Abstract
Background
Patients and clinicians need reliable, up‐to‐date information from randomised controlled trials (RCTs) on the costs and benefits of treatments. Recruitment difficulties arise when clinicians do not invite patients to participate in trials.
Objectives
Primary: to assess the evidence for the effect of disincentives and incentives on the extent to which clinicians invite eligible patients to participate in RCTs of healthcare interventions. Secondary: to assess the evidence in relation to stated willingness to invite participation.
Search methods
1. The Cochrane Methodology Register and Cochrane Database of Methodology Reviews were searched in May 2006 and Cochrane Central Register of Controlled Trials, National Research Register and ClinicalTrialsGov in April 2005. 2. EMBASE, MEDLINE, CINAHL, PsycINFO and AMED were searched in April 2005. 3. Reference lists of included studies were checked.
Selection criteria
Studies exploring the effect of (dis)incentives on clinicians' views and recruitment‐related activity.
Data collection and analysis
The information about included studies was insufficient for a full assessment of quality. Data on (dis)incentives were extracted and association with recruitment tested.
Main results
No RCTs of interventions were identified. Eleven observational studies were included ‐ two medical records reviews, one matched pair study, one clinician interview study, two studies documenting clinicians' decisions and five postal surveys. Three measures of recruitment were used, invitation to participate, entry into RCT and reported entry to RCT. Five studies explored the effect of patient characteristics. The effect of age and prognosis varied between trials. Six studies considered the association between clinicians' views and recruitment. Clinicians who agreed to participate because they were acquainted with the researchers were less likely to participate than those otherwise motivated (1 study, 2‐sided p = 0.04 Fisher's exact test) and (Odds Ratio [OR] 0.4, 95% Confidence Interval [CI] 0.2 to 0.9, 1 study). Clinicians who had recruited were more likely to report some difficulties including "trials involve extra work" (OR 92.94, 95% CI 4.54 ‐ 1902.11; p ≤ 0.01, 1 study) and "inviting patients to participate is embarrassing" (chi‐square 15.55, df = 1, p < 0.0001, 1 study). The effect of the need to discuss clinical uncertainty was unclear but concern that the doctor‐patient relationship would be adversely affected by participation was a deterrent (chi‐square = 7.25, df = 1, p = 0.007, 1 study).
Authors' conclusions
The impact of factors varied across studies. Researchers need to be aware that aspects of the design and conduct of trials can affect clinicians' willingness to invite patients to participate. Further research is needed.
Plain language summary
Incentives and disincentives to participation by clinicians in randomised controlled trials
Randomised controlled trials (RCTs) are needed to provide robust evidence of the relative efficacy and safety of treatments. In many RCTs, clinicians (i.e. healthcare professionals inviting patients to take part in an RCT in which they provide at least one of the interventions) only invite a small proportion of the people who are eligible for trials to take part. Observational studies have been conducted to explore reasons for this but the results do not identify any factors that appear to have a consistent impact on recruitment.
Background
Patients and clinicians need reliable, up‐to‐date information on the efficacy, safety and adverse effects of alternative treatments to be able to select the most appropriate therapies. Such evidence is required as soon as new treatments become available and before early impressions obscure clinical equipoise and reduce the likelihood that research results will influence practice. In one study less than half of the oncologists surveyed reported that they would rely on published data if it conflicted with their clinical experience (Fallowfield 1997).
According to the hierarchy of methods for evaluating treatment effects (Guyatt 1995), randomised controlled trials (RCTs) with definitive results should be the most robust way to compare treatments. However in practice many RCTs are abandoned (Easterbrook 1992) or do not produce unequivocal evidence because of difficulties with recruitment. A study of trials in the US, for example, found that a third recruited less than 75% of their planned sample thereby reducing the statistical power to detect any differences between interventions (Charlson 1984). A more recent study of trials in which no significant difference was found between interventions concluded that most cancer clinical comparisons with negative results are affected by poor accrual (Costa 2004). An overview of 13 trials that were sponsored by the National Heart Lung and Blood Institute found that enrolment was completed within the planned recruitment period in only 2 trials (Shea 1992). The effect of recruitment difficulties in reducing the timeliness and strength of evidence should not be underestimated. Collins et al suggest that delays in recruitment to the ISIS‐2 trial of treatment for acute myocardial infarction resulted in up to 10,000 unnecessary deaths of patients treated in routine practice before the trial results were available (Collins 1992).
In multicentre trials the researchers are often clinicians in routine clinical practice who recruit and follow up patients under their care. Recruitment difficulties arise when clinicians do not recruit or only recruit a small proportion of the patients who are eligible for an RCT. For each clinician invited to participate in a trial, the decision to accept or decline that invitation is likely to be based on an appraisal of the (real or perceived) availability of the necessary resources and of the anticipated costs and benefits of participation both personally and for patients. A survey exploring attitudes towards trials reported that 76% of oncologists thought that the reluctance of clinicians to participate was a greater obstacle to successful completion of a trial than the reluctance of patients (Fallowfield 1997). It is imperative therefore that the reasons behind this reluctance are understood so that attempts can be made to overcome them.
Many trial reports highlight a wide disparity between the number of clinicians who agree in principle to take part in a trial and the number that recruit participants. In the year following completion of a survey on trials, only 35% of clinicians who had agreed to take part in a trial had recruited any patients (Taylor 1994). One possible explanation for this is that when considering recruitment of a particular patient the disincentives become more salient, the incentives less attractive and/or additional disincentives are encountered or anticipated. The cost of equipping clinicians to take part in a trial will often consume a considerable proportion of a trial budget making it vital that the reasons for this disparity are identified.
A number of concerns about lack of resources and anticipated costs of participation have already been identified in a HTA report (Prescott 1999). The aim of that report was to assemble a comprehensive bibliography covering the diversity of factors that might limit the quality, number and progress of RCTs. The authors did not, therefore, distinguish between the prevalence of concerns and the impact of those concerns on the decision to enrol participants into a trial. However this distinction must be taken into account in any assessment of the reasons why clinicians do not invite patients to participate in trials. In one study 73% of clinicians agreed with the statement that "excessive time is required for patient follow‐up on study" but only 26% cited this as the reason they had not entered patients into trials (Benson 1991).
A broader review of randomised and quasi‐randomised trials of strategies to improve recruitment generally did not find any trials of interventions designed to increase the extent to which clinicians participated in trials (Mapstone 2007). In the absence of such studies, there is a need to consider other types of evidence for the effect of incentives and disincentives on clinicians' decisions to invite patients to participate in RCTs. This knowledge can then be used to increase recruitment into RCTs either by increasing incentives and decreasing disincentives for all clinicians or by identifying, and targeting resources towards, those clinicians whose appraisal of the status quo is sufficiently favourable for them to invite patients to participate.
Objectives
The primary objective is to assess the evidence for the effect of disincentives and incentives on the extent to which clinicians invite eligible patients to participate in randomised controlled trials of healthcare interventions. Where no evidence for the effect of an incentive or disincentive on clinicians' behaviour is identified, a secondary objective is to assess the evidence for the effect on the stated willingness of clinicians to recruit participants into randomised controlled trials of healthcare interventions.
Definition of terms to be used in the review:
(Dis)incentive: The term (dis)incentive will be used to denote "disincentives, incentives or both".
Recruitment: The focus of the review is on the role of clinicians in trials and, in keeping with other literature in this area, the term recruitment will be used to mean "offer patients the opportunity to take part in a trial".
Clinician: For this review, the term clinician will be taken to include any healthcare professional recruiting patients into RCTs in which they provide at least one of the interventions.
Methods
Criteria for considering studies for this review
Types of studies
Study designs for the primary objective:
1. Intervention Studies: Randomised trials of interventions designed to increase recruitment by increasing incentives or reducing disincentives. 2. Non‐randomised intervention studies: Studies investigating the effects of interventions designed to increase recruitment by increasing incentives or reducing disincentives. 3. Observational Studies: Studies investigating the relationship between perceived levels of (dis)incentives and recruitment to RCTs.
