Abstract
Neurological complications have emerged as a significant cause of morbidity and mortality in the ongoing COVID-19 pandemic. Beside respiratory insufficiency, many hospitalized patients exhibit neurological manifestations ranging from headache and loss of smell, to confusion and disabling strokes. COVID-19 is also anticipated to take a toll on the nervous system in the long term. Here, we will provide a critical appraisal of the potential for neurotropism and mechanisms of neuropathogenesis of SARS-CoV-2 as they relate to the acute and chronic neurological consequences of the infection. Finally, we will examine potential avenues for future research and therapeutic development.
Iadecola et al. review and discuss the acute and chronic neurological consequences of COVID-19, potential mechanisms for neuropathogenesis, and the outstanding questions to minimize its harmful nervous system involvement.
Introduction
There is increasing evidence that the nervous system is frequently involved in patients hospitalized with coronavirus disease 2019 (COVID-19). This is not surprising, because neurological manifestations have also long been described in infections from other respiratory viruses, including coronaviruses (Bergmann et al., 2006). However, the neurological manifestations of COVID-19 are common and disabling enough to have attracted widespread attention in the scientific and lay press for their short- and long-term impact on population health (Pleasure et al., 2020; Wenner Moyer, 2020). A large body of clinical data from tertiary referral centers is rapidly accumulating on this topic worldwide, often with conflicting observations, partly reflecting the preliminary and incomplete nature of the available data. Here, we provide a succinct summary of the nervous system involvement in COVID-19. In particular, we will focus on the mechanisms of pathogenicity, on the acute and delayed neurological manifestations reported to date, and on how the nervous system involvement compares to that of other respiratory viruses. Finally, we will attempt to flesh out caveats and unanswered questions that may help gain a better appreciation of this critical aspect of COVID-19 and chart a path forward to minimize its harmful nervous system involvement.
COVID-19 and Mechanisms of SARS-CoV-2 Pathogenesis
Beta-coronaviruses are a common cause of self-limited respiratory tract infections, but the strains responsible for the Middle Eastern respiratory syndrome (MERS-CoV), the severe acute respiratory syndrome (SARS-CoV-1), and COVID-19 (SARS-CoV-2) cause more severe disease (Gerges Harb et al., 2020). Although COVID-19 seems to have a lower case-fatality rate (≈0.7%) than SARS (≈10%) and MERS (≈30%) (Gerges Harb et al., 2020), the large number of patients affected has caused over 0.8 million deaths worldwide thus far, with many more expected based on current trends. The male sex is more susceptible to the infection, and disease severity and mortality are higher in older individuals (Goyal et al., 2020; Grasselli et al., 2020; Wang et al., 2020a). Besides the pulmonary disease, extra-pulmonary manifestations are being increasingly appreciated, including neurological involvement.
