Supplemental Digital Content is available in the text.
Keywords: 2019-nCoV, D-dimer, severe COVID-19, mortality
Abstract
The 2019 novel coronavirus, declared a pandemic, has infected 2.6 million people as of April 27, 2020, and has resulted in the death of 181,938 people. d-dimer is an important prognostic tool, is often elevated in patients with severe coronavirus disease-19 (COVID-19) infection and in those who suffered death. In this systematic review, we aimed to investigate the prognostic role of d-dimer in COVID-19-infected patients. We searched PubMed, Medline, Embase, Ovid, and Cochrane for studies reporting admission d-dimer levels in COVID-19 patients and its effect on mortality. Eighteen studies (16 retrospective and 2 prospective) with a total of 3682 patients met the inclusion criteria. The pooled weighted mean difference (WMD) demonstrated significantly elevated d-dimer levels in patients who died versus those who survived (WMD, 6.13 mg/L; 95% confidence interval [CI] 4.16–8.11; P < 0.001). Similarly, the pooled mean d-dimer levels were significantly elevated in patients with severe COVID-19 infection (WMD, 0.54 mg/L; 95% CI 0.28–0.80; P < 0.001). The risk of mortality was fourfold higher in patients with positive d-dimer versus negative d-dimer (risk ratio, 4.11; 95% CI, 2.48–6.84; P < 0.001) and the risk of developing severe disease was twofold higher in patients with positive d-dimer levels versus negative d-dimer (risk ratio, 2.04; 95% CI, 1.34–3.11; P < 0.001). Our meta-analysis demonstrates that patients with COVID-19 infection presenting with elevated d-dimer levels have an increased risk of severe disease and mortality.
The 2019 novel coronavirus (2019-nCoV) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first originated in late 2019 in Wuhan, China, and has since caused a substantial impact on mankind.1 As of April 24, 2020, 2.6 million individuals have been infected with SARS-CoV-2 in 213 countries worldwide and 181,938 lives have been lost.2 On December 31, 2019, China reported the outbreak to the World Health Organization (WHO). Subsequently, WHO officially declared the coronavirus disease 2019 (COVID-19) epidemic as a public health emergency of international concern.3 The clinical features of COVID-19 vary from asymptomatic cases to severe infection, causing acute respiratory distress syndrome (ARDS), multisystem organ dysfunction, and death.4
There is uncertainty regarding the case fatality rate (CFR) of COVID-19 infection. The overall CFR for COVID-19 was reported at about 2% in China and 7.2% in Italy (likely due to the higher mean age of the overall population in the latter).5 The CFR is very high in patients with severe COVID-19 infection, as high as 50% or more in patients admitted to the intensive care unit (ICU).6 Due to high mortality in critically-ill COVID-19 patients, the detection of biomarkers that may help identify at-risk patients earlier in their course of illness becomes crucial. d-dimer is a biomarker that has emerged as an important prognostic tool, with findings of elevated levels in critically-ill patients and those deceased. In this systematic review, we aimed to investigate the prognostic role of admission d-dimer levels in patients hospitalized with COVID-19.
METHODS
Search Strategy
The reporting of this systematic review and meta-analysis complies with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines (Supplement Table 1, http://links.lww.com/CIR/A21).7
The initial search strategy was developed by 2 authors (S.S. and S.P.). We performed a systematic search, without language restriction, using PubMed, EMBASE, SCOPUS, Google Scholar, and 2 preprint servers (https://www.medrxiv.org and https://www.ssrn.com/index.cfm/en/coronavirus) from inception to April 16, 2020, for studies that reported d-dimer levels in COVID-19 patients. We utilized the “related articles” function in PubMed to find relevant articles that were missed by the initial search. Also, reference lists of the included studies were hand-searched to further locate relevant articles that were missed in the primary search. We used the following keywords and medical subject heading: “COVID-19,” “SARS-CoV-2,” “Wuhan coronavirus,” “Coronavirus 2019,” “2019 n-CoV,” “d-dimer,” “laboratory.”
Study Selection and Data Extraction
To be included in our systematic review and meta-analysis, the study had to fulfill the following criteria: (1) reported d-dimer levels in COVID-19 patients according to severity or including mortality as a clinical outcome; (2) included human subjects; and (3) studies in the English language. Single-arm studies, case reports, editorials, or systematic reviews were excluded. Two investigators (S.S. and S.P.) independently performed the literature search and screened all titles and full-text versions of all relevant studies that met study inclusion criteria.
