′-TGGACTCTGACACTGTGCCTC-3′;
XBP1s: 5′-GAGGCAACAGTGTCAGAGTCC -3’ and 5′-TGCTGAGTCCGCAGCAGGTG-3′;
XBP1u: 5′-GAGGCAACAGTGTCAGAGTC -3’ and 5′-CTCAGACTATGTGCACCTCTGC-3′.
Triglycerides assay
To determine the intracellular TG content, hepatocytes seeded in 12-well plates were washed twice with phosphate-buffered saline (PBS) and cellular lipids were extracted by 1 mL hexane:isopropanol (3:2) mixture. After centrifuging at 10,000 r/min for 5 min, 20 μL upper liquid was transferred into new tubes. Solvents were removed with Speedvac vacuum concentrators for 20–30 min. TG levels were determined by adding TG assay reagent (Triglycerides Assay Kit, MedTest Dx, Canton, MI) and incubated at 37°C for 5 min. The final TG levels were standardized with protein concentrations in cells undergoing the same treatment conditions.
Statistics
All data were expressed as mean ± SD. Statistical analysis was performed using a one-way ANOVA and was analyzed further by post hoc test with Fisher’s least significant difference (LSD) for statistical differences.
Results
Palmitate is an mTORC1 activator in hepatocytes
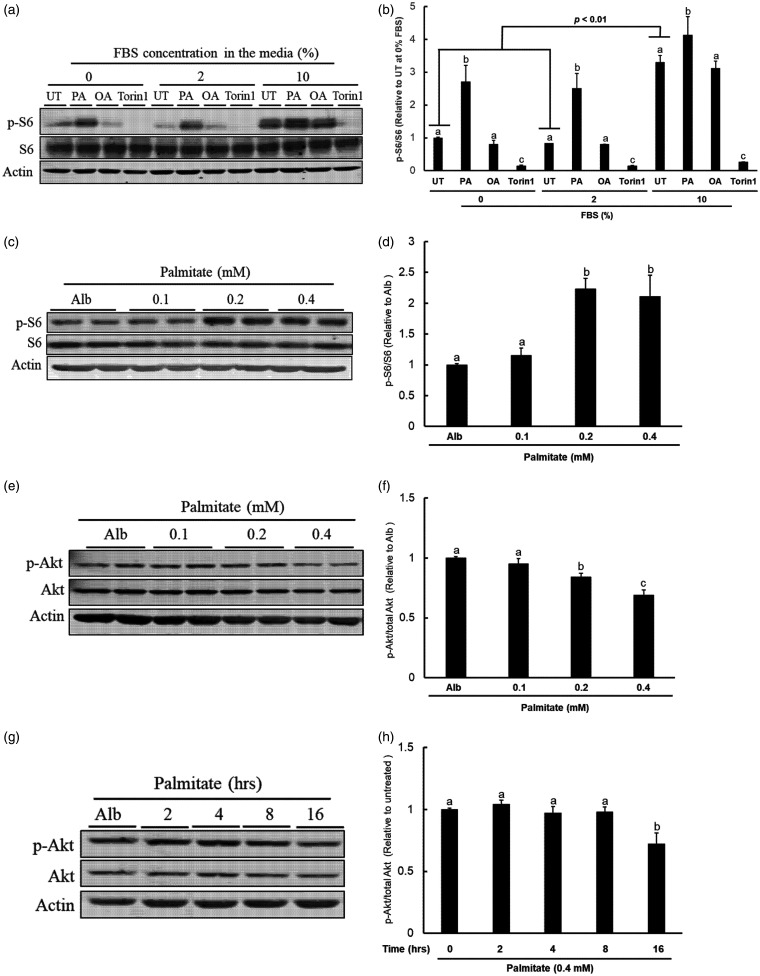
Growth factors strongly stimulate mTORC1 activation.13,14 In cell culture conditions, growth factors are exclusively derived from fetal bovine serum (FBS). The standard culture medium for AML12 hepatocytes contains 10% FBS. Therefore, we first examined the effects of FBS concentrations on mTORC1 activation in AML12 cells. Three concentrations of FBS (0, 2%, 10%, respectively) were used and mTORC1 activation status was determined by detecting protein abundance of phosphorylated S6 ribosomal protein (pS6), an indicator of mTORC1 activation, after a 16-h exposure of three treatments, palmitate (0.4 mM), oleate (0.4 mM), and torin-1 (0.25 μM). As expected, in comparison to lower concentrations of FBS (0% and 2%), the 10% FBS-containing media led to a robust S6 ribosomal protein phosphorylation, indicating a strong mTORC1 activation (Figure 1(a) and (b)). Palmitate and oleate (an 18-C monounsaturated fatty acid) are the two most abundant FFAs detected in the circulation. Unlike oleate, which had no effect on mTORC1 activation, palmitate exposure strongly stimulated S6 protein phosphorylation no matter what concentration of FBS was included in the media. Considering that the conspicuous mTORC1 activation under regular cultural condition (10% FBS) may mask the potential effect of the experimental treatments, the cell culture studies involving the determination of mTORC1 activation status were performed using DEME/F-12 media containing 2% FBS to optimize our observations. As shown in Figure 1(c) and (d), during a 16-h exposure period, palmitate at both 0.2 mM and 0.4 mM activated mTORC1. In line with many previous reports showing that chronic mTORC1 activation results in a feedback inhibition on Akt activity,25,26 both time-course (Figure 1(e) and (f)) and dose-dependent (Figure 1(g) and (h)) inhibition of Akt kinase (phosphorylation) were detected in response to palmitate exposure. These data altogether confirmed that palmitate, the most abundant circulatory SFA, is a nutrient activator of mTORC1 in hepatocytes.
Figure 1.