Brain Expression of SARS-CoV-2 Receptors and Related Proteins
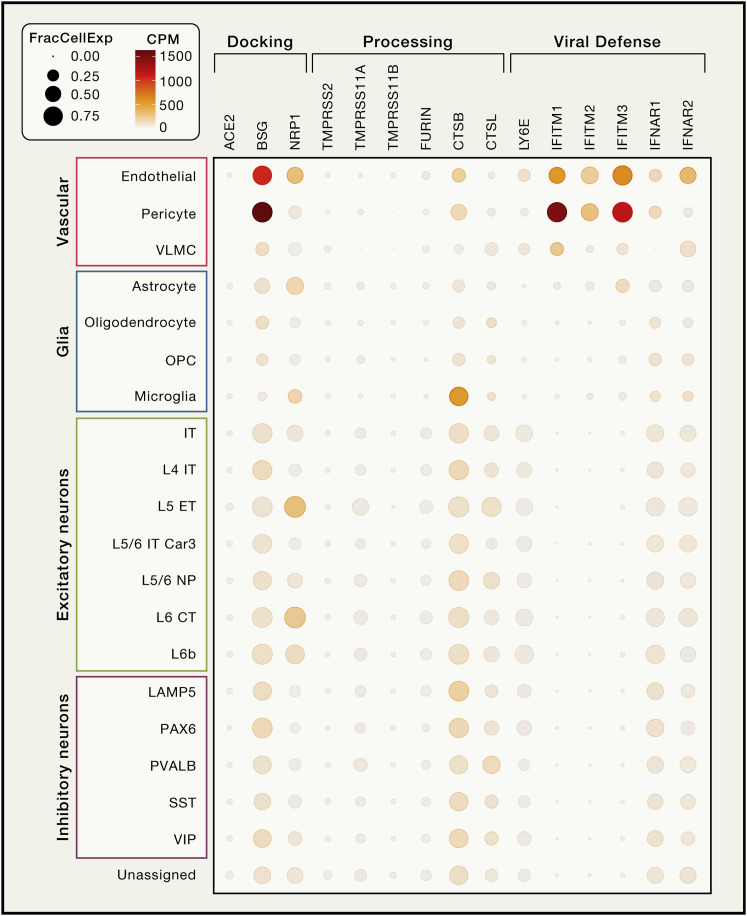
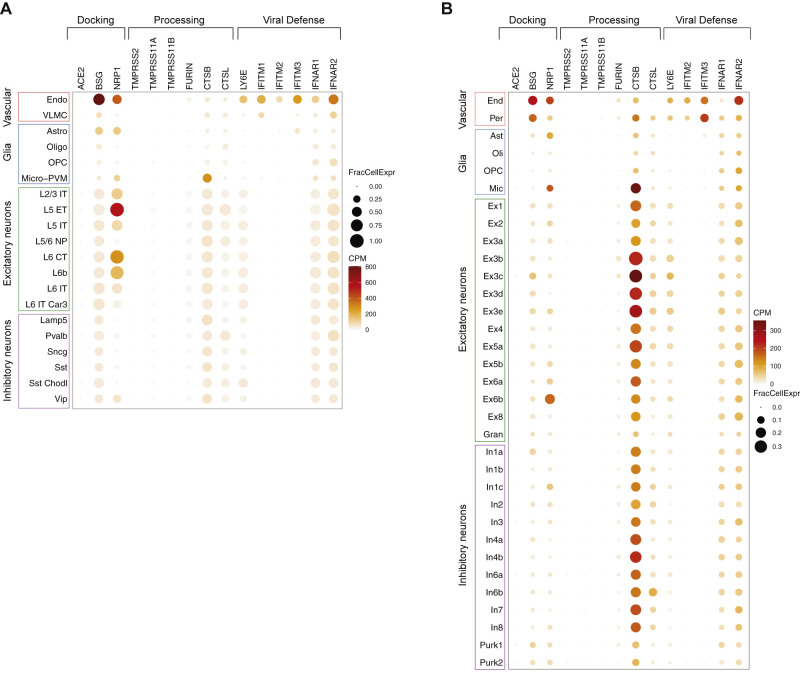
Similar to SARS-CoV-1, SARS-CoV-2 utilizes angiotensin converting enzyme-2 (ACE2) as the main docking receptor and needs proteolytic processing of the spike protein by transmembrane protease serine 2 (TMPRSS2) for efficient cell entry (Hoffmann et al., 2020). ACE2 protein has been observed in human brain vessels (Hamming et al., 2004), a finding recently attributed to expression in pericytes and smooth muscle cells in the vascular wall, but not in the endothelium lining cerebral vessels (He et al., 2020). However, data mining of human brain single-nuclear RNA sequencing (RNA-seq) data also found expression in the choroid plexus and neocortical neurons, although the number of positive neurons was small (∼2% or less) (Chen et al., 2020c). No expression in microglia, endothelial cells, and pericytes was observed (Chen et al., 2020c). Besides ACE2, SARS-CoV-2 may utilize basigin (BSG; CD147) (Wang et al., 2020b) and neuropilin-1 (NRP1) (Cantuti-Castelvetri et al., 2020) as docking receptors, while a range of proteases including TMPRSS11A/B, cathepsin B and L, and furin (FURIN) have been shown to facilitate viral cell entry and replication (Shang et al., 2020). Results from human single nuclei RNA-seq databases we mined (Hodge et al., 2019; Lake et al., 2018) are presented in Figures 1 and S1 . Collectively, the data suggest that vascular wall cells may express ACE2 in the human brain at low levels, but non-canonical SARS-CoV-2 receptors are present in several brain cell types making them vulnerable to the virus. However, there is also evidence for a strong antiviral defense system in the brain vasculature (Figures 1 and S1), which, in concert with the endothelium’s ability to sense circulating interferon (IFN) type I signals, would limit SARS-CoV-2 entry into the brain.
Figure 1.
Expression Profiles of Selected Genes Relevant to SARS-CoV-2 Brain Entry
In a single nuclear RNA-seq profile of human cortical brain tissue (https://celltypes.brain-map.org) (Hodge et al., 2019), there was no evidence of ACE2 expression in any brain cell type. Basigin (BSG) was prominently expressed in pericytes and endothelial cells, whereas neuropilin-1 (NRP1) was detected in endothelial cells and in several classes of excitatory neurons. Low expression of TMPRSS11A and FURIN was found in neurons, whereas CSTB was moderately expressed in most cell types with the exception of astrocytes and oligodendrocytes and their precursors. Endothelial cells and pericytes also express lymphocyte antigen 6 family member E (LY6E) and the interferon (IFN)-induced transmembrane proteins-1 and 3 (IFITM1, IFITM3), which have been shown to restrict SARS-CoV-2 cell entry (Hachim et al., 2020; Pfaender et al., 2020; Zhao et al., 2020). IFN type I receptors (IFNRA1 and IFNRA2) showed higher expression in endothelial cells than in other cell types. Cell cluster annotations are from (Hodge et al., 2019). CPM, transcript counts per million within the cell cluster; FracCellExpr, fraction of cells in which the transcript is detected.
Figure S1.
