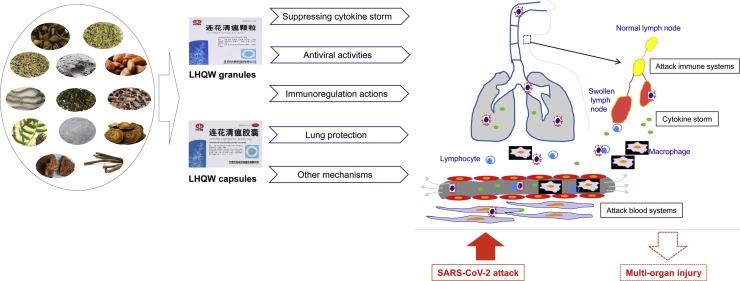
Graphical abstract
Abbreviations: ACE2, angiotensin-converting enzyme 2; ADRs, adverse drug reactions; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; AGE, advanced glycation end products; ALI, acute lung injury; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus Disease 19; EC50, median effective concentrations; HPLC, high performance liquid chromatography; IBV, influenza B virus; IFN, interferon; IL, interleukin; LHQW, Lianhua Qingwen; LPS, lipopolysaccharide; IP, interferon-inducible protein; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; NF-κB, nuclear factor-kappa B; RAGE, receptor of advanced glycation end products; SARS, Severe Acute Respiratory Syndrome; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; TCM, traditional Chinese medicine; UPLC-DAD-QTOF-MS, ultraperformance liquid chromatography coupled with diode-array detector and quadrupole time-of-flight mass spectrometry
Keywords: Coronavirus, SARS-CoV-2, Lianhua Qingwen, Antiviral activity, Complementary strategy
Abstract
Background
An outbreak of Coronavirus Disease 2019 (COVID-19) which was infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is still spreading and has led to unprecedented health emergency over the world. Though no specific drug has been developed so far, emerging agents have been confirmed effective or potentially beneficial to restrain it. Lianhua Qingwen (LHQW) is a commonly used Chinese medical preparation to treat viral influenza, including in the fight against SARS in 2002–2003 in China. Recent data also showed that LHQW played a vigorous role in COVID-19 treatment.
Purpose
This review will elucidate the pre-clinical and clinical evidence of LHQW in lung protection and antiviral activities, and provide timely data delivery for the exploration of effective treatment strategies in the therapy of COVID-19.
Study design and method
The research data were obtained from the academic databases (up to August 8, 2020) including Pubmed, CNKI and Web of Science, on ethnobotany and ethno medicines. The search keywords for screening the literature information were “virus”, “COVID-19”, or “SARS-CoV-2”, and “Lianhua Qingwen”. The documents were filtered and summarized for final evaluation.
Results
The collected evidence demonstrated that LHQW exhibited benefits against COVID-19. Impressively, LHQW in conjunction with conventional treatment could significantly improve COVID-19 patients as a synergetic strategy. The mechanisms were mainly involved the antiviral activity, and regulation of inflammation response as well as immune function.
Conclusion
Although the data were far from adequate, the latest advances had shown the benefits of LHQW in COVID-19, especially in combination with other antiviral drugs. This review provides comprehensive evidence of LHQW as a complementary strategy for treating COVID-19. Nevertheless, imperious researches should be conducted to clarify the unconfirmed effects, regulatory mechanisms and adverse reactions of LHQW in treating COVID-19 by means of well designed randomized controlled trials.
1. Introduction
The serious attack of Coronavirus Disease 2019 (COVID-19), infected by Severe Acute Respiratory Syndrome (SARS) Coronavirus 2 (SARS-CoV-2), has quickly spread around the globe [[1], [2], [3]]. Till August 8, 2020, over 19,000,000 people have been confirmed infected and more than 700,000 people have died during this severe viral prevalence. COVID-19 pandemic has caused a serious threat to public health and become the greatest challenge facing the world.
Recent advances have discovered that COVID-19 is a systematic disease targeting the lungs, vasculatures, and immune system, and involves severe lung inflammation and immune deficiency in terms of cytokine storm, neutrophil extracellular traps, as well as imbalance of lymphocyte subsets and so forth [[4], [5], [6]]. Cytokine storm is an excessive immune response of the body to external stimuli such as viruses, which will stimulate the secretion of a large number of inflammatory factors and lead to cytokine cascade reaction [4,6]. Patients with severe cases will develop acute respiratory distress syndrome and septic shock, and eventually develop multi-organ failure and death [5]. The basic clinical treatments for COVID-19 include anti-infectious, anti-proinflammatory cytokines, nonspecific antiviral as well as life support therapies [[6], [7], [8]]. There are multiple drug trials going on, but there are no specific anti-SARS-CoV-2 drugs or vaccines till now. It is urgent to develop effective drugs to contain and treat COVID-19.