Study designs for the secondary objective:
1. Intervention Studies: Randomised trials of interventions designed to increase clinicians' intentions to recruit by reducing disincentives or increasing incentives. 2. Non‐randomised intervention studies: Studies investigating the effects of interventions designed to increase incentives or reduce disincentives on clinicians' intentions to recruit. 3. Observational studies: Studies investigating the relationship between perceived levels of (dis)incentives and intention to recruit to RCTs.
Types of data
Data from studies with a pre‐stated aim to explore the factors that affect clinicians' decisions to recruit patients into randomised controlled trials and which report empirical data on clinician (dis)incentives.
Studies should relate to recruitment to randomised trials of healthcare interventions in which the clinician who invited patients to participate was responsible for providing at least one of the interventions and for the continuing care of the patient.
The unit of randomisation into the healthcare RCTs should be individual patients. Healthcare RCTs which used cluster randomisation to allocate patients to different forms of care will therefore be excluded.
Study designs which incorporate a short pre‐randomisation phase during which tolerability is tested or investigations are required to check eligibility will be included. For studies in which recruitment is to a non‐randomised study with the possibility of subsequent entry to a randomised trial only data relating to recruitment to the randomised phase will be included.
Types of methods
Any interventions, including those designed to increase recruitment and surveys comparing clinicians' recruitment behaviours (or, for the secondary objective, recruitment intentions) with their perceptions of incentives and disincentives.
Types of outcome measures
For the primary objective: The recruitment of patients into RCTs.
Search methods for identification of studies
The Cochrane Methodology Register and Cochrane Database of Methodology Reviews were searched in May 2006 and Cochrane Central Register of Controlled Trials, National Research Register and ClinicalTrialsGov in April 2005. EMBASE, MEDLINE, CINAHL, PsycINFO and AMED were searched in April 2005. Reference lists of included studies and of reviews of relevant studies were checked. For the full search strategy, see appendices (Appendix 1; Appendix 2; Appendix 3).
Data collection and analysis
Identification of studies
The titles and, where available, abstracts of all studies identified by the searches were checked by one reviewer (JMR) and any that did not refer to recruitment to randomised controlled trials by clinicians were excluded. The remaining studies were checked independently by two reviewers (JMR and RKM) to identify those that meet the inclusion criteria. Copies of all papers that appeared relevant or could not be excluded from available information were obtained. Copies of appropriate review papers were obtained at this stage and the reference lists were checked. The final selection of papers for inclusion was made by agreement between the two reviewers. The selection of studies is illustrated using a flow chart as recommended by the University of York Centre for Research and Dissemination (CRD Report 4 2001) (Figure 1).
1.
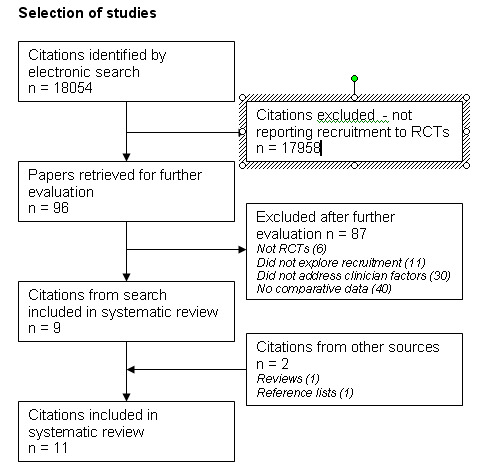
Selection of studies.
Assessment of methodological quality
For each study of recruitment, aspects of the design, conduct and analysis that could affect the internal or external validity of the results were considered. Aspects included selection of participating clinicians, completeness of reporting of results, the possibility of measurement bias (including recall bias), the handling of confounding factors, the number of cases and for cohort studies, loss to follow up.
Data extraction
A selection of papers that met the inclusion criteria was used to develop a data collection form based on the list of (dis)incentives identified by the HTA report (Prescott 1999). Two reviewers extracted data independently. Data extracted included the design of the study, details of the RCT for which recruitment was being studied and evidence for the effect of (dis)incentives.
Presentation of results
The evidence from observational studies is likely to be less reliable than that from randomised intervention studies because of the increased potential for bias. These results have not therefore been treated as evidence for the impact of (dis)incentives but may be used to:
inform the design of future studies of (dis)incentives
identify any erroneous beliefs that appear to limit recruitment
highlight areas where recruitment might be increased by training.
It was not possible to investigate publication bias (or other biases relating to small studies) using Funnel Plots.
Analysis
For cross tabulations chi‐square has been calculated except for analyses where the total sample size was less than 20 and those where the sample size was less than 40 with one or more of the expected values less than 5. For these analyses Fisher's exact test was used. For two studies (de Wit 2001; Siminoff 2000) which did not report raw data, the odds ratios included in the papers have been reported in this review. For continuous data the weighted mean differences have been calculated. Analysis of data from the matched pair study (Kemeny 2003) used Liddell's exact test.
Results
Description of studies
Eighteen thousand and fifty four references were identified by the electronic search after removal of duplicates (see Figure 1). Ninety six studies were selected for consideration by two authors of which eleven met the criteria for inclusion in the review. The searches did not identify any intervention studies.
Eleven observational studies reporting comparisons between the views of clinicians or clinician/patient characteristics and a measure of recruitment met the inclusion criteria for the primary objective (Antman 1985; de Wit 2001; Kemeny 2003; Kuyvenhoven 1997; Lee 1980; Richardson 2002; Siminoff 2000; Simon 1999; Simon 2004; Taylor 1984; Wilson 2000). All trials were conducted in Western settings and were initiated in response to concerns about low recruitment rates in past or current trials. Four (Antman 1985; Kemeny 2003; Simon 1999; Simon 2004) looked for the presence of selection bias based on demographic and disease‐related factors.
Five studies compared characteristics of patients offered participation in trials of treatment for cancer with those of patients not invited to take part (Antman 1985; Kemeny 2003; Lee 1980; Simon 1999; Simon 2004). Three (Kemeny 2003; Simon 1999; Simon 2004) looked at RCTs of treatment for breast cancer, one (Antman 1985) for sarcoma and one (Lee 1980) for lung cancer. Three were retrospective studies: Antman 1985 and Lee 1980 reviewed the medical records of patients who met the eligibility criteria for a trial and Kemeny 2003 used a matched pair design to investigate the effect of patient age on the clinician's decision to offer participation. The two studies by Simon et al. (Simon 1999; Simon 2004) were prospective studies in which clinicians completed a questionnaire related to their treatment recommendation for each new patient with a diagnosis of breast cancer.
Six studies compared clinicians' perceptions about trials with their recruitment activity.
Four studies related to trials carried out in primary care settings (de Wit 2001; Kuyvenhoven 1997; Richardson 2002; Wilson 2000). One study reported recruitment to a combined cohort study/RCT of treatment for dyspepsia in primary care (de Wit 2001). Participants who were recruited to the cohort study could subsequently be invited to participate in an RCT. The study was a retrospective comparison of the views of clinicians who had recruited less than 2 participants into the RCT with those of clinicians who recruited 2 or more. The other three studies in primary care all compared those who recruited at least one patient with those who did not recruit. Kuyvenhoven 1997 studied recruitment to a trial of treatment for sore throats and Richardson 2002 management of dyspepsia. Wilson 2000 considered recruitment to trials conducted by a research network. All were postal surveys conducted prior to (Kuyvenhoven 1997) or during (Richardson 2002; Wilson 2000) the recruitment phase of the trial.
The remaining studies were both of trials for breast cancer (Siminoff 2000; Taylor 1984). Taylor 1984 involved a retrospective postal survey of the principal investigators for an RCT and compared the views of clinicians who reported having recruited all eligible patients with those who reported recruiting some or none. Siminoff 2000 involved retrospective interviews with clinicians which focused on the medical records of four patients who had been eligible to participate in a trial. This study considered the factors that influenced the decision to offer or not offer participation but did not report how clinicians were classified as recruiters or non‐recruiters.
Risk of bias in included studies
None of the studies reported sufficient information to allow full assessment of quality but some general observations can be made. Nearly all studies reported comparisons between a number of (dis)incentives and a measure of recruitment activity but no allowance was made for multiple comparisons in the assessment of significant differences.