The data from included studies were extracted using a standardized protocol and a data extraction form. Any discrepancies between the 2 investigators were resolved through consultation with the senior investigator (J.G.). Two independent reviewers (S.S. and S.P.) extracted the following data from the eligible studies: author name, study design, publication year, follow-up duration, number of patients, age, gender, diabetes mellitus (DM), hypertension, coronary artery disease (CAD), acute cardiac injury, arrhythmias, shock, and outcomes. The Newcastle Ottawa Risk bias assessment tool was used to appraise the quality of the included studies (Supplement Table 2, http://links.lww.com/CIR/A21).
OUTCOMES
Clinical Outcomes
The primary outcome of interest in our study was all-cause mortality and severity of COVID-19.
STATISTICAL ANALYSIS
For binary data, the Mantel-Haenszel (MH) risk ratio (RR) random-effects model (DerSimonian and Laird method) was used to summarize data between the 2 groups.8 For continuous data (e.g., d-dimer), studies that reported as the median and interquartile range, we first used the Wan method to estimate the mean and standard deviations.9 We then calculated the pooled weighted difference in means (WMD) using a random-effects model to evaluate the association of levels of d-dimer between the 2 groups. Higgins I-squared (I2) statistic was used to assess the test of heterogeneity. A value of I2 of 0%–25% represented insignificant heterogeneity, 26%–50% represented low heterogeneity, 51%–75% represented moderate heterogeneity, and more than 75% represented high heterogeneity.10 A prespecified random-effects metaregression analysis was conducted for the primary outcome in relation to the baseline demographics, comorbid conditions, biomarkers to test the relationship between d-dimer and disease severity, and all-cause mortality. Publication bias was formally assessed using funnel plots and Egger’s linear regression test of funnel plot asymmetry. A 2-tailed P < 0.05 was considered statistically significant. Statistical analysis was performed using Comprehensive Meta-Analysis version 3.0 (Biostat Solutions, Inc. [BSSI], Frederick, MD).
RESULTS
Search Results
A total of 920 citations were identified during the initial search (Fig. 1). Nine hundred and two records were excluded. After a detailed evaluation of these studies, 12 studies met the inclusion criteria. We also included 6 manuscripts from 2 preprint servers (https://www.medrxiv.org and https://www.ssrn.com/index.cfm/en/coronavirus), to accommodate the rapidly evolving nature of information for COVID. We acknowledge that the manuscripts from these 2 sources are not peer reviewed. Eighteen articles of 3682 patients were included in the final analysis.
FIGURE 1.
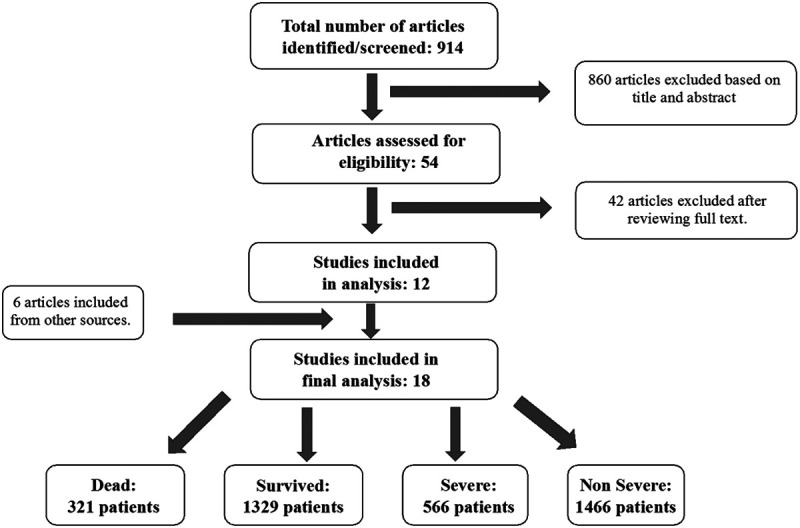
Flow diagram illustrating the systematic search of studies
Study Characteristics
This systematic review and meta-analysis of 18 studies incorporated a total of 3682 patients. Six articles compared d-dimer levels upon admission in patients who survived versus those who died,11–16 1 article compared patients with elevated d-dimer level with those with normal d-dimer levels,17 and 11 articles compared severe versus nonsevere COVID-19 patients.18–28 All studies were retrospective12–27 except 2, which were prospective,11,28 and all were conducted in China in the year 2020.