For thousands of years, traditional Chinese medicine (TCM) plays an important role in treating human diseases [[9], [10], [11], [12], [13], [14]]. Lianhua Qingwen (LHQW) is a classical Chinese medical preparation, while one of its prescription compositions Maxingshigan Decoction has a history of more than thousand years to treat plague, and exhibits antiviral and lung protection actions [15,16]. LHQW is now officially recorded in Chinese Pharmacopeia (2015 Edition) and has been used to treat viral influenza for more than one decade, including in the fight against SARS in 2002–2003 in China [17]. This review has introduced the advances of LHQW in anti-viral treatment, intending to provide ideas for the timely treatment of COVID-19 patients.
2. Methods
The literature materials were collected from the scientific databases including the Pubmed, CNKI and Web of Science (up to August 8, 2020), on ethnobotany and ethno medicines. The search keywords for screening the literature information were “virus”, “COVID-19”, or “SARS-CoV-2”, and “Lianhua Qingwen”. The documents were filtered and summarized for final evaluation.
3. Advances of LHQW in laboratory researches and clinical trials
LHQW prescription (capsules type, or granules type) consists of 11 herbs, gypsum and menthol [18]. It includes Forsythia suspensa (Thunb.) Vahl. (Lianqiao), Lonicera japonica Thunb. (Jinyinhua), Ephedra sinica Stapf (Mahuang), Armeniacae Amarum Semen (Kuxingren), Gypsum Fibrosuum (Shigao), Isatis tinctoria L. (Banlangen), Dryopteridis Crassirhizomatis Rhizoma (Mianmaguanzhong), Houttuynia cordata Thunb. (Yuxingcao), Pogostemon cablin (Blanco) Benth. (Guanghuoxiang), Rheum palmatum L. (Dahuang), Rhodiola rosea Linn. (Hongjingtian), Mentha haplocalyx Briq. (Bohe), Glycyrrhiza uralensis Fisch. (Gancao) with a herbal ratio of 170 g: 170 g: 57 g: 57 g: 170 g: 170 g: 170 g: 170 g: 57 g: 34 g: 57 g: 5 g: 57 g, which is recorded in Chinese Pharmacopeia (2015 Edition).
Jia and colleagues established a rapid ultraperformance liquid chromatography coupled with diode-array detector and quadrupole time-of-flight mass spectrometry (UPLC-DAD-QTOF-MS) to analyze the major constituents of LHQW capsule, and the results demonstrated that 12 representative compounds were quantified as chemical marker, including salidroside, chlorogenic acid, forsythoside E, cryptochlorogenic acid, amygdalin, sweroside, hyperin, rutin, forsythoside A, phillyrin, rhein, and glycyrrhizic acid [17]. Recent study then showed that the main components of LHQW included chlorogenic acid, caffeic acid, isochlorogenic acid B, isochlorogenic acid C, phillyrin, forsythiaside A, and rutin by high performance liquid chromatography (HPLC) analysis [19]. Emerging data have also shown its beneficial effects in treating diverse diseases such as acute respiratory infection, influenza, pneumonia, hand-foot-mouth disease, chronic obstructive pulmonary disease (COPD), as well as COVID-19 [[20], [21], [22], [23]].
3.1. In vitro studies with LHQW
3.1.1. In vitro studies on influenza virus infection
In vitro, LHQW capsules could inhibit the proliferation of influenza viruses of various strain with the 50 % inhibitory concentration ranging from 0.35 to 2 mg/mL [24]. It blocked the early stages (0−2 h) of virus infection, and reduced virus-induced nuclear factor-kappa B (NF-κB) activation and the gene expression of interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, interferon-inducible protein (IP)-10, and monocyte chemoattractant protein (MCP)-1 [24]. In addition, LHQW treatment efficiently impaired the nuclear export of the viral RNPl [24].
LHQW capsules also showed a multi-access effects on anti-human influenza A virus H3N2, including the prevention effect on virus adsorption, inhibition on virus proliferation after adsorption and direct killing of the virus [25]. Meanwhile, the median effective concentrations (EC50) in above-mentioned effects were 0.042, 0.031, 0.051 and 0.050 mg/mL, respectively, while the prevention effect on virus adsorption was the strongest [25]. Moreover, LHQW capsule also remarkably decreased the infectivity of H3N2 in vitro [25]. In addition, LHQW capsules could inhibit both Victoria and Yamagata lineages of influenza B virus (IBV) with the 50 % inhibitive concentrations ranging from 0.228 ± 0.150 to 0.754 ± 0.161 mg/mL [26]. LHQW capsules not only had hemagglutination inhibition activity against B/Guangzhou/0215/2012 of influenza B virus, but also inhibited the over-expression of RANTES, IL-6, IL-8, IP-10, TNF-α, MCP-1, macrophage inflammatory protein (MIP)-1β, and interferon (IFN)-λ at the mRNA level and prevented a severe inflammatory response [26].