Medical records reviews
For the two studies involving retrospective reviews of medical records (Antman 1985; Lee 1980) there is the possibility that relevant information was not recorded by the clinician.
Questionnaire based studies
For all surveys, the possibility that respondents see non‐recruitment as failure must be considered. Where this is the case responses may be affected by self‐serving bias (Miller 1975) such that respondents are more likely to attribute non‐recruitment to the failures of others ‐ e.g. the researchers who designed the study badly rather than to their own shortcomings ‐ e.g. personal difficulty with initiating discussion about RCTs.
In the two prospective studies (Simon 1999; Simon 2004) it is possible that recruitment decisions were affected by the requirement to complete the questionnaire. Neither study reported recruitment rates prior to or after the study to allow assessment of this. None of other five postal surveys (de Wit 2001; Kuyvenhoven 1997; Richardson 2002; Taylor 1984; Wilson 2000) used an existing validated questionnaire. A particular concern in questionnaire development is the possibility of confirmatory bias (Nickerson 1998) arising from a positive test strategy (Klayman 1987) in which researchers only explore issues that they believe to be relevant. The results then appear to confirm this belief. In the absence of information about the way in which (dis)incentives were selected for inclusion, it is not possible to gauge whether the results were subject to this bias.
In the four studies in which clinicians' views on (dis)incentives were sought during or after the recruitment period of the trial (de Wit 2001; Richardson 2002; Taylor 1984; Wilson 2000) there is the possibility that responses were affected by recall bias (Sackett 1979).
Four studies (de Wit 2001; Kuyvenhoven 1997; Richardson 2002; Wilson 2000) compared clinicians' views with the number of patients recruited rather than the proportion of eligible patients. It is possible therefore that differences in recruitment arose from differences in casemix.
Response rates for the questionnaires were generally high with only one below 50% (42% Wilson 2000), one over 75% (78% de Wit 2001) and three 90% or over (90% Kuyvenhoven 1997, 97% Richardson 2002 and Taylor 1984) but no details were given of the extent of missing data within returned questionnaires or how this was handled.
Interview‐based study
In the study by Siminoff 2000 the interview focused on the factors that clinicians took into account when deciding whether to discuss RCTs with four patients. The interviews were retrospective and responses may therefore have been affected by recall bias (Sackett 1979). It was not clear how the selection of patients was made or how recruitment was assessed.
Matched pair design
The study by Kemeny 2003 reported a number of confounding factors ‐ patients in the matched pairs differed according to marital status, retirement status and functional limitations and co‐morbidity. Data were reported for only 67/77 (87%) matched pairs because recruitment data for at least one of the remaining 10 pairs was missing.
Effect of methods
Notes
For Wilson (Wilson 2000) the recruitment status of 45 (of 636 respondents) was not reported. In converting the percentages reported in the text into numbers the total number of respondents was taken to be 591.
For Taylor (Taylor 1984) the categories recruitment of "all" and recruitment of "some but not all" eligible patients have been combined and compared to reported non‐recruitment.
de Wit 2001(de Wit 2001) reported data related to participation in a cohort study and in a randomised trial, only the latter data have been included
General views on Research
Orientation towards research
Taylor et al. used clinicians' responses to open questions to classify them as being orientated either towards a clinical, pragmatic approach to their clinical practice based on personal experience or towards a scientific approach relying on published evidence. Only 2 of the 12 clinicians classed as taking a pragmatic approach reported having recruited patients compared to the 57 of the other 79 respondents (2‐sided p = 0.0004 Fisher's exact test) (Taylor 1984). An association between interest in research and recruitment was also found by Wilson. Three hundred and sixteen of the 424 clinicians who reported "wishing to learn more about research" recruited compared to 106/167 of those who did not report this (chi‐square 7.17, df = 1, p = 0.007; 591 participants) (Wilson 2000). However previous research experience was not found to be associated with recruitment. Clinicians who reported previous experience were no more likely to have recruited at least 2 participants into the RCT than those without experience (Odds Ratio [OR] 1.5, 95% Confidence Interval (CI) 0.6 to 3.6; 128 participants) (de Wit 2001).
The study by Siminoff reported that surgeons who use trial results were no more likely to recruit (OR 1.20, 95% CI 0.46 ‐ 3.12; ns, 107 participants) and that oncologists who used results were less likely to recruit (OR 0.06, 95% CI 0.004 ‐ 1.01; p ≤ 0.05, 40 participants). There was no difference in recruitment rates of oncologists according to agreement with the statement that clinicians could "gain knowledge by trial participation" (OR 2.13, 95% CI 0.21‐22.10; ns, 40 participants) nor were surgeons who disagreed with randomisation significantly less likely to recruit than those did not disagree (OR 1.14, 95% CI 0.23‐ 5.56; ns, 107 participants) (Siminoff 2000).
Reasons for agreeing to participate
Three studies considered associations between the factors that motivated clinicians to agree to participate in a trial and their subsequent recruitment activity. Only one factor, participation of the academic research group, was found to be positively associated with recruitment. Clinicians who ranked the participation of the academic research group as one of the top three (out of eight) reasons for participating in the combined study were more likely to have recruited at least 2 participants into the RCT than those who did not (OR 2.2, 95% CI 1.0 to 4.8; 128 participants) (de Wit 2001).
For two factors there was a negative association with recruitment. Four of the 8 clinicians whose decision to participate was based on "personal acquaintance with the researchers or having a colleague who had decided to participate" recruited to the trial compared to 49 of the 58 other respondents. Those motivated by professional relationships were less likely to recruit (2‐sided p = 0.05 Fisher's exact test [data not reported for 5 respondents]) (Kuyvenhoven 1997). Clinicians who ranked the personal appeal by the research group as one of the top three (of eight) reasons for participating in the study were less likely to have recruited at least 2 participants into the RCT than those who did not (OR 0.4, 95% CI 0.2 to 0.9; 128 participants) (de Wit 2001).
No effect was found for a further five factors. There was no difference in recruitment into the RCT between those who ranked professional obligation as one of the top three (of eight) reasons for participating in the study and those who did not (OR 1.7, 95% CI 0.7 to 3.7; 128 participants (de Wit 2001). There was no difference in recruitment into the RCT between those who rated the participation of the sponsor (a pharmaceutical company) (OR 3.1, 95% CI 0.7 to 14.7; 128 participants) or the participation of a clinical research organisation (OR 2.8, 95% CI 0.6 to 13.4; 128 participants) in the top 3 out of 8 reasons for participating in the combined study (de Wit 2001). There was no difference between those involved in Continuing Medical Education or College of Family Physicians activities and those who were not in recruitment of at least 2 participants into the RCT (OR 2.1, 95% CI 0.9 to 5.0; 128 participants) (de Wit 2001). Of 444 clinicians who "wished to support their Royal College" 324 recruited compared to 98/147 of the other respondents. Those wishing to support their College were no more likely to have recruited than those who did not report this (chi‐square 2.15, df = 1, p = 0.14; 591 participants) (Wilson 2000).
Trial specific factors
Two studies considered factors that were related to specific trials.
The clinical question
No difference in recruitment was found according to views about the research topic and trial interventions. There was no difference in recruitment into the RCT between those who ranked research topic as one of the top three (out of eight) reasons for participating in the combined study and those who did not (OR 1.0, 95% CI 0.5 to 2.2; 128 participants) (de Wit 2001). No significant difference was found between recruitment rates of clinicians who thought that there was already an established treatment and those who did not (surgeons (OR 2.87, 95% CI 0.73‐11.35; ns, 107 participants and oncologists OR 0.17, 95% CI 0.01‐2.30; ns, 40 participants) (Siminoff 2000).
No difference was seen in recruitment rates between oncologists who stated that they did not know the best combination of drugs and those who did not express this uncertainty (OR 1.13, 95% CI 0.25 ‐ 5.22; ns, 40 participants) (Siminoff 2000).