Expression Profiles of Selected Genes Relevant to SARS-CoV-2 Brain Entry in Single Nuclear RNA-Seq Profiles of Human Brain Tissue, Related to “Brain Expression of SARS-CoV-2 Receptors and Related Proteins”
A, count matrix and cluster annotations are from https://portal.brain-map.org/atlases-and-data/rnaseq/human-m1-10x. The data set is composed of 76,533 total nuclei of the primary motor cortex derived from 2 post-mortem human brain specimens. B, count matrix and cluster annotations are from the Gene Expression Omnibus submission GSE97942 (Lake et al., 2018). The data set is composed of 35,289 nuclei collected from frontal cortex, visual cortex, and cerebellum from 6 post-mortem human brain samples. CPM, transcript counts per million transcripts within the cell cluster; FracCellExpr, fraction of cells in which the transcript is detected. Data analysis was performed in the R (Vers. 4.02) statistical environment using tidyverse (Vers. 1.3.0) and Seurat (Vers. 3.2.0) packages.
Nervous System Invasion
The possibility of CNS invasion for SARS-CoV-2 has been suggested by analogy with the neurotropism of other coronaviruses, mainly SARS-CoV-1, MERS-CoV, and OC43 (Bergmann et al., 2006). Organoids and in vivo studies in human ACE2 transgenic mice have shown that SARS-CoV-2 can infect neurons and cause neuronal death in an ACE2-dependent manner (Song et al., 2020). In brain cells derived from human pluripotent stem cells, dopaminergic neurons, but not cortical neurons or microglia, were particularly susceptible to SARS-CoV-2 infection (Yang et al., 2020). Clinical-pathological studies that have tested for the presence of the virus in the brain or the cerebrospinal fluid (CSF) have had mixed results. Some studies have shown SARS-CoV-2 RNA in brain post-mortem or in the CSF in patients with encephalopathy or encephalitis, but at very low levels (Moriguchi et al., 2020; Solomon et al., 2020). Other studies could not detect viral invasion, even though there was evidence of CSF inflammation (Bernard-Valnet et al., 2020; Ye et al., 2020). Considering the inconsistent data and the low levels of viral RNA, when detected, the possibility of artifact or contamination has been raised (Solomon et al., 2020).
Potential Routes of Brain Entry
Examination of how the virus could enter the nervous system may help assess the likelihood for direct invasion and pathogenicity. Based on other coronaviruses, several potential routes of entry for SARS-CoV-2 have been proposed (Bergmann et al., 2006).
Olfactory Route
Infection of olfactory system is consistent with the observation that loss of smell is a frequent neurological manifestation in COVID-19 (see Neurological Manifestations of COVID-19) and with evidence of increased MRI signal in the olfactory cortex suggestive of infection (Politi et al., 2020). The virus could be internalized in nerve terminals by endocytosis, transported retrogradely, and spread trans-synaptically to other brain regions, as described for other coronaviruses (Dubé et al., 2018). ACE2 and TMPRSS2 have been detected in the nasal mucosa at the RNA and protein levels, but they seem to be localized to epithelial cells (sustentacular cells), not olfactory neurons (Brann et al., 2020), although another report suggests neuronal involvement (Nampoothiri et al., 2020). Therefore, it is unclear if the virus is restricted to the olfactory epithelium or reaches olfactory neurons.
Blood-Brain Barrier
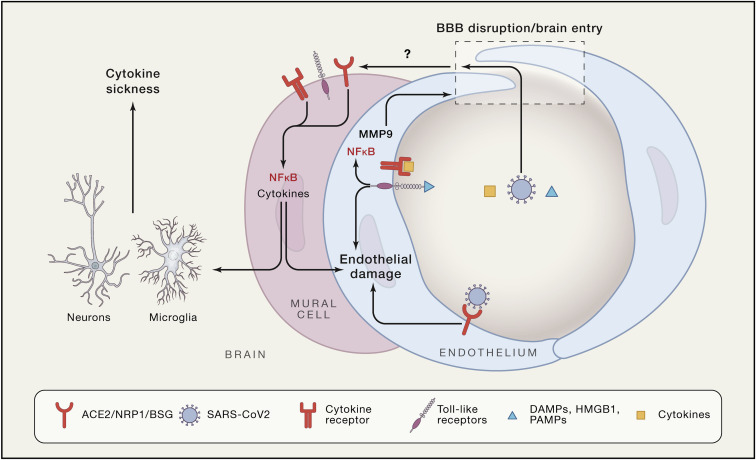
The blood-brain barrier (BBB) is a common route of entry of blood-borne viruses into the brain (Bergmann et al., 2006). In COVID-19, dissemination of the virus into the blood has been described, albeit with widely ranging frequencies (1% to 41%) (Wang et al., 2020c; Zheng et al., 2020), and the virus could access the brain by crossing the BBB. Crossing the intact BBB would require internalization and transport of the virus across the cerebral endothelium, in which the expression of SARS-CoV-2 docking proteins remains unclear (Figure 1). ACE2 immunoreactivity was observed in brain vessels of a patient who died with multiple ischemic infarcts but the cellular localization was not determined (Bryce et al., 2020). The possibility of entry through other putative SARS-CoV-2 receptors expressed more widely in the cerebral vasculature, such as NRP1 and BSG, cannot be ruled out (Cantuti-Castelvetri et al., 2020). On the other hand, SARS-CoV-2-associated cytokines, including interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF), and IL-17 disrupt the BBB (Erickson and Banks, 2018) and could facilitate the entry of the virus (Figure 