3.1.2. In vitro studies on SARS-CoV-2 infection
The recent network pharmacology studies showed that the effects of LHQW prescription was related to virus infection, inflammation and immunity, moreover, its main active ingredients were verified by molecular docking with angiotensin-converting enzyme 2 (ACE2), one receptor of SARS-CoV-2, so as to have a therapeutic effect on COVID-19 [[27], [28], [29]]. Further evaluation revealed that LHQW significantly inhibited SARS-CoV-2 replication in Vero E6 cells, markedly reduced pro-inflammatory cytokines (TNF-α, IL-6, CCL-2/MCP-1 and CXCL-10/IP-10) production and resulted in abnormal particle morphology of virion in cells [30].
These in vitro studies indicate that LHQW shows a wide range of antiviral activities including its anti-SARS-CoV-2 role. Its effects are partly due to its regulation on immunity functions, and inhibitory effects on virus replication and pro-inflammatory cytokines release.
3.2. In vivo studies with LHQW
3.2.1. In vivo studies on influenza virus infection
To clarify the in vivo effects of LHQW, Gao and colleagues recently showed that LHQW could treat influenza A virus infection in H1N1 mouse model by improving pathologic alterations and body weight loss, and reducing virus replication, lung lesions as well as inflammation [19]. Furthermore, they identified two important metabolites, prostaglandin F2α and arachidonic acid, and their arachidonic acid metabolism pathway, as vital indicators of LHQW in treating influenza by using serum metabolomics analysis [19]. LHQW also decreased the influenza viral titers in the lungs of mice, with decreased inflammatory cytokine levels in the early stages of infection [24]. In mice after infected with the flu virus FM1, LHQW capsule obviously increased IFN-γ content in lung homogenate and significantly reduced the levels of TNF-α, IL-1β and IL-6 in lung tissues, and had inhibiting effect on the marked decrease of CD4+ and CD4+/CD8+ [31,32].
Recent data indicated that orally administered LHQW capsules (100−400 mg/kg/day) could alleviate IBV-induced pathological change and decrease the abnormal increased inflammatory cells in lung tissues, but did not reduce the lung viral load and mortality in mice [26]. Further investigation confirmed that the combination treatment of LHQW capsules (200 mg/kg/day) with oseltamivir (2 mg/kg/day) markedly reduced IBV infection over the individual administration of either alone in vivo, proving that LHQW capsules could be used as an assistant drug to enhance the effect of oseltamivir against IBV infection [26].
3.2.2. In vivo studies on lung injury
In a lipopolysaccharide (LPS)-induced acute lung injury animal model, LHQW could efficiently decrease the chemotaxis of monocytes toward the pulmonary infection foci, and thus blocked the disease development by down-regulating MCP-1 expression [33]. In treating fine particulates PM2.5 (7.5 mg/kg)-exposed model rats, LHQW significantly relieved the pathological changes of thickened alveolar septum, congestion of capillary, interstitial edema and infiltration of lymphocyte and neutrophil surrounding bronchiole [34]. In addition, LHQW had antagonist effects on the pulmonary oxidative lesions that induced by fine particulates PM2.5 in rats [34].
These in vivo data further demonstrate the antiviral effects of LHQW as well as its lung protection role. Nevertheless, none of the in vivo studies are directly focused on Corona virus or COVID-19.
3.3. Clinical trials with LHQW
3.3.1. Clinical trials on common cold
A recent clinical trial including 36 common cold with wind-heat syndrome patients demonstrated that LHQW (three times daily for four days) treatment could significantly reduce TCM symptom score, with the median time to fever relief of (4 ± 5) h and the median time to fever clearance of 36 h [35]. During the study, no serious adverse events were reported.
3.3.2. Clinical trials on influenza virus infection
Compared with oseltamivir, LHQW achieved a similar therapeutic effectiveness with reduction of the duration of illness and duration of influenza A virus H1N1 shedding [36]. Further clinical trial showed that LHQW was superior to oseltamivir in improving the symptoms of influenza A virus infection by a meta-analysis of randomized, controlled trials [37].