Palmitate activates mTORC1. (a) AML12 cells were cultured with media containing various concentrations of FBS (0%, 2%, and 10%, respectively) and treated with palmitate (0.4 mM), oleate (0.4 mM), and torin-1 (0.25 µM) for 16 h. mTORC1 activation status was determined by detecting abundance of phosphorylated and total S6 ribosomal protein (p-S6) by Western blotting; (b) Quantitative analysis of the ratio of p-S6 to S6 for the indicated treatments. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly within groups with the indicated FBS concentrations (P < . 05); (c) AML12 cells cultured with 2% FBS media were treated with the indicated concentrations of palmitate for 16 h. Protein abundance of p-S6 and total S6 were detected by Western blotting; (d) Quantitative analysis of the ratio of p-S6 to S6 for the indicated palmitate concentrations. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05); (e) AML12 cells cultured with 2% FBS media were treated with the indicated concentrations of palmitate for 16 h. Protein abundance of p-Akt and total Akt were detected by Western blotting; (f) Quantitative analysis of the ratio of p-Akt to total Akt for the indicated palmitate concentrations. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05); (g) AML12 cells cultured with 2% FBS media were treated with 0.4 mM palmitate for the indicated experimental durations. Protein expressions of p-Akt, total Akt, and actin were determined by Western blotting; (h) Quantitative analysis of the ratio of p-Akt to total Akt at the indicated time. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05).
Palmitate metabolism is required for its mTORC1-activating effect
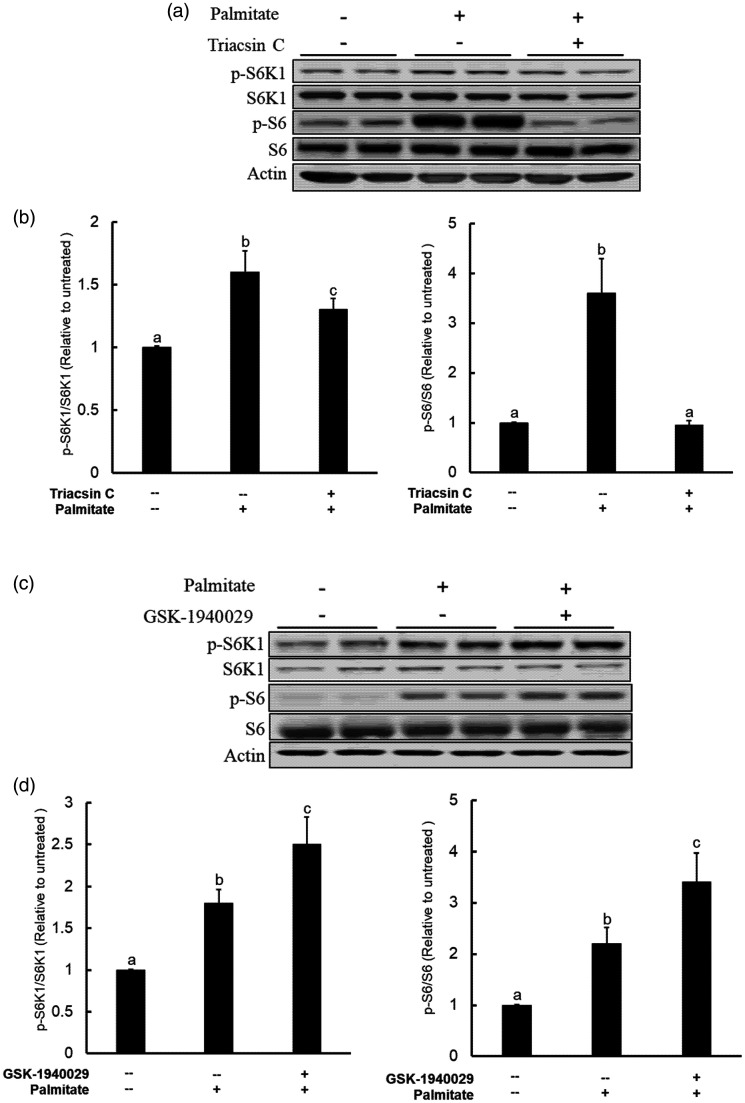
Metabolic intermediates of palmitate contribute to its detrimental impact.27 The first step of intracellular palmitate metabolism is catalyzed by acyl-CoA synthetase, the enzyme converting palmitate to palmitoyl-CoA. To test the hypothesis that the acyl-CoA synthetase activity is required for mTORC1 activation upon palmitate exposure, we pretreated AML12 cells with Triacsin C (10 μM), an inhibitor of long fatty acid acyl-CoA synthetase, for 2 h before a 16-h palmitate exposure. Our data clearly showed that acyl-CoA synthetase inhibition attenuated palmitate-instigated mTORC1 activation, evidenced by blunted phosphorylation of both S6K1 and its direct target S6 protein when Triacsin C was present (Figure 2(a) and (b)).
Figure 2.
Intracellular metabolism of palmitate is required for mTORC1 activation and lipotoxic effects. (a) AML12 cells were pretreated either long-chain fatty acyl-CoA synthetase inhibitor (Triacsin C at 10 µM) for 2 h prior to palmitate exposure (0.4 mM). mTORC1 activation status was determined by Western blot detection of S6K1 and S6 protein phosphorylation after 16-h palmitate exposure; (b) Quantitative analysis of the ratios of p-S6K1 to S6K1 and p-S6 to S6 for the indicated treatments. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05); (c) AML12 cells were pretreated SCD-1 inhibitor GSK1940029 (20 µM) for 2 h before palmitate addition (0.4 mM). mTORC1 activation status was determined by Western blot detection of S6K1 and S6 protein phosphorylation after 16-h palmitate exposure; (d) Quantitative analysis of the ratios of p-S6K1 to S6K1 and p-S6 to S6 for the indicated treatments. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05).