The protocol
Oncologists who thought that trial entry requirements were too stringent were less likely to have recruited than those who did not (OR 0.01, 95% CI 0.00005‐ 0.62; p ≤ 0.05, 40 participants) but no difference was found for this comparison for surgeons (OR 1.29, 95% CI 0.22‐7.68; ns, 107 participants) (Siminoff 2000)
Practical Considerations
The evidence for the effect of clinicians' views of practical aspects of participating in trials was mixed. In the study by Siminoff, oncologists who considered that "trials involve extra work" were more likely to have recruited (OR 92.94, 95% CI 4.54 ‐ 1902.11; p ≤ 0.01, 40 participants) but those who thought that paperwork is too time consuming were less likely to have done so (OR 0.001, 95% CI 0.00002 ‐ 0.06; p ≤ 0.01, 40 participants). Recruitment was not affected by surgeons' views on practical aspects ‐ extra work (OR 1.58, 95% CI 0.42 ‐ 5.94; ns, 107 participants) and paperwork (OR 0.56, 95% CI 0.15 ‐ 2.16; ns, 107 participants) (Siminoff 2000). Two of the 6 clinicians who referred to practical difficulties associated with the trial reported having recruited compared to 57 of the other 85 respondents. No significant difference in reported recruitment was between those who referred to practical difficulties and those who did not (2‐sided p = 0.18 Fisher's exact test) (Taylor 1984).
Support staff
There was some evidence for an association between recruitment and having the staff available to help with trials. Twenty six of the 46 clinicians who had assistance with the trial from a practice nurse recruited compared to 16 of the 49 who did not have help. Those with nurse assistance were more likely to recruit (chi‐square = 5.48, df = 1, p = 0.024) (Richardson 2002). In contrast oncologists who reported that they had "no staff support available" were not less likely to have recruited than those who did not report this (OR 4.99, 95% CI 0.64‐38.63; ns, 40 participants) (Siminoff 2000).
Financial considerations
Reimbursement for time spent on recruitment was not associated with recruitment. Clinicians who ranked the per patient reimbursement given for time taken to complete the research protocol as one of the top three (of 8) reasons for participating in the combined study were no more likely to have recruited at least 2 participants into the RCT than those who did not (OR 2.0, 95% CI 0.6 to 6.4; 128 participants) (de Wit 2001).
Recruitment was associated with the belief that trials were expensive. Oncologists who had recruited were more likely than those who had not to consider that trials were expensive (OR 33.66, 95% CI 1.61‐705.12; p ≤ 0.05, 40 participants) (Siminoff 2000).
Patient factors
Five studies investigated the effect of the patient factors, both demographic and disease‐related, on recruitment.
Age
Patient age was found to affect recruitment in some studies but not in others. Two studies reported that the mean age of participants invited to participate was lower than that of participants not invited but the difference was only significant for the larger study (Simon 2004). For the smaller study the mean age of the 48 patients invited to participate was 43.7 (standard deviation [sd] 18.7) compared to 50.80 (sd 22.40) for the 75 not invited (Weighted Mean Difference [WMD] ‐7.10, 95% CI ‐14.43 to 0.23, p = 0.06, 123 participants) (Simon 1999). For the other study the mean ages were 51.60 (sd 11.50) for the 106 invited and 56.90 (sd 14.00) for the 213 not invited (WMD ‐5.30, 95% CI ‐8.19 to ‐ 2.41, p = 0.0003; 317 participants) (Simon 2004). Siminoff et al. reported that the older a patient was the less likely she was to be referred (p ≤ 0.05) (Siminoff 2000).
In contrast Antman et al. found no difference in the likelihood of being invited to participate between the 44 patients under the age of 50 and the 46 patients aged 50 or over (chi‐square 0.68, df = 1, p = 0.41; 90 participants) (Antman 1985) and Kemeny et al. found no difference on the same measure for matched pairs of patients in which a patient under the age of 65 (mean age 48) was matched with a patient aged 65 or over (mean age 74) (Relative Risk [RR] 2.11, 95% CI 0.91 to 5.30; 2‐sided p = 0.09 Liddell test for matched pairs, 134 participants) (Kemeny 2003).
Gender
Two studies found no evidence that gender affected recruitment by clinicians ‐ Antman 1985 (M:F = 44:46, chi‐square 0.68, df = 1, p = 0.41; 90 participants) and Lee 1980 (M:F = 62:15, chi‐square = 0.835, df = 1, p = 0.36; 77 participants).
Disease factors
Four studies considered the effect of factors related to disease type and severity.
Two studies by Simon et al. found associations between disease stage and likelihood of being invited to participate. Simon et al. presented data for recruitment according to whether a patient had a lower (I, II or III) or higher (IV) stage disease. Forty three of the 94 patients with lower disease status were recruited compared to only 5 of the 29 with higher stage disease (chi‐square = 7.57, df = 1, p = 0.0059; 123 participants) (Simon 1999). Analysis using data for four disease stages found an association between disease stage and the likelihood of being invited to participate. Fourteen out of 74 patients with stage I disease were invited to participate compared to 49/99 stage II, 9/33 stage III and 28/86 stage IV (chi‐square = 18.76, df = 3, p = 0.0003; 292 participants [disease stage was not known for 27 participants]) but there was no linear trend (chi‐square for linear trend p = 0.426, df = 1, p = 0.514). Pairwise chi‐square tests found patients with stage II disease were more likely to be invited to participate than those with stage I (p < 0.0001), stage III (p = 0.026) or stage IV (p = 0.020) disease (Simon 2004). This study also found that patients were more likely to be invited to participate if their performance status (a measure of the extent to which a patient is physically able to perform the activities of daily living) (Oken 1982) was higher (0, I or II) rather than lower (III or IV) (chi‐square = 8.74, df = 1, p = 0.003; 288 participants [status not reported for 31 participants]).
Two studies found no association. The study by Antman et al. found that the proportion of patients invited to participate did not vary according to the site of the sarcoma classified as extremity, visceral or trunk/retroperitoneal (chi‐square = 5.13, df = 2, p = 0.0768; 90 participants), disease stage (IIB, IIIA, IIIB or IIIC/IVA (chi‐square = 2.32, df = 1, p = 0.509; 90 participants) or size of tumour (< 5cm, 5‐10cm or >10cm) (chi‐square = 0.70, df = 2, p = 0.703; 90 participants) (Antman 1985). No difference in recruitment rate was identified according to favourability of prognosis (5‐point scale) for either surgeons (OR 1.05, 95% CI 0.65 ‐ 1.69; ns, 107 participants) or oncologists (OR 1.11, 95% CI 0.67 ‐ 1.85; ns, 40 participants) (Siminoff 2000).
Ethnicity
Only one of the three studies that considered the impact of ethnicity on recruitment found a significant effect. Simon et al (Simon 2004) found that white patients were more likely to be invited to participate than black or other patients (chi‐square 13.01, df = 2, p = 0.0015; 293 participants [ethnicity was not recorded for 26 participants]). The invitation to participate did not differ according to ethnicity (white versus black) (chi‐square = 1.43, df = 1, p = 0.23; 77 participants) (Lee 1980). The proportion of patients invited to participate did not differ according to ethnicity (white/other versus black) (chi‐square = 0.00, df = 1, p = 1.0; 124 participants) (Simon 1999).
During the consultation
Four studies considered the relationship between clinicians' views on several components of the recruitment consultation and their level of recruitment. Twenty nine of the 72 clinicians who reported that they found recruitment difficult had recruited compared to 12 of the 18 who did not express difficulty. Those expressing difficulty were less likely to have recruited (chi‐square 4.04, df = 1, p = 0.04; 90 participants) (Richardson 2002).
Initiating discussion of trials
There was some evidence for an association between viewing the prospect of initiating discussion favourably and recruitment. Both surgeons and oncologists were less likely to have recruited if they thought there was a "lack of patient interest" (surgeons OR 0.1004, 95% CI 0.02 ‐ 0.49; p ≤ 0.01, 107 participants and oncologists OR 0.04, 95% CI 0.003 ‐ 0.65; p ≤ 0.05, 40 participants) (Siminoff 2000).
Clinicians who reported that the strong doctor‐patient relationship that exists in general practice either assisted or had no effect on recruitment were more likely to have recruited than those who did not report this (chi‐square 44.35, df = 1. p < 0.0001; 591 participants) (Wilson 2000).