A multi-center, randomized, double-blind, and placebo-controlled phase II clinical study, sponsored by Yiling Pharmaceutical Inc., was conducted to investigate the safety and efficacy of two doses of LHQW capsule compared to placebo in subjects with acute uncomplicated influenza in the U.S.A (https://www.clinicaltrials.gov/ct2/show/study/NCT02867358). A total of 420 participants with influenza (ages from 18 years to 65 years) were randomly divided into three groups: high dose LHQW capsule treatment (6 capsules each time, bid), low dose LHQW capsule treatment (4 capsules of LHQW + 2 capsules of placebo each time, bid) and placebo intervention (6 capsules of placebo each time, bid). The outcome measures included the alleviation of fever and other symptoms including nasal congestion, sore throat, cough, aches and pains, fatigue, headache, chills or sweats in subjects with acute uncomplicated influenza. Now, there are no study results posted on ClinicalTrials.gov for this study yet. The results are expected to enhance its effectiveness against viruses.
3.3.3. Clinical trials on COPD and pneumonia
COPD is characterized by a chronic inflammatory response and can be worsened by acute exacerbations. LHQW had been proven to accelerate the improvement of acute exacerbation of COPD (AECOPD) patients, especially for the high-risk subgroup, due to the decreased release of inflammatory mediators including IL-8, TNF- α, IL-17, and IL-23 [38]. The recent meta analysis reported 22 studies with a total of 2,007 patients, showing that the adjuvant therapy of LHQW with western medicine could improve the clinical efficiency and C-reactive protein index, shorten the time of antifebrile, cough time, rhondros duration and imaging time, as well as speed up the recovery of pneumonia patients [39]. Though the quality of the included literature was mixed, the lung protection effects of LHQW was demonstrated.
3.3.4. Clinical trials on COVID-19
As COVID-19 has led to serious death and lacks treatment strategies, the potential therapeutic drugs are increasingly being explored. Recently, a single center retrospective study showed that LHQW granules (6 g, tid) combined with conventional therapy (nutritional support therapy, symptomatic therapy, antiviral and antibiotic medication) could significantly relieve the symptoms like fever, cough, weakness and short breath in COVID-19 suspected cases with good safety [40]. Wang et al. conducted another single center clinical trial which was showed that seven days after treatment with LHQW granules (6 g, tid) in conjunction with conventional treatment for COVID-19 patients had a total effective rate of 92.73 % accompanied by significantly reduced major symptoms including fever, cough, fatigue and chest tightness [41]. Meanwhile, the network pharmacology analysis confirmed that its effects were mainly focused on the biological processes such as the response to LPS, the molecular response to bacterial origin, the response to metal ions and cell biological stimulation [41]. It may benefit the treatment of COVID-19 by the signaling pathways such as advanced glycation end products (AGE)-receptor of AGE (RAGE), TNF, sarcoma-associated herpesvirus infection, IL-17 and human cytomegalovirus infection [41]. The quadruple combination therapy including Ribavirin, Lopinavir/ritonavir, Umifenovir, and LHQW, could markedly improve the abnormal coagulation and leukocyte of severe COVID-19 patients with a better prognosis [42].
A multicenter retrospective study showed similar results, which revealed that combined application of LHQW granules (6 g, tid) could notably improve the clinical symptoms of fever, fatigue, cough, expectoration, chest tightness, and anorexia, and reduce the proportion of common to severe cases in COVID-19 patients with common type [20]. In another prospective multicenter randomized controlled trial in confirmed COVID-19 cases, Hu et al. showed that the usual treatment in combination with LHQW capsules (4 capsules thrice daily for 14 days) had significantly higher recovery rate, and shorter median time to symptom recovery, as compared with control group [43]. Additionally, LHQW group also had shorter time to recovery of fever, fatigue and coughing, and higher improvement rate in chest computed tomographic manifestations and clinical cure [43]. However, both groups did not differ in the rate of conversion to severe cases or viral assay findings.
Recently, Yu et al. recently published a clinical study on the treatment of mild COVID-19 with LHQW granules (6 g, tid) combined with abidor (0.2 g, tid) [44]. It was found that the total effective rate of LHQW granules combined with abidor treatment (LHQW group) was significantly higher than that of abidor group (80.95 % vs 64.86 %), while the rate of severe illness was markedly lower (14.29 % vs 23.65 %) [44]. After 7 days of treatment, the main TCM syndrome scores (fever, fatigue, cough, dry throat, chest tightness), C-reactive protein and procalcitonin levels in the LHQW group were significantly lower, and the white blood cell and lymphocyte counts were obviously higher than those in abidor group [44]. Besides, the effective rate of chest CT in the LHQW group was 69.39 %, which was higher than 62.84 % in abidor group [44]. Other related clinical studies also revealed that LHQW significantly improved the symptoms in COVID-19 patients and reduced the duration of fever, fatigue and cough and so forth, which provide preliminary research evidence for clinical treatment of COVID-19 [45,46].