Stearoyl-CoA desaturase (SCD)-1 is the primary enzyme catalyzing the intracellular desaturation of SFA, including palmitate. To determine the contribution of intracellular desaturation process of palmitate to its mTORC1-activating effects, we pretreated AML12 hepatocytes with GSK-1940029, a specific inhibitor of SCD-1, for 2 h before palmitate exposure and mTORC1 activation was determined by Western blotting for phospho-S6K1 and S6 ribosomal protein after a 16-h treatment. As shown in Figure 2(c) and (d), SCD-1 inhibition strengthened palmitate-induced mTORC1 activation.
mTORC1 activation contributes to palmitate-induced hepatocyte cell death
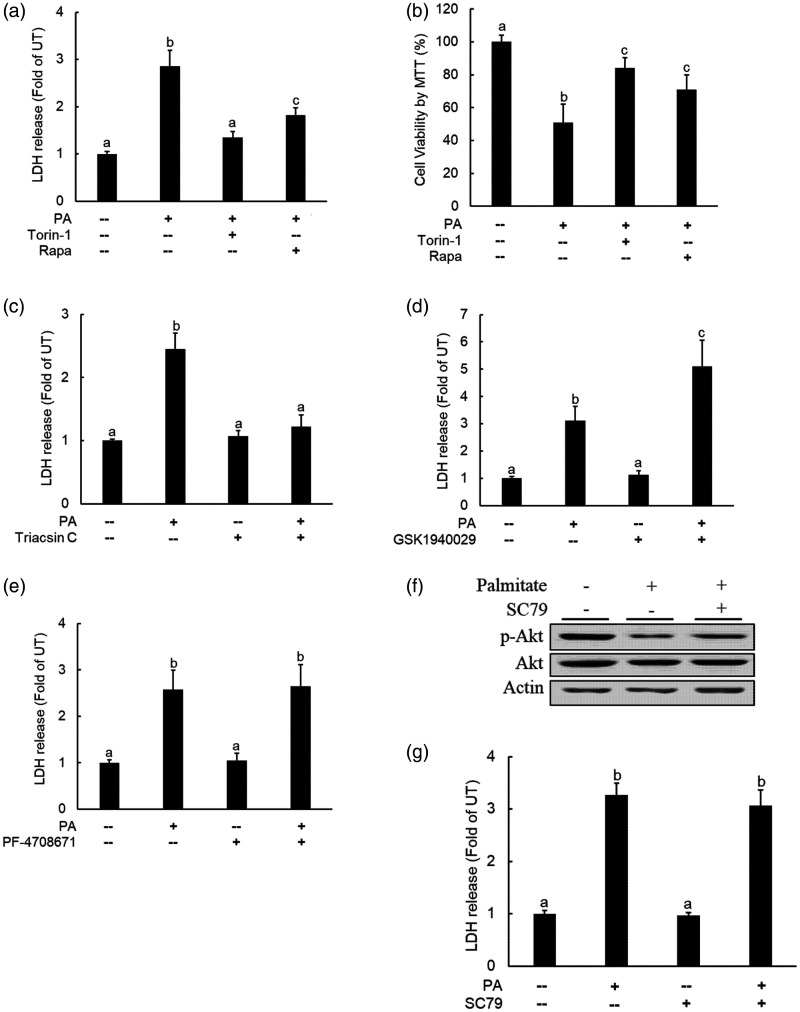
Palmitate exposure induced cell death in a variety of cell types, including hepatocytes.28 To test whether mTORC1 activation contributes to palmitate-induced hepatocyte cell death, AML12 cells were pretreated with either torin-1, a chemical inhibitor for both mTORC1 and mTORC2, or rapamycin, a specific inhibitor for mTORC1, for 2 h before palmitate addition (0.4 mM). Cell death was determined after a 16-h palmitate exposure by both LDH release and MTT assay. As shown in Figure 3(a) and (b), both inhibitors significantly suppressed cell death. In line with their opposite effects on palmitate-induced mTORC1 activation, Triacsin C pretreatment was protective against palmitate-induced cell death (Figure 3(c)), whereas SCD-1 inhibition by GSK-1940029 aggravated palmitate-elicited lipotoxic cell death (Figure 3(d)). Together, our data suggest that mTORC1 activation attributes to palmitate-induced cell death in hepatocytes.
Figure 3.
Inhibition of mTORC1 protects against palmitate-induced cell death in hepatocytes. AML12 cells were pretreated with either torin-1 (0.25 µM) or rapamycin (0.25 µM) for 2 h before palmitate (0.4 mM) exposure. Cell death was determined after 16-h palmitate exposure via both LDH release assay (a) and MTT measurement (b); (c) AML12 cells were pretreated with Triacsin C (10 µM), the long-chain fatty acyl-CoA synthetase inhibitor, for 2 h prior to palmitate exposure (0.4 mM). Cell death was determined 16-h later by LDH release; (d) AML12 cells were pretreated with GSK1940029 (20 µM), the stearoyl-CoA desaturase (SCD)-1 inhibitor, for 2 h before palmitate addition (0.4 mM). Cell death was determined after 16-h palmitate exposure by LDH release measurement. (e) AML12 cells were pretreated with PF-4708671 (10 nM), the S6K1 inhibitor, for 2 h before palmitate addition (0.4 mM). Cell death was determined after 16-h palmitate exposure by LDH release measurement. AML12 cells were pretreated with SC79 (10 µM), an Akt activator, for 2 h before palmitate addition (0.4 mM). Akt activation status and cell death were detected by Western blotting for p-Akt protein abundance (f) and LDH release measurement (g) 16 h later, respectively. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05).
Prolonged mTORC1 activation suppresses Akt activity via activating S6K1.25,26 We previously reported the inhibitory effect of palmitate on Akt activation in HepG2 cells.29 The similar observations were obtained in our current study, correlated with mTORC1 activation (Figure 1(c) and (d)). Given that Akt suppression is attributable to cell death in a variety of cell types under many different experimental conditions,29,30 we examined whether Akt inhibition may potentially contribute to palmitate-induced cell death. To test our hypothesis, we pretreated cells with either PF-4708671 (10 nM), a specific S6K1 inhibitor, or SC79 (10 μM), a specific Akt activator, before palmitate challenge. As shown in Figure 3(e) to (g), neither PF-4708671 nor SP79 exerted protection against palmitate-induced cell death after a 16-h exposure.