Positive d-dimer was defined as a value above the normal reference range. Five studies11,12,14,19,26 considered levels ≥ 0.5 mg/L as abnormal, 5 studies13,17,18,23,27 used > 0.5 mg/L as an abnormal value, 3 studies15,20,28 considered levels > 0.55 mg/L as abnormal, 2 studies21,24 considered levels > 0.243 mg/L as abnormal, and 3 studies16,22,25 gave only mean values of d-dimer (which were then used to calculate pooled WMD). The assay used to measure d-dimer was mentioned in only 1 study.17 Wherever necessary, the unit for d-dimer was converted to mg/L. Severe COVID-19 disease was defined in a patient with a respiratory rate ≥ 30 beats/min (resting state) or a mean oxygen saturation of ≤ 93% on room air or an arterial blood oxygen partial pressure (Pao2)/oxygen concentration (Fio2) ≤ 300 mm Hg and was consistent across all studies. The severe group included patients with severe COVID-19 and/or those needing ICU care for acute respiratory failure requiring mechanical ventilation, or for shock, or multiorgan failure. Survived patients were defined as those who were discharged from the hospital following recovery, were still in-hospital at the end of follow-up period (6 studies),11–13,15–17 or those patients who survived at least 28 days from admission (1 study).14
Table 1 summarizes the baseline characteristics of 6 studies,11–17 which compared dead versus survived patients, and 1 study which compared patients with elevated d-dimer versus normal d-dimer levels. Among the 6 studies which compared dead versus survived patients, the mean age of the study population in this group was 62.5 ± 14.8 years, and 56.3% were males. Overall, hypertension was the most common comorbidity (36.6%), followed by DM (16.8%) and CAD (11.7%). Shock was observed in 8.9% of patients.
Table 1.
Baseline Characteristics of Studies Included in the Meta-Analysis Comparing COVID-19-Infected Patients Who Died Versus Patients Who Survived
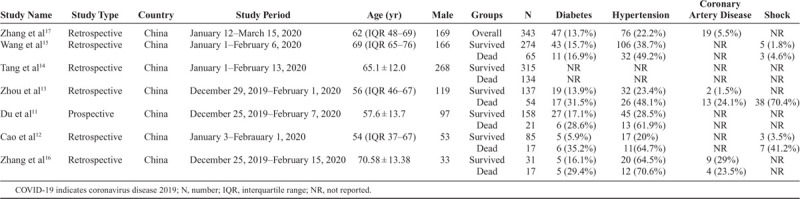
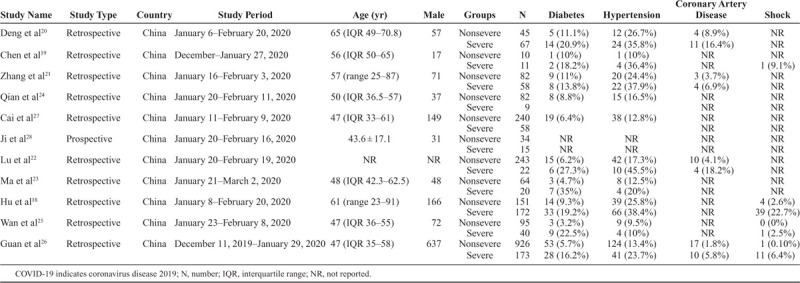
Table 2 summarizes the baseline characteristics of 11 studies18–28 that compared severe versus nonsevere COVID-19 patients. The mean age of the study population was 49.9 ± 17.2 years, and 54.6% were males. Overall, hypertension was the most common comorbidity (18.8%), followed by DM (9.2%) and CAD (3.9%). Shock was observed in 3.6% of patients, of which 2% of patients had septic shock. Shock was undefined in the other patients.
Table 2.