These data indicate that LHQW prescription protects against the virus attack, and it has been recommended in the treatment of COVID-19 in China [47]. Besides, on April 14, 2020, the National Medical Products Administration of China approved modifying the specification of LHQW (Yiling Pharmaceutical Inc.) by adding a new indication for COVID-19 treatment in mild and normal cases. However, the efficacy and safety of which shall be confirmed by more high-quality clinical trials.
4. Perspectives for LHQW use in COVID-19 therapy
COVID-19 has led to a great case fatality. The discovery of potential effective drugs on the market becomes one of the principal means to develop antiviral drugs for COVID-19. In response to the severe SARS-CoV-2 attack, TCM has gained high usage in recent several months especially in China [47]. The control and improvement of the present COVID-19 situation in China could not be separated from the extensive participation of TCM, which has been proven effective by the clinical studies in COVID-19 patients [41,44,48,49]. LHQW is one of the TCM formulations that have been officially approved to treat COVID-19 patients, which may be due to its antiviral, lung protection, and regulation of inflammation response as well as immune regulation actions (Fig. 1 ). Recent clinical evidence also showed that LHQW in combination with western medicines, such as the other antiviral drugs, is better than that not using LHQW during COVID-19 treatment, suggesting it would be beneficial as a supplementary strategy for treating COVID-19 [41,[44], [45], [46]].
Fig. 1.
Role of LHQW in the treatment of COVID-19. LHQW: Lianhua Qingwen.
Nevertheless, there are also potential limitations that need to be improved for future challenges. First, the data indicated that LHQW combined with conventional therapies could markedly improve the clinical symptoms, quality of life and absorption of lung infiltration during anti-viral therapy, but the level of evidence is weak due to the included small sample trial and lacking the classification of disease severity. Second, the herbs in LHQW are mostly grown in specific areas, the varied origin of herbs are usually together with different content of bioactive ingredients, thus the quality standard control of the included crude drugs should be encouraged [12]. Besides, the collected clinical trials involving TCM in the treatment of COVID-19 were mainly from China and published in Chinese, and thus further well-designed multicenter RCTs from different countries will be more attractive before a definitive conclusion can be drawn.
In addition to western medicine, the potential adverse drug reactions (ADRs) can not be ignored. It was demonstrated that the use of LHQW also brought the incidence of gastrointestinal reactions, as well as skin and its accessories injuries, including diarrhea, abdominal distension, gastrointestinal discomfort, rash, and itching, despite the low quality of the included documents [50,51]. The complex components of LHQW stand for multiple targets and pleiotropic effects, but may also lead to more ADRs. Hence, the researches on specific active components of LHQW are still important and the reduction of irrelevant ingredients may be related to less adverse reactions.
CFDA has recently approved adding a new indication for LHQW in mild and common COVID-19 patients, but whether it would bring benefits for the severe cases is unclear. Future related studies will provide accumulated evidence to clarify it. Besides, in view of the definite effects of LHQW in preventing severe viral infections such as SARS and COVID-19 [17,44,45,49,52,53], LHQW may represent a considerable and effective strategy against unknown viral epidemics.
In conclusion, this review has summarized the benefits of LHQW for treating virus infection, especially its anti-SARS-CoV-2 role as an alternative candidate and complementary strategy. We hope this review will not only present comprehensive evidence for LHQW use in COVID-19 therapy, but also fuel antiviral researches and put forward promising insights for further antiviral drug development. Nevertheless, pressing researches should be conducted to illuminate the unconfirmed effects, regulatory mechanisms and adverse reactions of LHQW in treating COVID-19.