Palmitate promotes hepatocyte triglycerides secretion
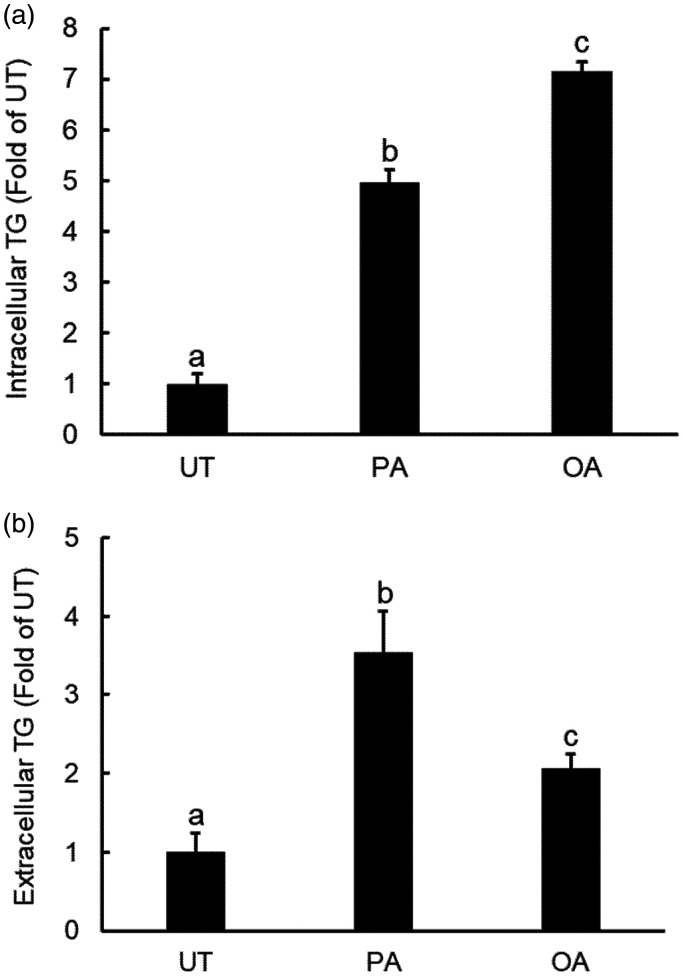
The liver plays a pivotal role in regulating lipid homeostasis. Hepatocytes generate and export lipids, primarily triglycerides, in the form of VLDL. The effects of palmitate and oleate on its accumulation and extracellular secretion of TG were directly determined via measuring its concentrations both inside hepatocytes and in culture media after a 10-h exposure of individual fatty acid. As shown in Figure 4(a), the long-term exposure of hepatocytes with both palmitate and oleate resulted in intracellular TG accumulation; however, at the same concentration (0.4 mM), oleate was a more potent inducer of intracellular TG accumulation in comparison to palmitate. Interestingly, whereas oleate exposure slightly increased TG levels in culture media after a 10-h exposure, palmitate exposure with the same concentration and duration led to a dramatic increment of extracellular TG levels(Figure 4(b)), suggesting that palmitate is a stronger promoter of hepatocyte TG secretion when compared with oleate.
Figure 4.
Palmitate promotes hepatocyte triglycerides secretion. AML12 cells were exposed with either palmitate (0.4 mM) or oleate (0.4 mM) for 10 h. Both intracellular TG (a) and extracellular TG concentrations were determined (b). All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05).
mTORC1 activation is required for palmitate-induced TG secretion in hepatocytes
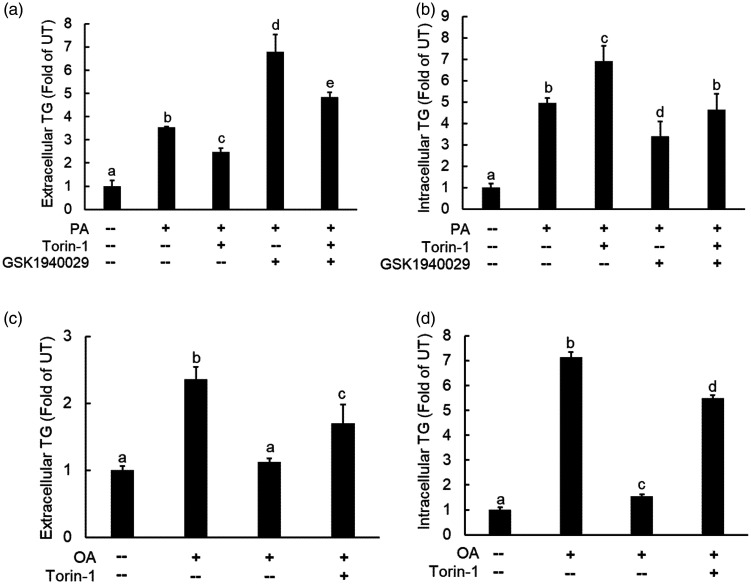
The differential regulation of palmitate and oleate on mTORC1 activation and TG secretion impelled us to hypothesize that the mTORC1 activation may contribute to incremental TG secretion under the circumstance of palmitate exposure. To test our hypothesis, we pretreated AML12 cells with torin-1 for 2 h before palmitate addition to the media and TG concentrations in the media were measured 10 h later. As shown in Figure 5(a), palmitate-induced extracellular TG increases were alleviated by mTORC1 inhibitor. Meanwhile, the pretreatment with GSK1940029 (an SCD-1 inhibitor), which strengthened palmitate-induced mTORC1 activation, as shown in Figure 2(b), aggravated palmitate-induced extracellular TG increment. On the other hand, palmitate exposure elevated intracellular TG levels, which was further increased by torin-1 pretreatment (Figure 5(b)). When same concentration of oleate was employed, the extracellular TG levels were increased to a much less extent when compared to that with palmitate, which was similarly attenuated by torin-1 pretreatment (Figure 5(c)). In contrast, torin-1 pretreatment alleviated oleate-induced intracellular TG levels (Figure 5(d)). These data altogether indicate that mTORC1 activation contributes to palmitate-triggered TG secretion from hepatocytes.