2 ). SARS-CoV-2 has been postulated to induce endothelial infection and inflammation in peripheral vessels (Teuwen et al., 2020), but direct evidence in cerebral endothelial cells has not been thus far provided. Rather, a lack of florid cerebrovascular inflammation has been noted in several autopsy studies (Bryce et al., 2020; Kantonen et al., 2020; Reichard et al., 2020; Solomon et al., 2020). Comorbidities often seen in COVID-19, including cardiovascular risk factor or pre-existing neurological diseases, could, alone or in combination with cytokines, increase BBB permeability (Erickson and Banks, 2018). For example, in a COVID-19 patient with Parkinson’s disease, electron microscopy revealed viral particles in frontal lobe microvessels and neurons, suggesting trans-endothelial entry (Paniz-Mondolfi et al., 2020). Another Parkinson’s disease patient with obesity, hypertension, and diabetes, exhibited at autopsy, in addition to hypoxic-ischemic neuronal damage, microhemorrhages, white matter lesions, and enlarged perivascular spaces, but no evidence of SARS-CoV-2 in the brain (Kantonen et al., 2020). SARS-CoV-2 could also enter the brain through the median eminence of the hypothalamus and other circumventricular organs, brain regions with a leaky BBB due to openings (fenestrae) in the capillary wall (Kaur and Ling, 2017). Although the size of the viral particle (80–120 nm) is larger than endothelial fenestrae (Sarin, 2010), preliminary data suggest that median eminence capillaries and tanycytes express ACE2 and TMPRSS, which could allow virus entry into the hypothalamus (Nampoothiri et al., 2020). Owing to its widespread connection, the hypothalamus could serve as a gateway to the entire brain.
Figure 2.
Potential Mechanisms of Vascular Damage and Brain Entry of SARS-CoV-2
Circulating virus, cytokines, DAMPs, and PAMPs could act on endothelial cells, leading to inflammation and opening of the BBB. Once in the perivascular space and these factors could induce inflammation in vascular mural cells and brain resident myeloid cells (microglia and macrophages). The resulting cytokine production could affect neuron neuronal function leading to the cytokine sickness, a potential cause of encephalopathy in COVID-19.
Infiltration of Infected Immune Cells
Viruses can enter the brain carried by infected immune cells, which can also serve as reservoir (Bergmann et al., 2006). Monocytes, neutrophils, and T cells traffic into the brain through the vasculature, the meninges, and the choroid plexus (Engelhardt et al., 2017), and these sites could be entry points for infected immune cells. Conclusive evidence of infection of immune cells by SARS-CoV-2 has not been provided thus far (Merad and Martin, 2020). SARS-CoV-2 nucleocapsid protein (NP) immunoreactivity was observed in CD68+ cells in lymphoid organs (Chen et al., 2020), while single-cell RNA seq data showed viral RNA in macrophages in bronchoalveolar lavage of COVID-19 patients (Bost et al., 2020). However, it remains unclear if this is due to actual virus propagation in macrophages or to phagocytic uptake of virus infected cells or extracellular virions (Bost et al., 2020; Merad and Martin, 2020). Furthermore, several autopsy series have revealed a notable lack of immune cell infiltration (Kantonen et al., 2020; Reichard et al., 2020; Solomon et al., 2020).
In summary, SARS-CoV-2 can infect neurons in vitro and cause neuronal death, but data from CSF and autopsy studies do not provide consistent evidence of direct CNS invasion. However, effects on the median eminence and other circumventricular organs cannot be ruled out and may play a role in the systemic manifestations of the disease.
Indirect Brain Effects of Systemic Factors
Several major organs are targeted by COVID-19 resulting in life threatening systemic complications.
Lung Damage and Respiratory Failure
The lung is the organ most affected in COVID-19, with massive alveolar damage, edema, inflammatory cell infiltration, microvascular thrombosis, microvascular damage, and hemorrhage (Carsana et al., 2020). SARS-CoV-2 has been detected mainly in pneumocytes and epithelial progenitors (Bost et al., 2020; Carsana et al., 2020). The respiratory failure resulting from lung damage leads to severe hypoxia (acute respiratory distress syndrome [ARDS]) requiring assisted ventilation (Grasselli et al., 2020). Consistent with hypoxic brain injury, autopsy studies in COVID-19 have shown neuronal damage in brain regions most vulnerable to hypoxia, including neocortex, hippocampus, and cerebellum (Kantonen et al., 2020; Reichard et al., 2020; Solomon et al., 2020).