Author contribution statement
Liu-Cheng Li was responsible for drafting the manuscript. Jiao Qu revised the manuscript. Lian-Di Kan, Ye-Cheng Jin and Hong-Mei Fang were responsible for the conception and design of the review. Zhi-Hui Zhang and Wen-Cheng Zhou collected literatures and made the illustration. Jie Chen, Qin Chen and Hua-Qian Jin analyzed literatures. All authors approved the final version of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81503129, No. 81703740), the Zhejiang Provincial Natural Science Foundation of China (No. LYY19H280006, No. LQ15H280003), the Clinical Research Projects of Zhejiang Medical Association (No. 2019ZYC-A85), and the Scientific Research Projects of Hospital Pharmacy of Zhejiang Pharmaceutical Association (No. 2017ZYY07). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Li J.Y., You Z., Wang Q., Zhou Z.J., Qiu Y., Luo R., Ge X.Y. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect. 2020;22:80–85. doi: 10.1016/j.micinf.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren Y., Yao M.C., Huo X.Q., Gu Y., Zhu W.X., Qiao Y.J., Zhang Y.L. Study on treatment of "cytokine storm" by anti-2019-nCoV prescriptions based on arachidonic acid metabolic pathway. Zhongguo Zhong Yao Za Zhi. 2020;45:1225–1231. doi: 10.19540/j.cnki.cjcmm.20200224.405. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Ding Y.Q. From SARS to COVID-19: pathogens, receptor, pathogenesis and principles of the treatment. Zhonghua Bing Li Xue Za Zhi. 2020;49:E012. doi: 10.3760/cma.j.cn112151-20200318-00220. [DOI] [PubMed] [Google Scholar]
- 6.Yang C.X., Qu J., Liu Y.T., Meng S.W., Wang B.L., Feng M.Q., Sun Y. Immune imbalance mechanism and intervention strategy in patients with coronavirus disease 2019 (COVID-19) Chin. Pharmacol. Bull. 2020;36:445–453. [Google Scholar]
- 7.Chakraborty C., Sharma A.R., Sharma G., Bhattacharya M., Lee S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4016–4026. doi: 10.26355/eurrev_202004_20871. [DOI] [PubMed] [Google Scholar]
- 8.Salvi R., Patankar P. Emerging pharmacotherapies for COVID-19. Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R., Sun W.J., Liang Z.Q., Cao Y.M., Cao Y.B. Protection against COVID-19 injury by qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dang Y., Xu J., Yang Y., Li C., Zhang Q., Zhou W., Zhang L., Ji G. Ling-gui-zhu-gan decoction alleviates hepatic steatosis through SOCS2 modification by N6-methyladenosine. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.109976. [DOI] [PubMed] [Google Scholar]
- 11.Kong Q., Wu Y., Gu Y., Lv Q., Qi F., Gong S., Chen X. Analysis of the molecular mechanism of Pudilan (PDL) treatment for COVID-19 by network pharmacology tools. Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L.C., Kan L.D. Traditional Chinese medicine for pulmonary fibrosis therapy: progress and future prospects. J. Ethnopharmacol. 2017;198:45–63. doi: 10.1016/j.jep.2016.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y., Yang J., Wang X., Ma Z., Li S., Liu Z., Fan X. Research progress in use of traditional Chinese medicine for treatment of spinal cord injury. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110136. [DOI] [PubMed] [Google Scholar]
- 14.Tian S., Song X., Wang Y., Wang X., Mou Y., Chen Q., Zhao H., Ma K., Wu Z., Yu H., Han X., Wang H., Wang S., Ji X., Zhang Y. Chinese herbal medicine Baoyuan Jiedu decoction inhibits the accumulation of myeloid derived suppressor cells in pre-metastatic niche of lung via TGF-β/CCL9 pathway. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110380. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh C.F., Lo C.W., Liu C.H., Lin S., Yen H.R., Lin T.Y., Horng J.T. Mechanism by which ma-xing-shi-gan-tang inhibits the entry of influenza virus. J. Ethnopharmacol. 2012;143:57–67. doi: 10.1016/j.jep.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 16.Zhong Y., Zhou J., Liang N., Liu B., Lu R., He Y., Liang C., Wu J., Zhou Y., Hu M., Zhou J. Effect of Maxing Shigan Tang on H1N1 influenza A virus-associated acute lung injury in mice. Intervirology. 2016;59:267–274. doi: 10.1159/000458726. [DOI] [PubMed] [Google Scholar]