Baseline Characteristics of Studies Included in the Meta-Analysis Comparing Severe Versus Nonsevere COVID-19-Infected Patients
All-Cause Mortality
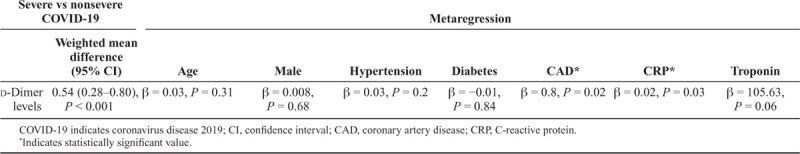
The data for d-dimer levels were available in 5 studies.11,13–16 The pooled mean d-dimer levels were significantly elevated in patients who died versus those who survived (WMD, 6.13 mg/L; 95% CI, 4.16–8.11; P ≤ 0.001; I2 = 81.41%) (Fig. 2). No publication bias was observed (Egger’s P = 0.39, Supplement Figure 1, http://links.lww.com/CIR/A20). A meta-regression analysis demonstrated no significant associations between age, male sex, hypertension, DM, CAD, C-reactive protein, and troponin in COVID-19-infected patients who died versus those who survived (Table 3).
FIGURE 2.
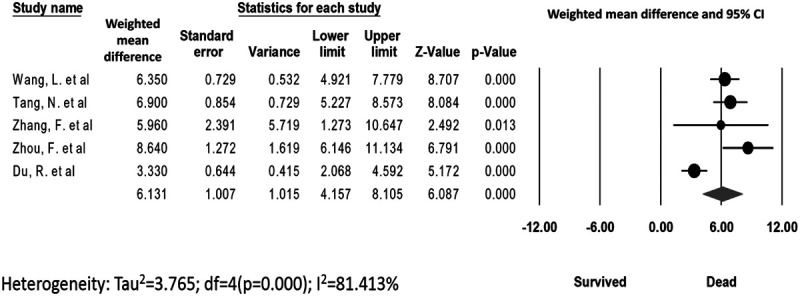
d-Dimer levels. The Forest plot for pooled weighted mean difference in d-dimer levels in dead versus survived COVID-19 patients. COVID-19 indicates coronavirus disease 2019.
Table 3.
Metaregression of Baseline Characteristics with Weighted Mean Difference in d-Dimer Levels in COVID-19 Patients—Dead Versus Survived

The risk of mortality was fourfold in patients with positive d-dimer versus negative d-dimer (21% vs 4.9%; RR, 4.11; 95% CI, 2.48–6.84; P ≤ 0.001, respectively). The test for heterogeneity was nonsignificant (I2 = 0%) (Fig. 3). No publication bias was observed (Egger’s P = 0.26; Supplement Figure 2, http://links.lww.com/CIR/A20).
FIGURE 3.
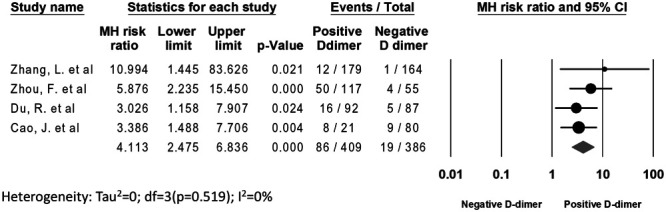
All-cause mortality. The Forest plot shows the outcomes of the individual trials as well as the aggregate.
Severity of COVID-19
The data for d-dimer levels were available in 9 studies.19–25,27,28 The pooled mean d-dimer levels were significantly elevated in patients with severe COVID-19 infection (WMD, 0.54 mg/L; 95% CI, 0.28–0.8; P ≤ 0.001; I2 = 90.74%) (Fig. 4A). No publication bias was observed (Egger’s P = 0.13; Supplement Figure 3, http://links.lww.com/CIR/A20). Meta-regression analysis showed a significant association between CAD, C-reactive protein, and severe COVID-19 disease, but the results were not significant for age, male sex, and comorbidities (hypertension, DM, troponin levels) (Table 4; Fig. 4B and 4C).
FIGURE 4.