Siminoff found that surgeons who reported feeling "comfortable explaining trials" to patients were more likely to have recruited than those who did not feel comfortable (OR 6.08, 95% CI 2.14 ‐ 17.28; p<=0.01, 107 participants). For oncologists the difference was not significant (OR 5.05, 95% CI 0.85 ‐ 29.91; ns, 40 participants) (Siminoff 2000).
However clinicians who reported having been "too embarrassed to ask" were more likely to have recruited than those who did not report this (chi‐square 15.55, df = 1, p < 0.0001; 591 participants) (Wilson 2000).
Informed Consent
Sixteen of the 25 clinicians who stated that "obtaining informed consent was an arduous task" reported recruiting compared to 43 of the other 66 respondents. No significant difference in reported recruitment was found between those who expressed this concern and those who did not (chi‐square 0.01, df = 1, p = 0.92) (Taylor 1984).
Discussing Uncertainty
Seven of the fifteen clinicians who expressed "difficulty in telling patients that they did not know which operation was better" recruited compared to 52 of the other 76 respondents. No significant difference in reported recruitment was found between those who expressed this difficulty and those who did not (chi‐square = 2.60, df = 1, p = 0.11) (Taylor 1984).
Oncologists who agreed that they had "difficulty in explaining medical uncertainty" were significantly more likely to have recruited than those who did not agree (OR 384.44, 95% CI 4.31‐34320.29; p ≤ 0.01, 40 participants) but there was no difference in recruitment by surgeons (OR 3.03, 95% CI 0.61‐15.15; ns, 107 participants) (Siminoff 2000).
Anticipated outcome of recruitment
Two studies reported the effect of clinician's views on the impact of recruitment the continuing patient care. Siminoff investigated the belief that treatments offered as part of the trial might benefit patients. No difference was found for agreement with the statement that trials enable clinicians to "give patients choice of treatment" (surgeons OR 0.44, 95% CI 0.08 ‐ 2.42; ns, 107 participants) or for agreement that trials "provide best, most current treatment" (surgeons OR 0.43, 95% CI 0.15 ‐ 1.24; ns, 107 participants and oncologists OR 1.75, 95% CI 0.39 ‐ 7.90; ns, 40 participants) (Siminoff 2000). The study also explored the effect of beliefs about the impact of trials on clinical freedom. Clinicians who reported fearing "loss of control over a patient's care" were no less likely to have recruited than who did not ‐ surgeons: (OR 0.28, 95% CI 0.05 ‐ 1.52; ns, 107 participants) and oncologists (OR 1.52, 95% CI 0.04 ‐ 54.21; ns, 40 participants) (Siminoff 2000).
Twenty‐five of the 48 clinicians who expressed concern that recruitment would have an adverse effect on the doctor‐patient relationship recruited compared to 34 of the other 43 respondents. Those expressing concern were less likely to report having recruited (chi‐square = 7.25, df = 1, p = 0.007) (Taylor 1984).
Concern that randomisation might not select the treatment subsequently shown to be optimal deterred some clinicians from recruiting eligible patients. None of the 5 clinicians who expressed the fear that they would feel "personally responsible if one of the treatments should be found to be more successful that the other" reported recruiting compared to 59 of the other 86 respondents. Those expressing this fear were less likely to report having recruited (2‐sided p = 0.004 Fisher's exact test) (Taylor 1984).
Discussion
The search did not identify any randomised studies of interventions to increase recruitment into healthcare RCTs by clinicians. The most striking feature of the results is the degree of variation between the included studies. In part, this may be an artefact of the way in which potential (dis)incentives were described in different studies. However this variation was observed even with clearly defined factors such as age and disease status. A number of reasons can be postulated to explain this. The recruitment period covered by the healthcare RCTs spans nearly a quarter of a century from 1974 (Lee 1980) to 1998 (Richardson 2002) during which time there have been major changes in the nature and delivery of medical care and in the legal and ethical regulations governing the conduct of trials. Another possible cause of heterogeneity is the difference between the diseases and interventions being studied. It is possible, for instance, that clinicians are less willing to consider random allocation when the interventions are very different or when one or more of the treatments cannot be reversed such as surgery. If this is the case it may mean that at least some (dis)incentives are confined to particular types of trial. In addition to this, three different measurements of recruitment activity were used, invitation to participate, actual recruitment and reported recruitment. The measure most relevant to this review is invitation to participate in a trial. It is possible that studies measuring actual recruitment may not have identified significant associations between clinicians' views about (dis)incentives and their attempts to recruit because the number of patients recruited is likely to be less than the number invited to participate. Reported recruitment has been shown to lead to overestimates of activity (Benjamin 2000; Taylor 1994) but it is unclear whether the degree of overestimation varies according to views about (dis)incentives or indeed actual recruitment rates.
A number of methodological limitations of the included studies need to be taken into account in the interpretation of the results of this review:
1. Most studies only report a small proportion of the data collected and therefore it is possible that there was some reporting bias in favour of results that were significant or were in accordance with researchers' understanding of the role of (dis)incentives.
2. The rationale for all the studies identified was concern about low recruitment rates and/or selection bias. It is therefore not possible to assess whether trials which meet or exceed recruitment targets are inherently different in terms of (dis)incentives or whether the success is due to the employment of effective strategies to lessen the impact of disincentives and increase that of incentives.
3. In all studies the sample size was determined by the number of clinicians belonging to a network or participating in specific trials and by response rates. They were not, therefore, powered to detect small differences in factors that could be easily modified and lead to worthwhile improvements in recruitment rates.
The limitations of the studies and heterogeneity of the designs mean that it is difficult to summarize the results concisely. However, a few findings are worthy of note. Two studies found a negative association between rating personal acquaintance with the researchers as a reason for agreeing to participate in an RCT and subsequent recruitment activity. This is perhaps a counterintuitive and surprising finding and it implies that researchers need to be alert to the possibility that some known clinicians may feel obliged to sign up to a trial without necessarily being motivated to recruit. Views on the relevance of the research topic or the nature of the interventions were not found to be associated with recruitment. The studies explored three areas which could offer an explanation for the high proportion of clinicians who agree to participate in an RCT but do not recruit ‐ characteristics of individual patients, difficulties with the process of inviting a patient to participate, obtaining informed consent etc and the anticipation that recruiting a patient will have adverse consequences for the patient. In all three areas there was some evidence that clinicians' behaviours were affected by these concerns. The strongest evidence was for the deterrent effect of the fear that recruitment would have an adverse effect on the doctor‐patient relationship.
The finding by Siminoff et al. of an association between holding the views that trials involve extra work and are costly and the level of recruitment to the trial highlights the difficulty of interpreting the results of cross‐sectional studies. One explanation of this finding is that only when clinicians actively recruit do they become aware of the time and costs associated with recruitment (Siminoff 2000).
Authors' conclusions
Implication for methodological research.
Further research is needed into the effect of potential disincentives and incentives on recruitment by clinicians. Interventions designed to increase the extent to which clinicians invite patients to participate in RCTs should be evaluated in well‐designed randomised trials. Possible interventions include ways to facilitate discussions between clinicians and patients about uncertainty, randomisation and informed consent. Studies comparing the views of clinicians with their recruitment activity should ensure that views are measured prior to the start of recruitment. Reports of RCTs of healthcare interventions should report recruitment rates in more details to allow exploration of differences between RCTs with high and low recruitment rates could also provide valuable information.
What's new
| Date | Event | Description |
|---|---|---|
| 27 December 2007 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 20 February 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Heather Pretty and Sarah Stockton, Information Scientists at the Centre for Evidence Based Mental Health for assistance in developing the search strategy for the review and for conducting several of the database searches.