- 17.Jia W., Wang C., Wang Y., Pan G., Jiang M., Li Z., Zhu Y. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. Sci. World J. 2015;2015 doi: 10.1155/2015/731765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C.H., Zhong Y., Zhang Y., Liu J.P., Wang Y.F., Jia W.N., Wang G.C., Li Z., Zhu Y., Gao X.M. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol. Biosyst. 2016;12:606–613. doi: 10.1039/c5mb00448a. [DOI] [PubMed] [Google Scholar]
- 19.Gao D., Niu M., Wei S.Z., Zhang C.E., Zhou Y.F., Yang Z.W., Li L., Wang J.B., Zhang H.Z., Zhang L., Xiao X.H. Identification of a pharmacological biomarker for the bioassay-based quality control of a thirteen-component TCM formula (Lianhua Qingwen) used in treating influenza A virus (H1N1) infection. Front. Pharmacol. 2020;11:746. doi: 10.3389/fphar.2020.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng D.Z., Wang W.J., Li Y., Wu X.D., Zhou B., Song Q.Y. Analysis of curative effects of 51 patients with COVID-19 treated with Chinese medicine Lianhua Qingwen: a multicentre retrospective study. Tianjin J. Tradit. Chin. Med. 2020;37:509–516. [Google Scholar]
- 21.Cui L.F., Xu H.R., Li F.Z., Wang C.X. A systematic review on the efficacy of Traditional Chinese Medicine on influenza. Glob. Tradit. Chin. Med. 2019;12:1449–1454. [Google Scholar]
- 22.Niu Q.Q., Chen Y., Liu Y., Mao S.Z., Wang H., Zheng W.K., Zhang J.H. Efficacy and safety of Lianhua Qingwen capsule for influenza: a systematic review. Zhongguo Zhong Yao Za Zhi. 2017;42:1474–1481. doi: 10.19540/j.cnki.cjcmm.2017.0044. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X.P. Progress on the efficacy and clinical application of Lianhua Qingwen capsule (LHQW) Inform. Tradit. Chin. Med. 2016;33:107–111. [Google Scholar]
- 24.Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q., Cheng P.L., Yutao W., Zheng J., Yang Z., Zhang F. The Chinese prescription Lianhua Qingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017;17:130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo H.Y., Ke C.W., Zheng J.P., Zhong N.S. Anti-viral effects of Lianhua Qingwen capsule against influenza A virus in vitro. Tradit. Chin. Drug Res. Clin. Pharmacol. 2007;18:5–9. [Google Scholar]
- 26.Yang C., Wang Y., He J., Yan W., Jiang H., Chen Q., Li L., Yang Z. Lianhua-Qingwen displays antiviral and anti-inflammatory activity and synergistic effects with oseltamivir against influenza B virus infection in the mouse model. Evid. Based Complement. Altern. Med. 2020;2020 doi: 10.1155/2020/3196375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. (Lond.) 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 28.Ling X.Y., Tao J.L., Sun X., Yuan B. Exploring material basis and mechanism of Lianhua Qingwen prescription against coronavirus based on network pharmacology. Chin. Tradit. Herb. Drug. 2020;51:1723–1730. [Google Scholar]
- 29.Wang L., Yang Z.H., Zhang H.R., Yu H.X., Yang K., Fu B.H., Yang H.T. Study on the network pharmacology and evidences of Lianhua Qingwen in the treatment of Novel Coronavirus (2019 nCoV) Pneumonia. J. Chin. Med. Mater. 2020;43:772–778. [Google Scholar]
- 30.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo H., Zhang Q.H., Yang J., Gong J.N., Zhao Y.S., Zhou X.P. Effect of Lianhua Qingwen capsule on immunity of mice infected with flu virus. J. Nanjing Univ. Tradit. Chin. Med. (Nat. Sci.) 2007;23:106–108. [Google Scholar]
- 32.Mo H.Y., Yang Z.F., Zheng J.P., Mo Z.Y., Li Y.M., Xiao Z.L. Experimental study on prevention and treatment of influenza virus FM1 infection in mice by Lianhua Qingwen capsule. J. Chin. Med. Mater. 2008;32:1230–1233. [Google Scholar]
- 33.Li Q., Yin J., Ran Q.S., Yang Q., Liu L., Zhao Z., Li Y.J., Chen Y., Sun L.D., Wang Y.J., Weng X.G., Cai W.Y., Zhu X.X. Efficacy and mechanism of Lianhua Qingwen Capsules(LHQW) on chemotaxis of macrophages in acute lung injury (ALI) animal model. Zhongguo Zhong Yao Za Zhi. 2019;44:2317–2323. doi: 10.19540/j.cnki.cjcmm.20190210.001. [DOI] [PubMed] [Google Scholar]