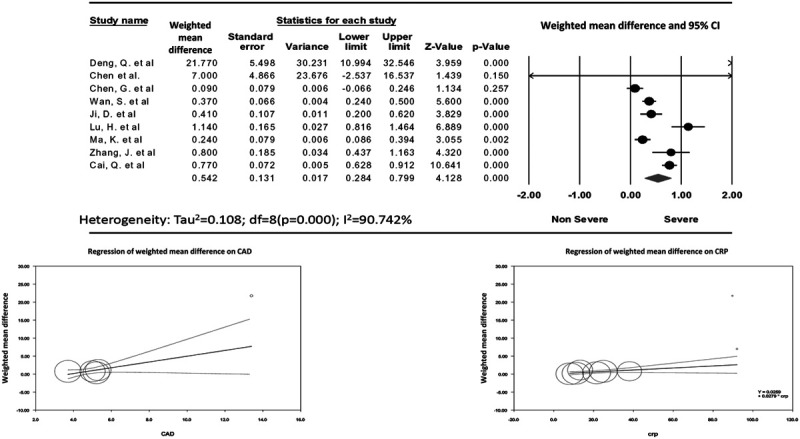
Disease severity. A, The Forest plot for pooled weighted mean difference in d-dimer levels in severe versus nonsevere COVID-19 patients, followed by random-effects meta-regression analysis plots depicting the relationship between weighted mean differences in d-dimer levels (on y-axis) and (B) CAD and (C) CRP. Each included study is represented by a circle, the size of which is proportional to its respective weight in the analysis. The line indicates the predicted effects (regression line). There was significant association between CAD (β = 0.8, P = 0.02), and CRP levels (β = 0.02, P = 0.03) and mean differences in d-dimer levels. COVID-19 indicates coronavirus disease 2019; CAD, coronary artery disease; CRP, C-reactive protein.
Table 4.
Metaregression of Baseline Characteristics with Weighted Mean Difference in d-Dimer Levels in Severe Versus Nonsevere COVID-19-Infected Patients
The risk of developing severe disease was twofold higher in patients with a positive d-dimer level versus negative d-dimer level (40.74% vs 21.98%; RR, 2.04; 95% CI, 1.34–3.11; P ≤ 0.001; I2 = 81.83%, respectively) (Fig. 5). No publication bias was observed (Egger’s P = 0.16; Supplement Figure 4, http://links.lww.com/CIR/A20). A sensitivity analysis was performed by removing 1 study at a time (n-1 analysis) to investigate the high heterogeneity. No significant change in the findings was observed with the sensitivity analysis.
FIGURE 5.
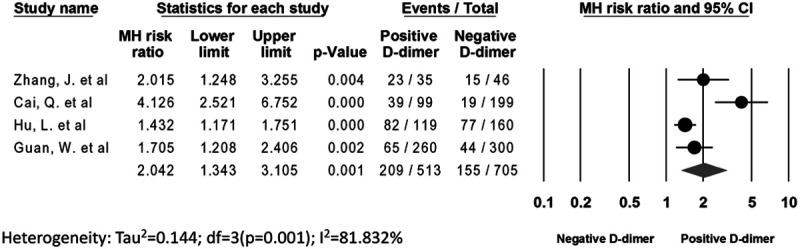
The Forest plot demonstrating the risk ratio of positive d-dimer with severity.
DISCUSSION
Elevated d-dimer is one of the abnormal laboratory parameters found in patients with COVID-19 infection. d-dimer is the fibrin degradation product released upon cleavage of cross-linked fibrin by plasmin, often utilized in diagnosing disseminated intravascular coagulation in those with low and intermediate pretest probability for deep vein thrombosis (DVT) and pulmonary embolism (PE).29 Historically, the role of d-dimer is limited due to its nonspecificity, with elevated levels often seen with advanced age, African American race, female sex, active malignancy, surgery, pregnancy, immobility, cocaine use, connective tissue disorders, end-stage renal disease, and prior thromboembolic disease.30 More recently, d-dimer has been explored to identify patients thought to develop severe COVID-19 infection earlier in their course of illness. A previous meta-analysis comprising 4 studies demonstrated elevated d-dimer levels in patients with severe COVID-19 infection compared with those with the nonsevere disease.31 However, this meta-analysis was limited by a relatively small sample size, and it failed to answer the clinically relevant question regarding the prognostic value of d-dimer in predicting severe COVID-19 infection and mortality. Our meta-analysis comprising 18 studies evaluated the prognostic role of d-dimer in COVID-19-infected patients, and is the largest to date, to the best of our knowledge. The key findings of our pooled analysis are: (1) the d-dimer levels were higher in patients with severe COVID-19 infection and those who succumbed to death, compared with nonsevere disease and those who survived, respectively; (2) patients with elevated d-dimer levels were at an increased risk of developing severe COVID-19 infection and increased all-cause mortality compared with those with normal d-dimer levels.