Appendices
Appendix 1. NRR and ClinicalTrialsGov search strategy
The National Research Register (NRR) and ClinicalTrialsGov were searched in April 2005 using the following strategy: 1. trial* and (accrual or recruit* or participat*) 2. (barrier* or disincent* or incent* or obstacle*) 3. 1 or 2
Appendix 2. CMR, CDMR and CENTRAL search strategy
The Cochrane Methodology Register (CMR), Cochrane Database of Methodology Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), were searched in April 2006 using:
#1 (trial* or study or studies or research or rct*) and (accrual or enlist* or enrol* or enter* or entry or join or joins or joining or participat* or recruit*) in All Fields in all products #2 barrier* or disincent* or incent* or obstacle* in All Fields in all products #3 (#1 OR #2) #4 difficult* or problem* or deter or deters or deterrent or discourage* or adverse* or impediment or failure or impede or attitude* or decision* or process* or strateg* or reason* or factor* or benefit* or willing* or ready or able or readiness or agree* or consent or permission or assent or volunteer* or permit* or choose or choice or chose or commitment or committed or accept or acceptance or nonacceptance or offer or offers or offering or offered or facilitat* or motivate* or incentive* or maximise* or technique* or enhance or selection or preselection or improve or improves or improved or improving or increase* or eligible or eligibility or refus* or decline* or coerce or unwilling* or discourage* or reluctan* or decreas* or decreasing in All Fields in all products #5 trial* or study or studies or research or rct* in All Fields in all products #6 clinician* or physician* or practitioner* or ((general or family) near practic*) or (family near doctor*) or GP* in All Fields in all products #7 (#4 AND #5 AND #6) #8(#3 OR #7)
Appendix 3. EMBASE, MEDLINE, CINAHL, PsycINFO and AMED search strategy
EMBASE (1980‐2005), MEDLINE (1966‐April 2005), CINAHL (1982‐April 2005), PsycINFO (1872‐April 2005) and AMED (1985‐April 2005) were searched using the following strategy:
Interface ‐ DIALOG Datastar
Part #1 Recruitment: 1.PATIENT‐SELECTION#.DE. 2.RANDOM‐ALLOCATION#.DE. 3.PATIENT‐PARTICIPATION#.DE. 4.(ACCRUAL$ OR RECRUIT$ OR PARTICIPAT$ OR ENLIST$ OR ENROL$ OR NONPARTICIPAT$ OR REFER).TI,AB. 5.(BARRIER$ OR INCENTIVE$ OR DISINCENTIVE$).TI,AB. 6.SAMPLE‐SIZE#.DE. 7.MOTIVATION#.W..DE. 8.1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 Physicians: 9.PHYSICIANS#.W..DE. 10.(CLINICIAN$1 OR PHYSICIAN$ OR PRACTITIONER$ OR (GENERAL OR FAMILY) ADJ PRACTICE$ OR FAMILY DOCTOR$ OR GP$).TI,AB. 11.9 OR 10 RCTs: 12.CLINICAL‐TRIALS#.W..DE. 13.CONTROLLED‐CLINICAL‐TRIALS#.W..DE. 14.CROSS‐OVER‐STUDIES#.W..DE. 15.DOUBLE‐BLIND‐METHOD#.W..DE. 16.PLACEBOS#.W..DE. 17.RANDOM‐ALLOCATION#.W..DE. 18.RANDOMIZED‐CONTROLLED‐TRIALS#.W..DE. 19.SINGLE‐BLIND‐METHOD#.W..DE. 20.PT=CLINICAL‐TRIAL$ 21.PT=CONTROLLED‐CLINICAL‐TRIAL 22.PT=RANDOMIZED‐CONTROLLED‐TRIAL 23.12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 24.CLINICAL ADJ TRIAL$ 25.SINGL$ OR DOUBL$ OR TREBL$ OR TRIPL$ 26.BLIND$ OR MASK$ OR DUMMY 27.25 NEAR 26 28.PLACEBO$ 29.RANDOM$ 30.23 OR 24 OR 27 OR 28 OR 29 31.ANIMAL=YES 32.HUMAN=YES 33.31 NOT (31 AND 32) 34.30 NOT 33 Recruitment, Physicians & RCTs 35.8 AND 11 AND 34
Part #2 Recruitment: 36.(DIFFICULT$ OR PROBLEM$ OR OBSTACLE$ OR BARRIER$).TI,AB. 37.(DETER OR DETERS OR DETERRENT OR DISCOURAG$ OR ADVERSE$ OR IMPEDIMENT OR FAILURE OR IMPEDE).TI,AB. 38.(ATTITUDE$ OR DECISION$ OR PROCESS$ OR STRATEG$ OR REASON$ OR FACTOR$ OR INCENTIVE$ OR BENEFIT$).TI,AB. 39.(WILLING$ OR READY OR ABLE OR READINESS OR AGREE$ OR CONSENT OR PERMISSION OR ASSENT OR VOLUNTEER$ OR PERMIT$ OR CHOOSE OR CHOICE OR CHOSE).TI,AB. 40.(COMMITMENT OR COMMITTED OR ACCEPT OR ACCEPTANCE OR NONACCEPTANCE OR OFFER OR OFFERS OR OFFERING OR OFFERED).TI,AB. 41.(FACILITAT$ OR MOTIVAT$ OR INCENTIVE$ OR MAXIMISE$ OR TECHNIQUE$ OR ENHANC$) NEAR (JOIN OR JOINS OR JOINING OR JOINED OR ENTER OR ENTERS OR ENTERED OR ENTRY).TI,AB. 42.(SELECTION OR PRESELECTION OR IMPROVE OR IMPROVES OR IMPROVED OR IMPROVING OR INCREAS$ OR ELIGIBLE OR ELIGIBILITY).TI,AB. 43.((REFUS$ OR DECLIN$ OR COERCE OR UNWILLING$ OR DISCOURAG$ OR RELUCTAN$ OR DECREAS$ OR DECREASING).TI,AB. 44.36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 45.(JOIN OR JOINS OR JOINING OR ENTER OR ENTERS OR ENTERED OR ENTRY).TI,AB. 46.44 NEAR 45 Physicians 47.11 (as above) RCTs 48.(TRIAL$ OR STUDY OR STUDIES OR RESEARCH OR RCTS).TI,AB. Recruitment, Physicians & RCT 49.(46 NEAR 48) AND 47
Result set from Parts 1 or 2: 50.35 OR 49
Data and analyses
Comparison 1. Age of participant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Invited to participate | 2 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐5.54 [‐8.23, ‐2.85] |
| 1.1 Simon 1999 | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐14.43, 0.23] |
| 1.2 Simon 2004 | 1 | 317 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐8.19, ‐2.41] |
1.1. Analysis.
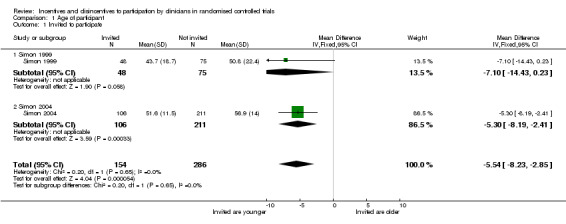
Comparison 1 Age of participant, Outcome 1 Invited to participate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antman 1985.
| Methods | Method: Retrospective review of the medical records of patients presenting with sarcoma who fulfilled the criteria for participation in an RCT. Patients with sarcoma were identified from a registry. Setting: Sarcoma services in Boston, Massachusetts in 1987. RCT: Adjuvant chemotherapy for sarcoma. | |
| Data | Recruitment data: Clinician's decision to invite or not invite individual patients to participate in the trial. (Dis)incentive data: Demographic and prognostic factors. | |
| Comparisons | Comparison of patients invited to participate with those not invited to particiapte according to age (dichotomised as under 50 versus 50 and over). | |
| Outcomes | The decision of the clinician to invite or not invite individual patients to participate in the trial. | |
| Notes | Six clinicians (2 medical oncologists, 2 surgeons and 2 radiotherapists) were included in the study on recruitment. 90 patients met the entry criteria for entry into the trial of whom 66/90 (73%) were invited by their clinician to participate and 42/90 (47%) were recruited. The study also reported data on the numbers of invited patients who agreed to participate, the choice of treatment for non randomised patients and compared the disease‐free survival time of trial participants with that of non‐paricipants. This data is not covered in the review. | |
de Wit 2001.