Figure 5.
mTORC1 activation is required for palmitate-induced TG secretion in hepatocytes. AML12 cells were pretreated with SCD-1 inhibitor, GSK1940029 (10 µM) for 2 h, followed by palmitate (0.4 mM) ± torin-1 (0.25 µM) addition. Both extracellular TG concentrations (a) and intracellular TG concentrations (b) were measured after a 10-h period. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05). (c and d) AML12 cells were pretreated with torin-1 (0.25 µM) for 2 h, followed by palmitate (0.4 mM) exposure. Both extracellular TG concentrations (c) and intracellular TG concentrations (d) were measured after a 10-h period. All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05).
mTORC1 induction contributes to palmitate-triggered IRE1α activation
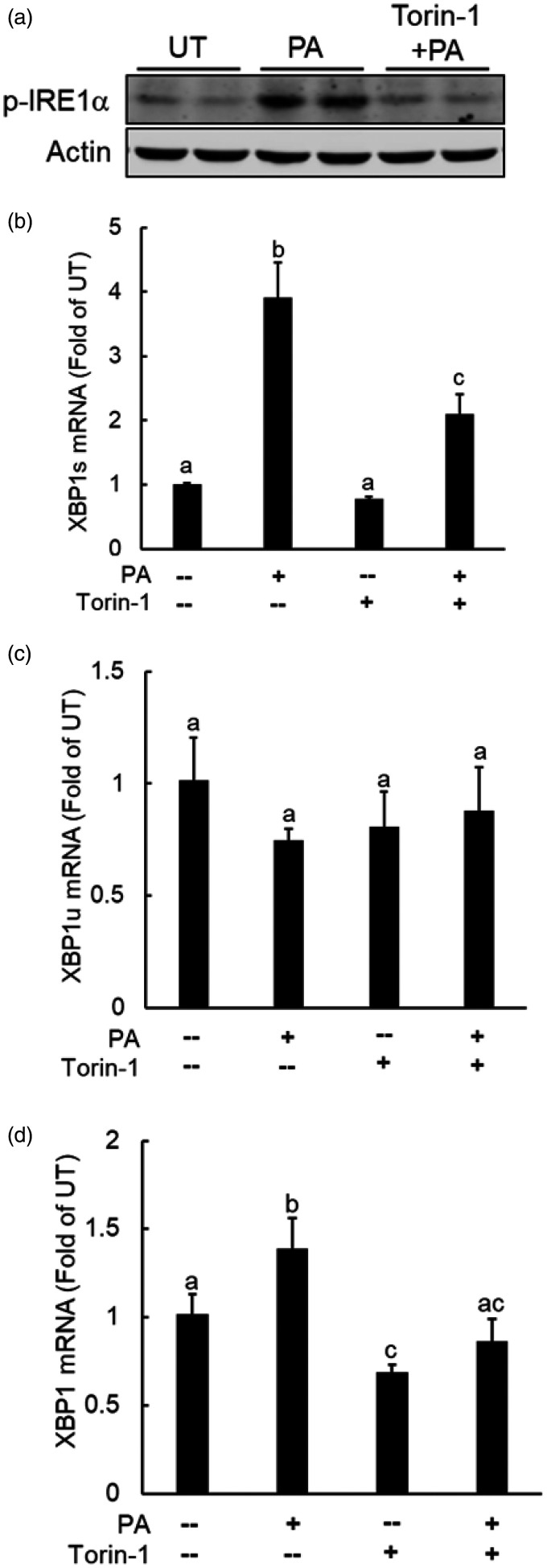
We previously reported the activation of IRE1α, one of three canonical UPR pathways, to be implicated in palmitate lipotoxicity in hepatoyctes.31 The stimulatory effect of palmitate on mTORC1, as observed in this study, prompted us to further examine the possible role of mTORC1 in palmitate-triggered IRE1α activation. To test this notion, we pretreated AML12 cells with torin-1 before palmitate exposure and IRE1α activation was determined via Western blotting determination of p-IRE1α protein abundance and real-time RT-PCR measurement of spliced XBP1 gene expressions. As shown in Figure 6(a), the hepatocytes exposed palmitate exhibited enhanced p-IRE1α protein abundance in comparison to untreated cells, which was ablated by torin-1 pretreatment. Palmitate exposure induced XBP1 splicing, evidenced by a robust increase of spliced XBP1 (XBP1s) gene expression (Figure 6(b)), while the un-spliced (XBP1u) and total XBP1 were either unaffected (Figure 6(c)) or slightly increased (Figure 6(d)) in response to palmitate exposure. Importantly, torin-1 pretreatment prevented palmitate-induced XBP1 splicing (Figure 6(b) to (d)). These data altogether indicate that mTORC1 induction is mechanistically involved in palmitate-instigated IRE1α activation in hepatocytes.
Figure 6.
mTORC1 activation contributes to palmitate-triggered IRE1α activation. AML12 cells were treated with 0.4 mM palmitate with/without a 2-h torin-1(0.25 µM) pretreatment for 16 h. IRE1α activation was determined by Western blot detection of p-IRE1α protein abundance (a) or XBP1 splicing by real time RT-PCR (b–d). All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05).