Systemic Inflammation and Immune Dysregulation
A key feature of COVID-19 is a maladaptive immune response characterized by hyperactivity of innate immunity followed by immunosuppression (Diao et al., 2020; Qin et al., 2020; Vabret et al., 2020; Zhou et al., 2020). Improvement of T cell function coincides with remission of symptoms and declining viral loads (Thevarajan et al., 2020), attesting to the link between immuno-suppression and disease severity. In patients with severe disease, the cytokine release syndrome can develop (Qin et al., 2020; Xu et al., 2020). Most COVID-19 patients exhibit increased circulating levels of IL-6, IL-1β, and TNF, as well as IL-2, IL-8, IL-17, G-CSF, GM-CSF, IP10, MCP1, and MIP1α2, and serum levels of IL-6 and TNF reflect disease severity (Diao et al., 2020). Even in the absence of SARS-CoV-2 brain invasion, viral proteins shed in the circulation and molecular complexes from damaged cells, such as the nuclear protein high mobility group box 1 (HMGB1) (Chen et al., 2020b), could enter the brain through a compromised BBB (Figure 2). After brain entry, these molecules could act as pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patters (DAMPs), and induce an innate immune response in pericytes, brain-resident macrophages, and microglia, which express toll-like receptors (TLR) (Figure 2). TLR2 mediates the pro-inflammatory effects of SARS-CoV spike protein on human macrophages through nuclear factor κB (NF-κB) (Dosch et al., 2009). Such innate immune response increases cytokine production and impair brain function (Dantzer, 2018). In mice, viral infections increase circulating levels of IFNα/β leading to activation IFNR1 on cerebral endothelial cells and CXCL10-CXCR3-mediated cognitive impairment (cytokine sickness behavior) (Dantzer, 2018). An IFN type I response does occur in COVID-19 and is thought to be protective (Merad and Martin, 2020), but could contribute to the alterations in consciousness (see Neurological Manifestation of COVID-19).
The Hypothalamus: Target and Culprit of Immune Dysregulation
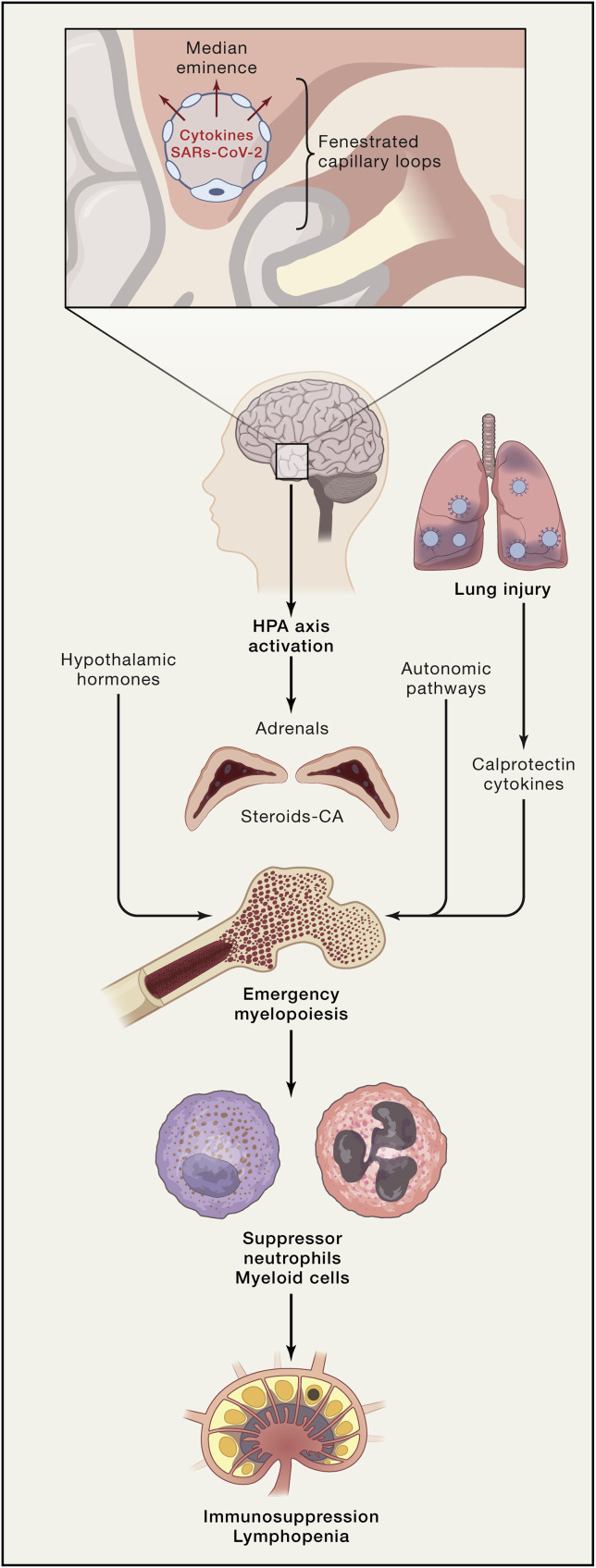
The brain, the hypothalamus in particular, could also contribute to the immune dysregulation (Figure 3 ). Several cytokines upregulated in COVID-19 (IL-6, IL-1β, and TNF) are powerful activators of the hypothalamic-pituitary-adrenocortical (HPA) axis (Dantzer, 2018). The HPA axis is central to the regulation of systemic immune activity and is activated by BBB dysfunction and neurovascular inflammation (Dantzer, 2018). As mentioned above, COVID-19 is associated with immunosuppression and lymphopenia. In stroke and brain trauma, adrenergic stress involving β-adrenergic receptors results in massive systemic immunosuppression (Iadecola et al., 2020). The mechanisms of these effects involve activation of the HPA, leading to the release of norepinephrine and glucocorticoids. These mediators act synergistically to induce splenic atrophy, T cell apoptosis, and natural killer (NK) cell deficiency. In the bone marrow, tyrosine hydroxylase and norepinephrine trigger a response in mesenchymal stromal cells, most likely through β3-adrenergic receptors, resulting in a reduction of cell retention (Iadecola et al., 2020). Downregulation of these factors, in concert with calprotectin release from damaged lungs, may increase hematopoietic stem cell proliferation skewed toward the myeloid lineage (emergency myelopoiesis) (Schulte-Schrepping et al., 2020; Silvin et al., 2020), which results in lymphopenia and neutrophilia, two key hematological features of COVID-19 (Chen et al., 2020a; Moriguchi et al., 2020; Qin et al., 2020) (Figure 3). Importantly, in SARS, HPA activation and glucocorticoid levels are correlated with neutrophilia and lymphopenia (Panesar et al., 2004).