| Methods | Method: Postal survey sent to family physicians who had participated in a combined randomised clinical trial/cohort study. Survey sent five months after completion of RCT. Setting: Family practices in the Netherlands in 1996‐8. RCT: Pharmacological treatment for dyspesia. | |
| Data | Recruitment data: Recruitment to RCT. (Dis)incentive data: Demographic data and motivational factors. Clinicians were presented with a list of 8 motivational factors and asked to select the 3 they considered to be most important. | |
| Comparisons | Comparison of demographic and motivational factors with level of recruitment. | |
| Outcomes | Recruitment to the RCT. The number of patients recruited per clinician was dichotomised with the cut off point at the 25th percentile of inclusion: 0 or 1 patients versus 2 or more. | |
| Notes | No details of the development of the questionnaire. Response rate 128/165 (78%). 527 patients were entered into the clinical trial. | |
Kemeny 2003.
| Methods | Method: Retrospective case matched pair study in which older patients who were eligible for a trial were matched for disease stage and date of diagnosis with a younger patient also eligible for the trial and under the care of the same clinician. Setting: The 10 institutions in an American Cancer and Leukaemia Group which had the highest rate of recruitment to breast cancer trials c. 2003. RCT: Trials of treatment for breast cancer. | |
| Data | Recruitment data: Clinician's decision to invite or not invite individual patients to participate in the trial. (Dis)incentive data: Age of patient. | |
| Comparisons | Comparison of patients invited to participate with those not invited to participate according to age (dichotomised as under 65 versus 65 and over). | |
| Outcomes | The decision of the clinician to invite or not invite individual patients to participate in the trial. | |
| Notes | Age data reported for both patients for 67/77 (87%) matched pairs Confounding factors: Older patients had more comorbid conditions and were more likely to be widowed retired and to have functional limitations. | |
Kuyvenhoven 1997.
| Methods | Method: Postal survey sent to general practitioners (GPs) who expressed interest in participating in the RCT. Setting: Network of 450 GPs active in education or reseach in c. 1997. RCT: Treatment of patients presenting with sore throats. | |
| Data | Recruitment data: Recruitment to RCT. (Dis)incentive data: Personal and practice of characteristics of clinicians. | |
| Comparisons | Comparison of personal and practice characteristics of clincians with recruitment. | |
| Outcomes | Recruitment to the RCT. | |
| Notes | No details of the development of the questionnaire. Descriptive data only reported for most factors. Response rate 93/107 (90%). 71 GPs agreed to participate in the trial of whom 17 (24%) did not recruit. | |
Lee 1980.
| Methods | Method: Retrospective review of the medical records of patients meeting the histopathology criteria for inclusion in an RCT. Setting: Radiology institute in St. Louis, Missouri. 1974 ‐ 8. RCT: Trial of radiotherapy for lung cancer. | |
| Data | Recruitment data: Clinicians' decision to invite or not invite individual patients to participate in the trial. (Dis)incentive data: Demographic factors. | |
| Comparisons | Comparison of demographic and prognostic factors of patients invited to participate in the trial with those who were eligible for the trial but not invited. | |
| Outcomes | The decision of the clinician to invite or not invite individual patients to participate in the trial. | |
| Notes | Twelve radiotherapists were included in the study on recruitment . 77 patients met the criteria for entry into the trial of whom 44/77 (57%) were invited by their clinician to participate and 41/77 (53%) were recruited. | |
Richardson 2002.
| Methods | Method: Retrospective postal survey sent to general practitioners (GPs) who agreed to recruit patients into an RCT in primary care. Setting: General Practice in New Zealand in c. 2000. RCT: Effect of addition of H. pylori testing to usual care for management of dyspespsia. | |
| Data | Recruitment : Recruitment to RCT. (Dis)incentive data: Demographic and practice characteristics of clincians. | |
| Comparisons | Comparison of personal and practice characteristics of clinicians with recruitment. | |
| Outcomes | Recruitment of at least one patient to the RCT. | |
| Notes | No details of the development of the questionnaire. Descriptive data only reported for most factors. Response rate 95/98 (97%). 71 GPs agreed to participate in the trial of whom fewer than half recruited. | |
Siminoff 2000.
| Methods | Method: Face‐to‐face interviews probing the criteria that clinicians used when making decisions about treatment. For each interviewee, researchers focused on four recent breast cancer patients who were eligible for the trial. Setting: Cancer services in Pennsylvania between 1993 and 1995. RCT: Adjuvant treatment for breast cancer. | |
| Data | Recruitment data: Referral of patient to RCT. (Dis) incentive data: Clinician demographics and attitudes to the trial. | |
| Comparisons | Comparison of clinician demographics and attitudes with referral to RCT. | |
| Outcomes | Recruitment of the four patients. | |
| Notes | Clinicians: 107 surgeons who had patients who were eligible to participate plus 40 oncologists to whom the patients were referred. 93/245 (38%) of eligible patient entered the trial. It was not clear whether recruiters were those who recruited some or all of the four eligible patients. | |
Simon 1999.
| Methods | Method: Survey completed for new breast cancer patients presenting at the practices of 4 oncologists. Setting: Clinical breast cancer service in Detroit, USA in 1994. RCT: Ongoing trials of treatment for breast cancer. | |
| Data | Recruitment data: Clinician's decision to invite or not invite individual patients to participate in the trial. Disincentive data: Age, race and disease status of patients. | |
| Comparisons | Comparison of patients invited to participate with those not invited to particiapte. | |
| Outcomes | The decision of the clinician to invite or not invite individual patients to participate in the trial. | |
| Notes | Surveys were completed for 136/161 (84%) of new patients. | |
Simon 2004.
| Methods | Methods: Survey completed for new breast cancer patients conducted in the practices of 11 oncologists. Setting: Academic faculty of the Karmanos Cancer Institute in Detroit, USA in 1996/7. RCT: Ongoing trials of treatment for breast cancer. | |
| Data | Recruitment data: Clinician's decision to invite or not invite individual patients to participate in the trial. (Dis)incentive data: Patient demographics and clinical data. | |
| Comparisons | Comparison of patients invited to participate with those not invited to particiapte. | |
| Outcomes | The decision of the clinician to invite or not invite individual patients to participate in the trial. | |
| Notes | Surveys completed for 319/344 (93%) of new patients of whom 106 (33%) were invited to participate. | |
Taylor 1984.
| Methods | Method: Postal questionnaire sent to the Principal Investigators at trial centres. Setting: Centres involved in the National Surgical Adjuvant Project for Breast and Bowel Cancer (NSAPB) in the USA and Canada. February 1980. RCT. Primary treatment for breast cancer. | |
| Data | Recruitment data: Respondents report of the proportion of patients entered into the trial. (Dis)incentive data: Views on the effect of the need for informed on recruitment and responses to open questions about obstacles to recruitment. | |
| Comparisons | Comparison of responses to open questions and reported recruitment. | |
| Outcomes | Reported recruitment by each Principal investigator of all, some or none of the eligible patients under his care. | |
| Notes | Questionnaire was pre‐tested. Response rate 91/94 (97%). | |
Wilson 2000.
| Methods | Methods: Postal survey sent to general practitioners (GPs) who had agreed to participate in recent or current research projects. Setting: 5 state based Research and Health Promotion Units of the Royal Australian College of General Practitioners. RCT: Trials in primary care. | |
| Data | Recruitment data: Recruitment of patients into trials. (Dis)incentive data: Reasons for being involved in research and factors affecting recruitment. | |
| Comparisons | Comparison of recruitment with motivation to participate and percieved difficulties. | |
| Outcomes | Recruitment of at least one patient into a trial. | |
| Notes | Response rate 636/1518 (41.9%) of whom 45 did not report recruitment status. Of the 591 for whom recruitment status was known 422(71%) clincians had recruited to the trial. | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hjorth 1996 | Comparison of views of principal investigators at centres participating in trials of adjuvant therapy for myeloma with recruitment. Excluded because recruitment was measured for the centre not for the responding principal investigator. |
| Winn 1984 | Explores clinicians stated willingness to recruit to hypothetical trials. No direct measure of recruitment to actual trials. |
Contributions of authors
JR identified studies and extracted data in collaboration with RM and wrote the first draft of the review. All three reviewers collaborated on the final version.
Sources of support
Internal sources
Department of Psychiatry, University of Oxford, UK.
External sources
No sources of support supplied
Declarations of interest
Jennifer Rendell, John Geddes and Rowena Merritt are not aware of any potential conflicts of interest.