- 34.Ping F., Li Z., Zhang F., Li D., Han S. Effects of Lianhua Qingwen on pulmonary oxidative lesions induced by fine particulates (PM2.5) in rats. Chin. Med. Sci. J. 2016;31:233–238. doi: 10.1016/s1001-9294(17)30006-8. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y., Zhang Z., Wei L., He S., Deng X., Ji A., Zhou B., Jiang D., Li M., Wang Y. Efficacy and safety of Reduqing granules in the treatment of common cold with wind-heat syndrome: a randomized, double-blind, double-dummy, positive-controlled trial. J. Tradit. Chin. Med. 2017;37:185–192. doi: 10.1016/S0254-6272(17)30043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan Z.P., Jia Z.H., Zhang J., Liu S., Chen Y., Liang L.C., Zhang C.Q., Zhang Z., Sun Y., Zhang S.Q., Wang Y.Y., Wu Y.L. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin. Med. J. (Engl.) 2011;124:2925–2933. [PubMed] [Google Scholar]
- 37.Zhao P., Yang H.Z., Lv H.Y., Wei Z.M. Efficacy of Lianhuaqingwen capsule compared with oseltamivir for influenza A virus infection: a meta-analysis of randomized, controlled trials. Altern. Ther. Health Med. 2014;20:25–30. [PubMed] [Google Scholar]
- 38.Dong L., Xia J.W., Gong Y., Chen Z., Yang H.H., Zhang J., He J., Chen X.D. Effect of lianhuaqingwen capsules on airway inflammation in patients with acute exacerbation of chronic obstructive pulmonary disease. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/637969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Z.J., Wu L.Y., Mou Y.Y., Duan H.M., Chen R.C., Xiao Y., Zheng W.J. Meta-analysis and systematic review of efficacy and safety of Lianhua Qingwen in adjuvant treatment of adult pneumonia. Zhongguo Zhong Yao Za Zhi. 2020 doi: 10.19540/j.cnki.cjcmm.20200508.501. [DOI] [PubMed] [Google Scholar]
- 40.Lv R.B., Wang W.J., Li X. Combined with western medicine conventional therapy in the treatment of 63 suspected cases of Coronavirus Disease 2019. J. Tradit. Chin. Med. 2020;61:655–659. [Google Scholar]
- 41.Wang F.C., Shen B.X., He C.Y., Zhao W.C., Nie S.L. Clinical efficacy and mechanism of Lianhua Qingwen Granule on COVID-19 based on network pharmacology research. Pharmacol. Clin. Chin. Mater. Med. 2020;36:93–101. [Google Scholar]
- 42.Li X., Yang Y., Liu L., Yang X., Zhao X., Li Y., Ge Y., Shi Y., Lv P., Zhang J., Bai T., Zhou H., Luo P., Huang S. Effect of combination antiviral therapy on hematological profiles in 151 adults hospitalized with severe coronavirus disease 2019. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.105036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu P., Li Y.Z., Wan S.B., Wang Y. Efficacy of Lianhua Qingwen granules combined with abidor in the treatment of mild COVID-19. Chin. Pharm. J. 2020 http://kns.cnki.net/kcms/detail/11.2162.R.20200422.1429.002.html [Google Scholar]
- 45.Cheng D.Z., Li Y. Clinical effectiveness and case analysis in 54 NCP patients treated with Lanhuaqingwen granules. World Chin. Med. 2020;15:150–154. [Google Scholar]
- 46.Yao K.T., Liu M.Y., Li X., Huang J.H., Cai H.B. Retrospective clinical analysis on treatment of coronavirus disease 2019 with traditional Chinese medicine Lianhua Qingwen. Chin. J. Exp. Tradit. Med. Formulae. 2020;26:8–12. [Google Scholar]
- 47.Liu Y., Liu L., Cao M.M., Wen J., Zhou S., Jiang H., Zhang Y., Zhao R.S., Yang Y.H. Literature analysis of Chinese patent medicine treatment in the observation period of Clinical Management of Corona Virus Disease 2019 (trial 6th edition) Clin. Med. J. 2020;18:62–66. [Google Scholar]
- 48.Ni L., Zhou L., Zhou M., Zhao J., Wang D.W. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19. Front. Med. 2020;14:210–214. doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and western medicine treatment. Biosci. Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 50.Peng L.L., Li L., Shen L., Li X.L. Literature analysis of clinical application and adverse drug reaction/event of Lianhua Qingwen capsule. Chin. J. Pharmacovigilance. 2015;12:753–755. 759. [Google Scholar]
- 51.Wang S.H., Liu J.F., Zhang Y.L., Dong Z. Systematic review of efficacy and safety of Lianhua Qingwen capsules in treatment of viral influenza. Zhongguo Zhong Yao Za Zhi. 2019;44:1503–1508. doi: 10.19540/j.cnki.cjcmm.20190102.001. [DOI] [PubMed] [Google Scholar]
- 52.Li H.R., Chang L.P., Wei C., Jia Z.H. Theoretical research basis and clinical efficacy of Lianhua Qingwen in treating Novel Coronavious pneumonica. World Chin. Med. 2020;15:332–336. [Google Scholar]
- 53.Zhu S.Y., Li X.Y., Wei Y.L., Yang P.Y., Qin E.D. Inhibitory effects of three prescriptions of traditional Chinese medicine on SARS-associated coronavirus in vitro. Lett. Biotechnol. 2003;14:390–392. [Google Scholar]