The mTORC1-IRE1α pathway activation contributes to palmitate-induced cell death and TG secretion in hepatocytes
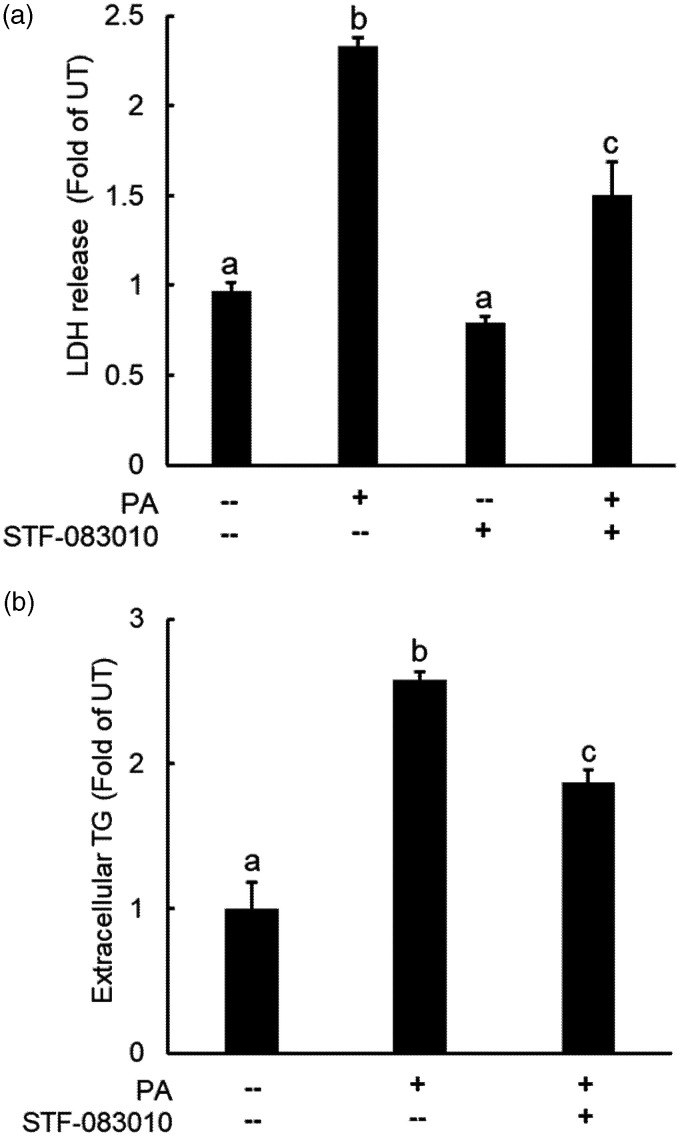
The critical roles of IRE1α activation in palmitate-induced cell death and TG secretion were subsequently determined via pretreating AML12 cells with STF-083010 (100 μM), a specific inhibitor of IRE1α, before palmitate addition. Extracellular LDH and TG levels were determined after 16-h and 10-h exposure period, respectively. As shown in Figure 7, IRE1α inhibition ameliorated both cell death (Figure 7(a)) and incremental TG secretion (Figure 7(b)) induced by palmitate exposure.
Figure 7.
IRE1α activation contributes to palmitate-induced cell death and TG overproduction in hepatocytes. (a) AML12 cells were pretreated with IRE1α inhibitor, STF083010 (50 µM), for 2 h before palmitate addition (0.4 mM). Cell death was determined 16 h later by measuring LDH release (a) and extracellular TG concentrations were determined 10 h later by a commercially available TG assay kit (b). All values are denoted as means ± SD from three or more independent experiments. Bars with different characters differ significantly (P < . 05).
Discussion
Oleate (18-C MUFA) and palmitate (16-C SFA) are the most abundant FFA found in human circulation. In this study, we have demonstrated that, unlike oleate, palmitate is a lipid nutrient inducer of mTORC1 in hepatocytes. Our data support that both metabolic conversation of palmitate to palmitoyl-CoA and the maintenance of its intracellular saturation status are required for the mTORC1-activating action. In comparison to the same quantities of oleate, exogenous palmitate exposure results in more TG secretion and induces hepatocyte cell death. Our further studies identify mTORC1 activation to be a molecular mechanism attributing to palmitate-induced incremental TG secretion and lipotoxic cell death in hepatocytes in that both changes induced by palmitate are ameliorated by mTORC1 inhibitors. Furthermore, our investigations uncover that IRE1α is a downstream target of palmitate-induced mTORC1 activation and IRE1α inhibition alleviates extracellular TG secretion and protects hepatocytes against palmitate-induced cell death. Taken together, our current study provides evidence that mTORC1-IRE1α signaling pathway activation contributes to palmitate-induced excessive TG secretion and lipotoxic cell death in hepatocytes.
A collection of metabolic abnormalities, including cellular dysfunction and/or cell death, resulting from the accumulation of lipid intermediates in non-adipose tissue is named as lipotoxicity. Lipotoxicity plays an important role in the pathogenesis of many metabolic disorders, including CVD, diabetes, and metabolic fatty liver diseases.1–3 The liver plays a central role in the regulation of intrahepatic and circulatory lipid homeostasis through very low-density lipoprotein (VLDL) production and secretion.9 Although much progress has been made over last several decades, the underlying cellular/molecular mechanisms behind lipotoxicity remain ambiguous in hepatocytes. It has been well-accepted that, unlike most unsaturated fatty acids, SFA are more harmful to cells, leading to not only cell death but also functional aberrations in a broad range of mammalian cell types, including hepatocytes.4–6 Palmitate is the most abundant SFA found in human circulation. Employing palmitate exposure of AML12 hepatocytes as an in vitro lipotoxicity model, we identified, via a small-scale screening of available chemical inhibitors, mTORC1 activation to be an important molecular mechanism underlying palmitate-induced lipotoxicity as mTOR inhibitors, torin1 (for both mTORC1 and mTORC2) and rapamycin (a selective inhibitor for mTORC1) ameliorated not only cell death but also incremental TG secretion in response to excessive palmitate exposure of hepatocytes. The comparable beneficial effects of these two inhibitors suggest that mTORC1 inhibition is the predominant contributor in the observed protective effect.