Unchanged
References
References to studies included in this review
Antman 1985 {published data only}
- Antman K, Amato D, Wood W, Carson J, Suit H, Proppe K, Carey R, Greenberger J, Wilson R, Frei E, III. Selection bias in clinical trials. J. Clin. Oncol. 1985;3(8):1142‐1147. [DOI] [PubMed] [Google Scholar]
de Wit 2001 {published data only}
- Wit NJ, Quartero AO, Zuithoff AP, Numans ME. Participation and successful patient recruitment in primary care. J.Fam.Pract. 2001;50(11):976. [PubMed] [Google Scholar]
Kemeny 2003 {published data only}
- Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, Bartlett N, Fleming G, Cohen HJ. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 2003;21(12):2268‐2275. [DOI] [PubMed] [Google Scholar]
Kuyvenhoven 1997 {published data only}
- Kuyvenhoven M, Dagnilie C, Melker R. Recruitment of general practitioners and patients in a sore throat study (Letter). Br.J.Gen.Pract 1997; Vol. 47, issue 415:126‐127. [PMC free article] [PubMed]
Lee 1980 {published data only}
- Lee JY, Marks JE, Simpson JR. Recruitment of patients to cooperative group clinical trials. Cancer Clin.Trials 1980;3(4):381‐384. [PubMed] [Google Scholar]
Richardson 2002 {published data only}
- Richardson A, Sutherland M, Wells E, Toop L, Plumridge L. Factors affecting general practitioner involvement in a randomised controlled trial in primary care. New Zealand Medical Journal 2002;115(1151):153‐155. [PubMed] [Google Scholar]
Siminoff 2000 {published data only}
- Siminoff LA, Zhang A, Colabianchi N, Sturm CM, Shen Q. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. Journal of Clinical Oncology 2000;18(6):1203‐1211. [DOI] [PubMed] [Google Scholar]
Simon 1999 {published data only}
- Simon MS, Brown DR, Du W, LoRusso P, Kellogg CM. Accrual to breast cancer clinical trials at a university‐affiliated hospital in metropolitan Detroit. Am J Clin Oncol 1999;22(1):42‐46. [DOI] [PubMed] [Google Scholar]
Simon 2004 {published data only}
- Simon MS, Du W, Flaherty L, Philip PA, LoRusso P, Miree C, Smith D, Brown DR. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol 2004;22(11):2046‐2052. [DOI] [PubMed] [Google Scholar]
Taylor 1984 {published data only}
- Taylor KM. The doctor's dilemma: physician participation in randomized clinical trials. Cancer Treatment Reports 1985;69(10):1095‐1100. [PubMed] [Google Scholar]
- Taylor KM, Margolese RG, Soskolne CL. Physicians' reasons for not entering eligible patients in a randomized clinical trial of surgery for breast cancer. New England Journal of Medicine 1984;310(21):1363‐1367. [DOI] [PubMed] [Google Scholar]
Wilson 2000 {published data only}
- Wilson I, McGrath B, Russell G, Bridges‐Webb C, Hogan C. General practitioners' views on patient care research. Australian Family Physician 2000;29(1):86‐88. [PubMed] [Google Scholar]
References to studies excluded from this review
Hjorth 1996 {published data only}
- Hjorth M, Holmberg E, Rodjer S, Taube A, Westin J. Physicians' attitudes toward clinical trials and their relationship to patient accrual in a Nordic multicenter study on myeloma. Controlled Clinical Trials 1996;17(5):372‐386. [DOI] [PubMed] [Google Scholar]
Winn 1984 {published data only}
- Winn RJ, Miransky J, Kerner JF, Kennelly L, Michaelson RA, Sturgeon SR. An evaluation of physician determinants in the referral of patients for cancer clinical trials in the community setting. Advances in cancer control: Epidemiology and research 1984;156:62‐73. [PubMed] [Google Scholar]
Additional references
Benjamin 2000
- Benjamin S, Kroll ME, Cartwright RA, Clough JV, Gorst DW, Proctor SJ, Ross JR, Taylor PR, Wheatley K, Whittaker JA, Stiller CA. Haematologists' approaches to the management of adolescents and young adults with acute leukaemia. British Journal of Haematology 2000;111(4):1045‐50. [DOI] [PubMed] [Google Scholar]
Benson 1991
- Benson AB, III, Pregler JP, Bean JA, Rademaker AW, Eshler B, Anderson K. Oncologists' reluctance to accrue patients onto clinical trials: an Illinois Cancer Center study. Journal of Clinical Oncology 1991;9(11):2067‐2075. [DOI] [PubMed] [Google Scholar]
Charlson 1984
- Charlson ME, Horwitz RI. Applying results of randomised trials to clinical practice: impact of losses before randomisation. BMJ 1984;289(6454):1281‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1992
- Collins R, Doll R, Peto R. Ethics in clinical trials. In: Williams CJ editor(s). Introducing New Treatments For Cancer: Practical, Ethical and Legal Problems. Chichester: John Wiley, 1992:49‐56. [Google Scholar]
Costa 2004
- Costa LJ, Xavier AC, Giglio A. Negative results in cancer clinical trials‐equivalence or poor accrual?. Controlled Clinical Trials 2004;25(5):525‐533. [DOI] [PubMed] [Google Scholar]
CRD Report 4 2001
- Centre for Research and Dissemination, University of York. Undertaking Systematic Reviews of Research on Effectiveness. http://www.york.ac.uk/inst/crd/report4.htm (accessed 28th February 2005).
Easterbrook 1992
- Easterbrook PJ, Matthews DR. Fate of research studies. Journal of the Royal Society of Medicine 1992;85(2):71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallowfield 1997
- Fallowfield LJ, Ratcliffe D, Souhami R. Clinicians' Attitudes to Clinical Trials of Cancer Therapy. European Journal of Cancer 1997;33(13):2221‐2229. [DOI] [PubMed] [Google Scholar]
Guyatt 1995
- Guyatt G, Sackett D, Sinclair J, Hayward R, Cook D, Cook R. Users' Guide to Medical Literature 9. A method for grading health‐care recommendations. JAMA 1995;274:1800‐1804. [DOI] [PubMed] [Google Scholar]
Klayman 1987
- Klayman J. Confirmation, disconfirmation and information in hypothesis testing.. Psychological Review 1987;94(2):211‐228. [Google Scholar]
Mapstone 2007
- Mapstone J, Elbourne D, Roberts I. Strategies to improve recruitment to research studies. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000013.pub3] [DOI] [PubMed] [Google Scholar]
Miller 1975
- Miller DT, Ross M. Self‐serving biases in the attribution of causality: Fact or fiction?. Psychological Bulletin 1975;82:213‐225. [Google Scholar]
Nickerson 1998
- Nickerson RS. Confirmation bias: A ubiquitous phenomenon in many guises.. Review of general psychology 1998;2:175‐220. [Google Scholar]
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET. Toxicity And Response Criteria Of The Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5:649‐655. [PubMed] [Google Scholar]
Prescott 1999
- Prescott R, Counsell C, Gillespie W, Grant A, Russell I, Kiauka S, Colthart IR, Ross S, Shepherd SM, Russell D. Factors that limit the quality, number and progress of randomised controlled trials. Health Technology Assessment 1999;3(20):1‐139. [PubMed] [Google Scholar]
Sackett 1979
- Sackett DL. Bias in analytical research. Journal of Chronic Diseases 1979;32:51‐63. [DOI] [PubMed] [Google Scholar]
Shea 1992
- Shea S, Bigger JT, Jr, Campion J, Fleiss JL, Rolnitzky LM, Schron E, Gorkin L, Handshaw K, Kinney MR, Branyon M. Enrollment in clinical trials: institutional factors affecting enrollment in the cardiac arrhythmia suppression trial (CAST). Controlled Clinical Trials 1992;13(6):466‐486. [DOI] [PubMed] [Google Scholar]
Taylor 1994
- Taylor KM, Feldstein ML, Skeel RT, Pandya KJ, Ng P, Carbone PP. Fundamental dilemmas of the randomized clinical trial process: results of a survey of the 1,737 Eastern Cooperative Oncology Group investigators. Journal of Clinical Oncology 1994;12(9):1796‐1805. [DOI] [PubMed] [Google Scholar]