Figure 3.
Potential Role of the HPA Axis in the Immune Dysregulation of COVID-19
Cytokine and SARS-CoV-2 entry into the median eminence of the hypothalamus could lead to activation of the autonomic nervous system and release of adrenal catecholamines and steroids. In analogy with stroke, brain trauma, and myocardial infarction (Iadecola et al., 2020), these neurohumoral effectors could act on the bone marrow to release immunosuppressor neutrophils and myeloid cells (emergency myelopoiesis), as described in COVID-19 (Schulte-Schrepping et al., 2020), leading to immunosuppression and lymphopenia. In addition, release of calprotectin and cytokines from damaged lungs could also contribute to emergency myelopoiesis (Silvin et al., 2020).
Hypercoagulable State
Another key feature of COVID-19 is a profound coagulopathy responsible for some of the most frequent and harmful complications of the disease. In a multicenter study, 88% of patients exhibited evidence of a hypercoagulable state (Helms et al., 2020b). COVID-19 coagulopathy is characterized by a distinctive pro-coagulant state with increased cloth strength, increased D-dimers (fibrin breakdown products indicative of intravascular thrombosis), and increased fibrinogen, without significant changes in the number of platelets or prolongation of clotting time parameters (Helms et al., 2020b). Coagulopathy and thrombosis may start in the lungs and other infected organs with endothelial damage, complement activation, the procoagulant action of IL-6, and neutrophil recruitment (Goshua et al., 2020; Ramlall et al., 2020). In turn, neutrophils release extracellular traps (NETs) in COVID-19 (Middleton et al., 2020), a lattice of chromatin and histones that activates clotting, which contributes to intravascular thrombosis by trapping cells and platelets in many organs including the brain.
Systemic Organ Failure
COVID-19 also damages other organs. Metabolic and pathological evidence of damage to the kidney, heart, liver, gastrointestinal tract, and endocrine organs has been provided (Goyal et al., 2020; Inciardi et al., 2020; Pal and Banerjee, 2020; Pan et al., 2020; Su et al., 2020). The resulting systemic metabolic changes, including water and electrolyte imbalance, hormonal dysfunction, and accumulation of toxic metabolites, could also contribute to some of the more non-specific nervous system manifestations of the disease, like confusion, agitation, headache, etc. Cardiac involvement could impact the brain by reducing cerebral perfusion or, as discussed in the next section, could be an embolic source leading to ischemic strokes.
Neurological Manifestations of COVID-19
Numerous neurological abnormalities have been described in patients with COVID-19. These involve the central and peripheral nervous system, range from mild to fatal, and can occur in patients with severe or otherwise asymptomatic SARS-CoV-2 infection. Neurological abnormalities have been described in ∼30% of patients who required hospitalization for COVID-19, 45% of those with severe respiratory illness and 85% of those with ARDS (Helms et al., 2020a; Mao et al., 2020). In patients with mild COVID-19, neurological symptoms are mostly confined to nonspecific abnormalities such as malaise, dizziness, headache, and loss of smell and taste (Mao et al., 2020), routinely observed in respiratory virus infections such as the influenza (Chow et al., 2019). While serious neurological complications have been reported in patients with otherwise mild COVID-19 (Oxley et al., 2020), the most severe complications occur in critically ill patients and are associated with significantly higher mortality (Merkler et al., 2020; Yaghi et al., 2020).