mTORC1 pathway integrates a wide range of extracellular and intracellular signals to regulate cell growth, anabolism, and protein synthesis. Both growth factors and nutrients are required for its activation.13,14 The mTORC1-activating effects of amino acids have been extensively studied and very well documented.32 Similarly, the activation of mTORC1 in response to glucose availability has also been widely reported, especially in the field of cancer research.33 Despite less investigated than other two macronutrients, palmitate has been reported to be a lipid activator of mTORC1 in podocytes.34 In line with these previous reports, we showed in this study that palmitate potently activated mTORC1 in hepatocytes, whereas oleate, an 18-C monounsaturated fatty acid, displayed incapability of doing so when the same quantity was employed. The metabolic conversation of palmitate to palmitoyl-CoA appears to be required for its stimulatory effect on mTORC1 activation as the inhibition of long fatty acyl CoA synthetase, the enzyme converting palmitate to palmitoyl-CoA, blunted mTORC1 activation upon palmitate exposure and protected hepatocytes against palmitate-induced cell death. On the other hand, the inhibition of stearoyl-CoA desaturase-1 (SCD-1), the enzyme to desaturate palmitate, strengthened palmitate-instigated mTORC1 activation, concomitant with aggravated palmitate-induced hepatocyte cell death and incremental TG secretion. These results altogether suggest that palmitate metabolism is required for its inductive effect on mTORC1 activation and resultant cytotoxic effects in hepatocytes.
ER stress represents a well-established mechanism implicating in palmitate‐induced lipotoxicity in a variety of cell types. Emerging evidence suggests that mTORC1-mediated signaling events and ER stress/UPR pathways are intertwined.23,24 A recent study using NRK-52E cells, a rat renal tubular epithelial cell line, showed that rapamycin protected against ER stress-induced cell death via specifically inhibiting IRE1α,24 suggesting an intimate link between mTORC1 activation and ER stress induction. We previously reported that IRE1α inhibition protected against palmitate-induced cell death in HepG2 cells, a human hepatoma cell line.31 In this study, our data unraveled that mTORC1 inhibitor mitigated palmitate-instigated IRE1α induction, suggesting that IRE1α is a downstream target of mTORC1 activation in response to palmitate exposure. Importantly, we showed that both palmitate-induced incremental TG secretion and increased cell death were ameliorated by IRE1α inhibition, providing evidence that mTORC1-IRE1α signaling pathway activation plays an important role in regulating palmitate-induced lipotoxic effects.
Although both palmitate- and oleate-induced extracellular TG increases were ameliorated by mTOR inhibition, torin-1 pretreatment aggravated palmitate-induced intracellular TG accumulation, whereas it attenuated oleate-induced TG increment in hepatocytes. The discrepant effects of mTOR inhibition on intracellular TG contents in response to different types of FFAs, palmitate and oleate in this study, are intriguing. It is noteworthy that the effects of mTORC1 on lipid metabolism in hepatocytes are multifaceted. Although mTORC1 activation is required for de novo lipogenesis via inducing SREBP-1c, a master regulator of de novo lipogenesis,35 the constitutive activation of mTORC1 in mouse liver is associated with decreased hepatic TG contents and increased VLDL secretion, due to a potential enhancement of phosphatidylcholine production.36,37 Furthermore, IRE1α activation has also been reported to promote TG secretion via activating microsomal triglyceride transfer protein activity.38 Therefore, it is plausible that, upon palmitate exposure, the activated mTORC1 and IRE1α in hepatocytes induce TG secretion both individually and cooperatively, while the basal mTORC1 activity is required for supporting de novo lipogenic process such as in the case of oleate exposure. Further investigation is warranted to clarify the discrepancy.
Given that excess palmitate exposure of hepatocytes gives rise to both cell death and TG overproduction, the likelihood exists that these two processes are mutually regulated. Increased cell death may lead to increased TG release into the cell culture media. On the other hand, TG/VLDL overproduction may conversely act as a cell death inducer. In the current study, the palmitate-induced TG increases in culture media were clearly observed (10-h palmitate treatment) before the occurrence of cell death, which usually takes place after a 10-h palmitate exposure, therefore it appears to be impossible that cell death contributes to observed incremental TG levels in the culture media in response to palmitate exposure. Similarly, the pretreatment of AML12 cells with CP346086, a selective chemical inhibitor of microsomal triglyceride transfer protein (MTTP), which is an essential component for hepatocyte VLDL secretion, failed to endow protection against palmitate toxicity (data not shown), excluding the possibility that TG overproduction is involved in palmitate-triggered cell death.
In conclusion, in this study we provide evidence that mTORC1-IRE1α signaling pathway activation in hepatocytes attributes to palmitate lipotoxicity. The critical involvement of mTORC1-IRE1α pathway activation in palmitate-induced TG secretion and cell injury in hepatocytes suggests the clinically beneficial potential via this pathway in the treatment of a variety of metabolic disorders. This is particularly tempting given that mTORC1 inhibitor rapamycin is clinically utilized for the treatment of certain cancers. However, as mTORC1 activity is essential for cell growth, proliferation, metabolism, and among others, inhibition mTORC1 by rapamycin has been reported to have adverse impact on the regenerative or repair process in organs and tissues, including the liver. Thus, further investigations will be necessary to clarify the underpinning mechanisms behind palmitate-induced mTORC1 activation as well as the critical link between mTORC1 and IRE1α activation in hepatocytes and to explore its clinical relevance.
Authors’ contributions
JW and YC designed and performed research, analyzed data, and wrote the original draft of the manuscript. QS and AG performed Western blot assay and edited the paper. ZS designed research, analyzed data, and edited the paper.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was in part funded by US NIH Grants NIAAA R21AA025363 (to Z. S.) and NIAAA R01AA026603 (to Z. S.).