Zhou et al13 reported that d-dimer levels >1 mg/L on admission in COVID-19-infected patients were independently associated with increased odds of mortality, a finding that echoes with our pooled analysis. Also, patients with advanced age, higher Sequential Organ Failure Assessment score, elevated troponin, and B-type natriuretic peptide have been associated with poor outcomes and mortality in COVID-19 infection.13,32,33 Furthermore, using a higher cutoff value of d-dimer (levels >2 mg/L) predicted in-hospital mortality even better, as noted by Zhang et al,17 with a sensitivity of 92.3% and a specificity of 83.3% after adjusting for age, gender, and comorbidities. Besides, studies have shown that rising d-dimer levels during the course of hospitalization were associated with worse long-term outcomes.12,13
The prognostic value of d-dimer, as seen in COVID-19 infection, has also been noted in sepsis and other infections (like pneumonia or influenza). Patients with sepsis who had elevated d-dimer levels on admission demonstrated a higher 28-day mortality.34 Likewise, elevated d-dimer levels may help predict severe community-acquired pneumonia.35 Elevated d-dimer was also noted in the 2009 novel influenza A (H1N1) infection among critically-ill patients and those who died.36 Other than infections, elevated d-dimer levels have been associated with adverse clinical outcomes in numerous cardiovascular conditions (like CAD and congestive heart failure).37,38 Nonetheless, in our analysis, patients who died as a result of COVID-19 infection were noted to have elevated d-dimer levels even after adjusting for age and comorbidities. However, no single cutoff value (both in COVID and non-COVID illnesses) has been identified to predict adverse outcomes consistently.
There has been evidence regarding an increased incidence of venous thromboembolic events (VTE), including DVT and PE, in patients with severe COVID-19 infection.39 One study proposed that d-dimer levels > 1.5 mg/L may help detect VTE events with a sensitivity of 85.0% and specificity of 88.5%; however, results should be interpreted with caution due to small sample size and lack of external validation.40 It remains unclear at this time if this is a direct consequence of SARS-CoV-2 infection or a due to cytokine storm resulting in the systemic inflammatory response syndrome, as seen in other viral infections.41–44 A similar pattern of changes in coagulation cascade with increased prothrombotic state and incidences of DVT and PE were also noted with the coronavirus responsible for Middle Eastern Respiratory Syndrome (MERS-CoV) and SARS-CoV-1.45 The risk of VTE is generally high in critically-ill patients, but the risk appears to be higher in patients infected with SARS-CoV-2.
While hypercoagulability could be one of the reasons for elevated d-dimer levels in severe COVID-19 infection, these patients may also have several other reasons for d-dimer elevation, including renal dysfunction, disseminated intravascular coagulation, atrial fibrillation, stroke, acute coronary syndrome, infection, and acute upper gastrointestinal bleed, especially among those admitted to the ICU. Furthermore, d-dimer has low specificity to detect VTE in critically-ill patients.46 Thus, imaging studies to diagnose DVT or PE should only be pursued if clinically warranted.40 Perhaps, empirically treating all COVID-19 patients with intermediate or full (therapeutic) doses of anticoagulation to prevent microvascular thrombosis14,47 might be beneficial (provided a thorough risk-benefit assessment is performed given these patients are also at-risk of spontaneous bleeding). However, our study was not designed to assess this difference.
Our study has a few important limitations. First, all studies included in our meta-analysis were from China, while currently the United States and Europe have the majority of COVID-19 cases. However, the preliminary reports from the United States and Europe have shown similar trends in COVID-19 infection in terms of clinical presentation and outcomes.5,48 Our pooled analysis provides the best available data regarding trends of d-dimer levels in patients with COVID-19 infection, and the likelihood of developing severe infection or mortality in patients with elevated d-dimer levels. Second, all studies included in our analysis were either prospective or retrospective reports, which is currently the best available evidence, and therefore, subject to potential confounding and publication bias. Third, high heterogeneity was observed between studies in our pooled analysis. Fourth, details on trends of d-dimer over the course of hospitalization were not available. Fifth, the normal reference range of d-dimer varied slightly among studies, and details regarding the assays used to measure d-dimer were not available in most studies. Sixth, trends in d-dimer levels for COVID-19 patients never hospitalized remain unknown. Finally, patient-level data to perform additional detailed analysis were not available.