Encephalopathy and Encephalitis
Alterations in mental status (confusion, disorientation, agitation, and somnolence), collectively defined as encephalopathy, have been consistently reported in various cohorts with COVID-19. Altered mental status occurs rarely (<5%), even in COVID-19 patients requiring hospitalization for respiratory illness (Mao et al., 2020), but affects the majority of critically ill COVID-19 patients with ARDS (Helms et al., 2020a). A key question is whether this alteration in mental status represents an encephalopathy caused by systemic illness or encephalitis directly caused by the SARS-CoV-2 virus itself. Several cases have been reported of COVID-19 patients (Efe et al., 2020; Farhadian et al., 2020; Huang et al., 2020b; Moriguchi et al., 2020; Pilotto et al., 2020) who appear to meet established diagnostic criteria for infectious encephalitis, which include altered mental status, fever, seizures, white blood cells in the CSF, and focal brain abnormalities on neuroimaging (Venkatesan et al., 2013). In at least two reported cases, SARS-CoV-2 was detected in the CSF (Huang et al., 2020b; Moriguchi et al., 2020), although, as discussed in the previous section (Nervous System Invasion), only modest amounts of viral RNA were detected. In at least one COVID-19 case, the diagnosis of temporal lobe encephalitis was confirmed by biopsy that showed perivascular lymphocytic infiltrates and hypoxic neuronal damage (Efe et al., 2020), but the presence of SARS-CoV2 or other viruses in brain or CSF was not documented. Indeed, most samples of CSF in patients with neurological abnormalities in the setting of COVID-19 have not revealed evidence of SARS-CoV-2 (Kandemirli et al., 2020), and most samples of brain tissue from autopsies of COVID-19 patients have not revealed evidence of encephalitis (see Nervous System Invasion). Besides encephalitis, most COVID-19 patients have other reasons for their altered mental status. Delirium, confusional states, and coma appear most common in COVID-19-related critical illness (Helms et al., 2020a; Mao et al., 2020; Rogers et al., 2020), which is often marked by hypoxia, hypotension, renal failure, the need for heavy doses of sedatives, and prolonged immobility and isolation (Cummings et al., 2020)—all factors well known to cause encephalopathy (Maas, 2020). The rarity of cases clinically consistent with encephalitis, the paucity of histopathological evidence of encephalitis, and the many alternative explanations for the altered mental status, suggest that SARS-CoV-2 brain invasion is a possible but rare cause of encephalopathy.
Ischemic Stroke
Stroke is not uncommon among patients hospitalized with COVID-19, with reported rates ranging from 1%–3% in hospitalized patients and up to 6% of critically ill patients (Mao et al., 2020; Merkler et al., 2020; Yaghi et al., 2020), 7-fold higher than in patients hospitalized with influenza even after adjustment for illness severity (Merkler et al., 2020). Early case reports described unusual embolic strokes in otherwise young healthy individuals with COVID-19 (Oxley et al., 2020), but in subsequent case series, patients were generally older and had numerous vascular comorbidities (Lodigiani et al., 2020). Therefore, it remains unclear whether these strokes were caused by SARS-CoV-2 or represented the background incidence of stroke in these high-risk populations that also happened to be infected at the time. It is plausible that SARS-CoV-2 infection does play some role in causing stroke, given that infections in general increases stroke risk (Parikh et al., 2020). The COVID-19-related hypercoagulability would be expected to increase susceptibility to cerebrovascular events, as reported in an autopsy series in which widespread microthrombi and patches of infarction were observed some brains (Bryce et al., 2020). Patients with COVID-19 may be at risk of cardioembolic stroke. Acute cardiac injury and clinically significant arrhythmias have been reported in approximately10% of hospitalized COVID-19 patients and 20%–40% of those requiring intensive care (Goyal et al., 2020; Huang et al., 2020a; Wang et al., 2020a). SARS-CoV-2 infection may rarely cause myocarditis and heart failure even in the absence of significant pulmonary involvement (Inciardi et al., 2020). Myocardial injury and arrhythmias, such as atrial fibrillation, in the setting of severe infection may result in cardiac embolism and brain infarction (Inciardi et al., 2020). A substantial proportion of critically ill patients with COVID-19 may also develop secondary bacteremia in addition to the primary viral illness. In one case series, approximately10% of patients requiring mechanical ventilation had bacteremia (Goyal et al., 2020), which increases the risk of stroke by over 20-fold (Dalager-Pedersen et al., 2014). Septic emboli to the brain often result in bleeding, and in a postmortem magnetic resonance imaging study, 10% of brains had evidence of hemorrhage (Coolen et al., 2020).Taken together, these clinical findings suggest that SARS-CoV-2 may adversely affect the brain via multiple pathophysiological pathways that culminate in vascular brain injury.
Post-infectious Neurological Complications
SARS-CoV-2 unleashes a dysregulated systemic immune response (see Systemic Inflammation and Immune Dysregulation), which can have delayed effects on the nervous system. These immune-mediated manifestations involve both the central and peripheral nervous system and occur typically after the acute phase of the infection subsides. In the CNS, reported cases in COVID-19 resemble classic post-infectious inflammatory conditions such as acute disseminated encephalomyelitis (Parsons et al., 2020) and acute necrotizing hemorrhagic encephalopathy (Poyiadji et al., 2020). Peripherally, several cases of Guillain-Barre syndrome, a neuropathy caused by an immune attack on peripheral nerves, have been reported in patients with recent COVID-19 (Toscano et al., 2020). Most reported cases describe classic features of this syndrome, such as generalized weakness, evidence of demyelination on nerve conduction studies, and elevated proteins without white blood cells in CSF (Toscano et al., 2020). The Miller-Fisher variant of Guillain-Barre syndrome, characterized by cranial nerve involvement, has also been reported, including at least one case with detectable anti-ganglioside antibodies suggesting an immune attack on the peripheral nerves (Gutiérrez-Ortiz et al., 2020). SARS-CoV-2 was not detected in any of the CSF samples (Toscano et al., 2020), supporting an immune mechanism rather than direct infection.