ORCID iD
Zhenyuan Song https://orcid.org/0000-0001-8750-5762
References
- 1.Sletten AC, Peterson LR, Schaffer JE. Manifestations and mechanisms of myocardial lipotoxicity in obesity. J Intern Med 2018; 284:478–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svegliati-Baroni G, Pierantonelli I, Torquato P, Marinelli R, Ferreri C, Chatgilialoglu C, Bartolini D, Galli F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic Biol Med 2019; 144:293–309 [DOI] [PubMed] [Google Scholar]
- 3.Escasany E, Izquierdo-Lahuerta A, Medina-Gomez G. Underlying mechanisms of renal lipotoxicity in obesity. Nephron 2019; 143:28–32 [DOI] [PubMed] [Google Scholar]
- 4.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 2003; 52:726–33 [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Wang Q, Huang L, Dong H, Lin L, Lin N, Zheng F, Tan J. Palmitate causes endoplasmic reticulum stress and apoptosis in human mesenchymal stem cells: prevention by AMPK activator. Endocrinology 2012; 153:5275–84 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem 2009; 284:14809–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D. Camilo Me Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr 2002; 21:219–23 [DOI] [PubMed] [Google Scholar]
- 8.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003; 100:3077–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamba V, Rader DJ. Obesity and atherogenic dyslipidemia. Gastroenterology 2007; 132:2181–90 [DOI] [PubMed] [Google Scholar]
- 10.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28:1225–36 [DOI] [PubMed] [Google Scholar]
- 11.Bergouignan A, Momken I, Schoeller DA, Simon C, Blanc S. Metabolic fate of saturated and monounsaturated dietary fats: the mediterranean diet revisited from epidemiological evidence to cellular mechanisms. Prog Lipid Res 2009; 48:128–47 [DOI] [PubMed] [Google Scholar]
- 12.Tholstrup T, Sandstrom B, Bysted A, Holmer G. Effect of 6 dietary fatty acids on the postprandial lipid profile, plasma fatty acids, lipoprotein lipase, and cholesterol ester transfer activities in healthy young men. Am J Clin Nutr 2001; 73:198–208 [DOI] [PubMed] [Google Scholar]
- 13.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124:471–84 [DOI] [PubMed] [Google Scholar]
- 14.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016; 529:326–35 [DOI] [PubMed] [Google Scholar]
- 16.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334:1081–6 [DOI] [PubMed] [Google Scholar]
- 17.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 2006; 291:E275–81 [DOI] [PubMed] [Google Scholar]
- 18.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab 2010; 298:E1027–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem 2009; 284:26591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006; 313:1137–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 2007; 27:901–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 2009; 119:1201–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwood EA, Hofmann C, Santo Domingo M, Bilal AS, Sarakki A, Stauffer W, Arrieta A, Thuerauf DJ, Kolkhorst FW, Muller OJ, Jakobi T, Dieterich C, Katus HA, Doroudgar S, Glembotski CC. ATF6 regulates cardiac hypertrophy by transcriptional induction of the mTORC1 activator, RHEB. Circ Res 2019; 124:79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato H, Nakajima S, Saito Y, Takahashi S, Katoh R, Kitamura M. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ 2012; 19:310–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of akt. Mol Cell 2006; 24:185–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breuleux M, Klopfenstein M, Stephan C, Doughty CA, Barys L, Maira SM, Kwiatkowski D, Lane HA. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther 2009; 8:742–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee JH, Kwon CH, Lee KW, Lee JH, Park CK, Chung WJ, Hwang JS, Yan JJ, Song DK, Tsujimoto Y, Lee MS. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res 2008; 49:84–97 [DOI] [PubMed] [Google Scholar]
- 28.Shen C, Dou X, Ma Y, Ma W, Li S, Song Z. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr Res 2017; 40:40–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Z, Song M, Lee DY, Liu Y, Deaciuc IV, McClain CJ. Silymarin prevents palmitate-induced lipotoxicity in HepG2 cells: involvement of maintenance of Akt kinase activation. Basic Clin Pharmacol Toxicol 2007; 101:262–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uriarte SM, Joshi-Barve S, Song Z, Sahoo R, Obejishvili L, Jala VR, Haribabu B, McClain C, Barve S. Akt inhibition upregulates FasL, downregulates c-FLIPs and induces caspase-8-dependent cell death in jurkat T lymphocytes. Cell Death Differ 2005; 12:233–42 [DOI] [PubMed] [Google Scholar]
- 31.Shen C, Ma W, Ding L, Li S, Dou X, Song Z. The TLR4-IRE1alpha pathway activation contributes to palmitate-elicited lipotoxicity in hepatocytes. J Cell Mol Med 2018; 22:3572–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goberdhan DC, Wilson C, Harris AL. Amino acid sensing by mTORC1: intracellular transporters mark the spot. Cell Metab 2016; 23:580–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol 2013; 15:555–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuda M, Tanaka Y, Kume S, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim Biophys Acta 2014; 1842:1097–108 [DOI] [PubMed] [Google Scholar]
- 35.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011; 146:408–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn WJ, 3rd, Wan M, Shewale SV, Gelfer R, Rader DJ, Birnbaum MJ. Titchenell PM. mTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J Clin Invest 2017; 127:4207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts JL, He B, Erickson A, Moreau R. Improvement of mTORC1-driven overproduction of apoB-containing triacylglyceride-rich lipoproteins by short-chain fatty acids, 4-phenylbutyric acid and (R)-alpha-lipoic acid, in human hepatocellular carcinoma cells. Biochim Biophys Acta 2016; 1861:166–76 [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, Davidson NO, Kaufman RJ. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab 2012; 16:473–86 [DOI] [PMC free article] [PubMed] [Google Scholar]