CONCLUSIONS
Our meta-analysis demonstrates that patients with COVID-19 presenting with elevated d-dimer levels have an increased risk of severe disease and mortality.
Supplementary Material
Footnotes
Disclosure: The authors have no conflicts of interest and funding to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.cardiologyinreview.com).
REFERENCES
- 1.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus disease 2019 (COVID-19) Situation Report—95. 2020. World Health Organization; Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200424-sitrep-95-covid-19.pdf?sfvrsn=e8065831_4. Accessed May 4, 2020. [Google Scholar]
- 3.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. [DOI] [PubMed] [Google Scholar]
- 6.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region—case series. N Engl J Med. 2020;382:2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 9.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a Prospective Cohort Study. Eur Respir J. 2020;55:2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;ciaa243 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, Yang D, Li J, et al. Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: a single center retrospective cohort study. medRxiv. 2020 [Google Scholar]
- 17.Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. Clin Infect Dis. 2020;ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020. Feb 19. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Ai J, Shen Y, et al. A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. medRxiv. 2020. [Epub ahead of print] [Google Scholar]
- 23.Ma KL, Liu ZH, Cao Cf, et al. COVID-19 myocarditis and severity factors: an Adult Cohort Study. medRxiv. 2020. [Epub ahead of print] [Google Scholar]
- 24.Qian GQ, Yang NB, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;hcaa089 doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Q, Huang D, Ou P, et al. 2020SSRN; . Available at: https://ssrn.com/abstract=3542163. Accessed May 4, 2020. [Google Scholar]
- 28.Ji D, Zhang D, Chen Z, et al. 2020SSRN; Available at: https://ssrn.com/abstract=3539674. Accessed May 4, 2020. [Google Scholar]
- 29.Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017;70:2411–2420. [DOI] [PubMed] [Google Scholar]
- 30.Kabrhel C, Mark Courtney D, Camargo CA, Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, Jiang D, Wen XS, et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li JW, Han TW, Woodward M, et al. The impact of 2019 novel coronavirus on heart injury: a systemic review and meta-analysis. Prog Cardiovasc Dis. 2020. doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodelo JR, De la Rosa G, Valencia ML, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30:1991–1999. [DOI] [PubMed] [Google Scholar]
- 35.Shilon Y, Shitrit AB, Rudensky B, et al. A rapid quantitative D-dimer assay at admission correlates with the severity of community acquired pneumonia. Blood Coagul Fibrinolysis. 2003;14:745–748. [DOI] [PubMed] [Google Scholar]
- 36.Wang ZF, Su F, Lin XJ, et al. Serum D-dimer changes and prognostic implication in 2009 novel influenza A(H1N1). Thromb Res. 2011;127:198–201. [DOI] [PubMed] [Google Scholar]
- 37.Morange PE, Bickel C, Nicaud V, et al. AtheroGene Investigators. Haemostatic factors and the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene study. Arterioscler Thromb Vasc Biol. 2006;26:2793–2799. [DOI] [PubMed] [Google Scholar]
- 38.Zorlu A, Yilmaz MB, Yucel H, et al. Increased d-dimer levels predict cardiovascular mortality in patients with systolic heart failure. J Thromb Thrombolysis. 2012;33:322–328. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bikdeli B, Madhavan MV, Jimenez D, et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borges AH, O’Connor JL, Phillips AN, et al. INSIGHT SMART Study Group; ESPRIT Study Group; SILCAAT Scientific Committee. Factors associated with D-dimer levels in HIV-infected individuals. PLoS One. 2014;9:e90978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramacciotti E, Agati LB, Aguiar VCR, et al. Zika and chikungunya virus and risk for venous thromboembolism. Clin Appl Thromb Hemost. 2019;25:1076029618821184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smither SJ, O’Brien LM, Eastaugh L, et al. Haemostatic changes in five patients infected with ebola virus. Viruses. 2019;11:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta P, McAuley DF, Brown M, et al. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minet C, Potton L, Bonadona A, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. 2015;19:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


