As the coronavirus disease 2019 (COVID-19) pandemic has progressed, the recognition of neurologic sequelae has increased. Herein, we report on a case of COVID-19–associated leukoencephalopathy with microhemorrhage.
A 59-year-old man with known persistent asthma (managed with inhaled steroids and long-acting beta agonists) presented with upper respiratory symptoms (10 days duration) and fever (7 days duration). COVID-19 was confirmed by means of polymerase chain reaction assay. He was intubated on hospital day 6. Severe agitation required high-dose analgesics (including dilaudid, fentanyl, and morphine), and blood pressure required intermittent pressor support (mean, 70–90 mm Hg, without sustained hypertension). Laboratory results included a platelet count of 175 000/μL, an activated partial thromboplastin clotting time of 34.7 seconds, an international normalized ratio of 1.3, a D-dimer level of 11 720 ng/mL, and a fibrinogen level of 693 mg/dL. Lumbar puncture was not performed.
Unenhanced head CT images showed subtle hypoattenuation of the bilateral cerebral hemispheric white matter and corpus callosum (Fig 1). T2-weighted fluid-attenuated inversion recovery MRI scans of the brain revealed diffuse, confluent posterior predominant white matter hyperintensities, scattered microhemorrhages predominantly in the corpus callosum, and apparent posterior circulation hyperperfusion (Fig 2), without diffusion restriction or abnormal enhancement.
Figure 1:
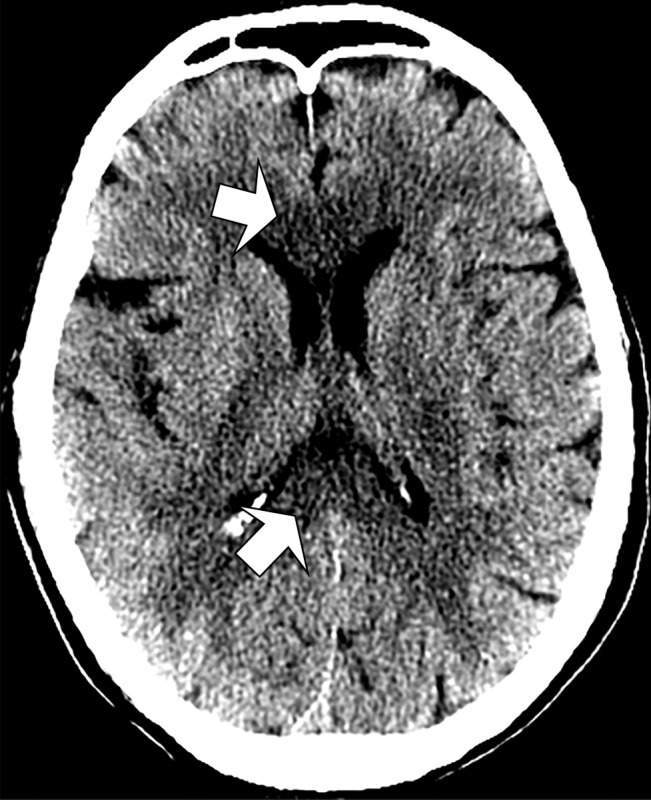
Unenhanced CT image of head demonstrates confluent hypoattenuation in genu (anterior) and splenium (posterior) of corpus callosum (arrows).
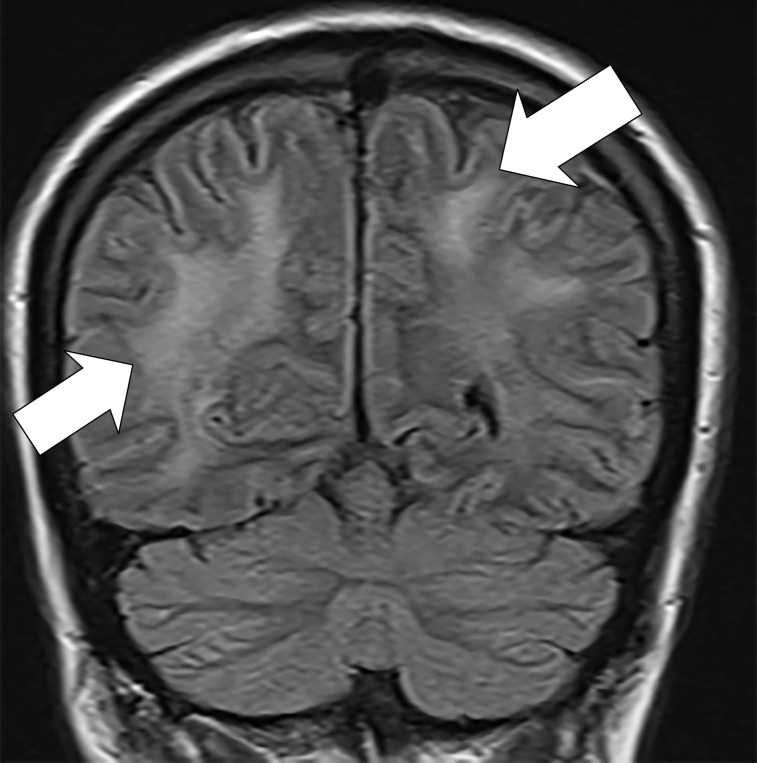
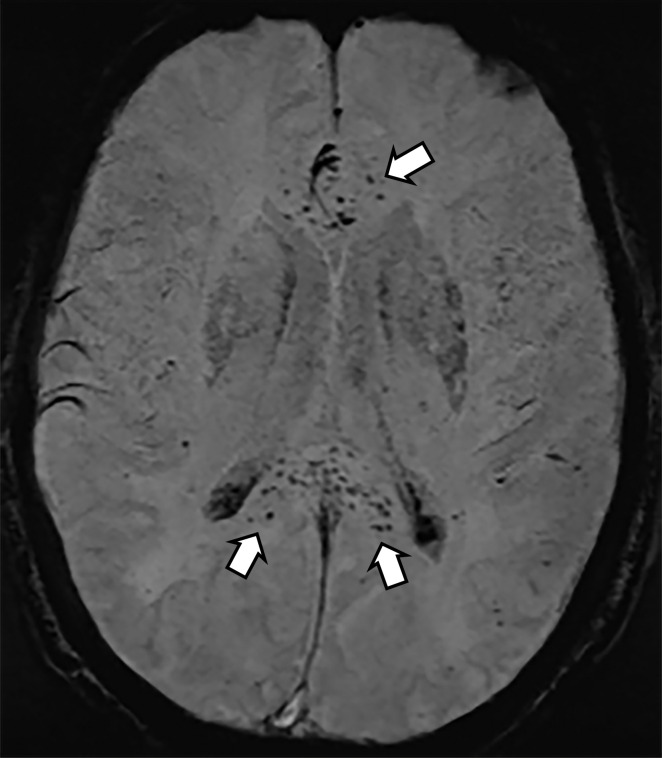
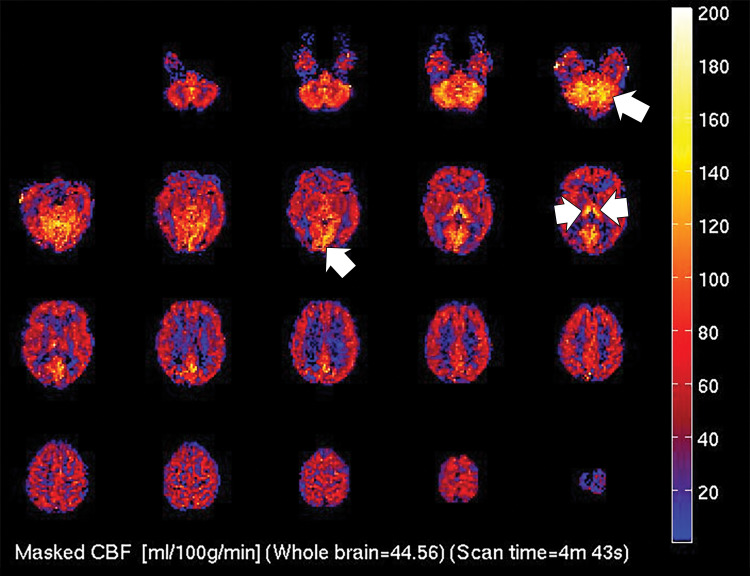
Figure 2a:
(a) T2-weighted fluid-attenuated inversion recovery MRI scan of brain shows extensive posterior greater than anterior white matter hyperintensity (arrows) without mass effect. (b) Susceptibility-weighted image demonstrates a cluster of microhemorrhages in corpus callosum (arrows). (c) Arterial-spin-labeling perfusion images are suggestive of posterior circulation hyperperfusion (arrows). CBF = cerebral blood flow.
Figure 2b:
(a) T2-weighted fluid-attenuated inversion recovery MRI scan of brain shows extensive posterior greater than anterior white matter hyperintensity (arrows) without mass effect. (b) Susceptibility-weighted image demonstrates a cluster of microhemorrhages in corpus callosum (arrows). (c) Arterial-spin-labeling perfusion images are suggestive of posterior circulation hyperperfusion (arrows). CBF = cerebral blood flow.
Figure 2c:
(a) T2-weighted fluid-attenuated inversion recovery MRI scan of brain shows extensive posterior greater than anterior white matter hyperintensity (arrows) without mass effect. (b) Susceptibility-weighted image demonstrates a cluster of microhemorrhages in corpus callosum (arrows). (c) Arterial-spin-labeling perfusion images are suggestive of posterior circulation hyperperfusion (arrows). CBF = cerebral blood flow.
These imaging findings are nonspecific and can accompany several well-established leukoencephalopathies. Confluent corpus callosal involvement can occur in acute disseminated encephalomyelitis (1) or acute hemorrhagic encephalomyelitis (2). Microbleeds can be seen with these or other vasculitides (3), as well as with posterior reversible encephalopathy syndrome (note that there was no documented hypertension in this case). Anesthesia-related toxicity is also possible. Although the mechanism of white matter injury and its relationship to COVID-19 is uncertain, possibilities include endotheliitis with thrombotic microangiopathy (4) as well as COVID-19–associated cytokine release syndrome, which may have implications for treatment (5).
Footnotes
Disclosures of Conflicts of Interest: J.R.S. disclosed no relevant relationships. K.W.G. disclosed no relevant relationships. D.E.S. disclosed no relevant relationships. A.P.S. disclosed no relevant relationships. D.W.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: occasionally provides expert testimony for medical-legal cases. Other relationships: disclosed no relevant relationships. J.H.B. disclosed no relevant relationships. T.G.W. disclosed no relevant relationships. C.P.G. disclosed no relevant relationships.
References
- 1.Banerjee A, Suthar R, Vyas S, Singh MP. Acute disseminated encephalomyelitis: complication of a vaccine preventable disease. BMJ Case Rep 2018;11(1):e225710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto PS, Taipa R, Moreira B, Correia C, Melo-Pires M. Acute hemorrhagic leukoencephalitis with severe brainstem and spinal cord involvement: MRI features with neuropathological confirmation. J Magn Reson Imaging 2011;33(4):957–961. [DOI] [PubMed] [Google Scholar]
- 3.Abdel Razek AA, Alvarez H, Bagg S, Refaat S, Castillo M. Imaging spectrum of CNS vasculitis. RadioGraphics 2014;34(4):873–894. [DOI] [PubMed] [Google Scholar]
- 4.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368(6490):473–474. [DOI] [PubMed] [Google Scholar]