Intensive Care-Related Neurological Manifestations
The relatively high frequency of altered mental status in hospitalized COVID-19 patients is congruent with the severity of their illness. Most critically ill COVID-19 patients require mechanical ventilation (Cummings et al., 2020) and an agitated confusional state (delirium) occurs in more than 80% of mechanically ventilated patients in intensive care units (Ely et al., 2001). Patients with ARDS, which frequently complicates severe COVID-19, are at particularly high risk of delirium, likely because of hypoxemia heavy doses of sedatives, administration of paralytic agents, or other causes (Hopkins et al., 2005; Ouimet et al., 2007).
Comparison with Other Viral Respiratory Infections
Many neurological abnormalities seen in COVID-19 mirror those of other viral respiratory illnesses. All of the reported COVID-19 related post-infectious inflammatory conditions of the nervous system, such as Guillain-Barre syndrome, acute necrotizing hemorrhagic encephalopathy, and acute disseminated encephalomyelitis, are classically seen after infections, including other coronaviruses (Gerges Harb et al., 2020). Influenza is occasionally associated with an encephalopathy or full blown encephalitis, with evidence of influenza virus in the cerebrospinal fluid (Surtees and DeSousa, 2006). Comparing the large numbers of patients infected by SARS-CoV-2 worldwide and the relative paucity of reported encephalitis cases, SARS-CoV-2 seems more similar to other common respiratory viral pathogens like influenza than to neurotropic pathogens that target specifically the brain, such as the herpes simplex virus. In general, however, COVID-19 is more debilitating than other common viral respiratory illnesses. Physicians have been struck by the frequency of thrombotic complications observed in critically ill COVID-19 patients, to the point that some hospitals instituted protocols for empiric, high-dose anticoagulation in patients with elevated D-dimer levels (Paranjpe et al., 2020). Emerging data seem to confirm this observation: in one multicenter study, patients with COVID-19 and acute respiratory distress syndrome had twice the incidence of thrombotic complications compared to a matched cohort with ARDS from other causes (Helms et al., 2020b). This also applies to thrombotic complications affecting the brain, because the proportion of COVID-19-related hospitalizations complicated by stroke seems much higher than that seen in influenza (Merkler et al., 2020). Based on neuroinflammation-associated abnormalities in the clotting cascade in brain (Han et al., 2008), activated protein C or thrombin inhibitors could also be of therapeutic value.
Future Directions and Conclusions
The findings reviewed above indicate that neurological manifestations are common in COVID-19 and constitute a defining aspect of the symptomatology of the disease. A caveat is that most clinical data are derived from case series on patients ill enough to require hospitalization at tertiary care centers, providing a biased representation of the frequency and type of the neurological manifestations. Similarly, basic science investigations exploring the mechanism of disease have largely emphasized concepts and findings that emerged from other coronaviruses, and there is limited new data on the interaction of SARS-CoV-2 with the brain and its vasculature. Therefore, conclusions based of existing literature have to be considered preliminary and subject to further scrutiny, verification, and validation. Here are some of the outstanding questions:
Do the neurological manifestations of COVID-19 reflect brain invasion? The encephalopathy is most likely a consequence of systemic factors, such as cytokine sickness, hypoxia, and metabolic dysfunction due to peripheral organ failure, while the strokes seem to be related more to hypercoagulability and endothelial injury than to SARS-CoV-2 vasculitis affecting brain vessels. The loss of taste and smell has been attributed to invasion of the olfactory neural system, but consistent evidence is lacking. In some cases, the possibility of a SARS-CoV-2 encephalitis could not be ruled out based on the potential for the virus to infect neurons (Song et al., 2020), but definitive clinical and pathological evidence of neurotropism is lacking. A major problem is that the molecular mechanisms of cellular entry for SARS-CoV-2 are not entirely clear. While ACE2 is thought to be the main receptors in some cell types, its expression levels do not seem to correlate with the infectivity potential. For example, the virus gains access to human pluripotent stem cell-derived dopaminergic neurons despite low levels of ACE2 (Yang et al., 2020). Systematic investigation of non-canonical docking and accessory proteins for SARS-CoV-2 (Figures 1 and S1), their cellular localization and function in human neurons, glia, and vascular cells would help address this question.
Does the brain contribute to the immune dysregulation? SARS-CoV-2 and inflammatory mediators may gain access to the median eminence and activate hypothalamic neurohumoral pathways that mediate immune dysregulation through the adrenergic system, as described in other brain diseases (Figure 3). Considering the importance of the immune dysregulation in COVID-19 severity and outcome, a better understanding of the contribution of the hypothalamus may suggest pharmacological approaches to dampen the immune dysregulation (Iadecola et al., 2020).
Does the brain contribute to respiratory failure and hypertension? Similarly, entry of the virus and/or proinflammatory molecules through the subfornical organ and the area postrema could also affect brainstem autonomic pathways controlling blood pressure and breathing (Kaur and Ling, 2017). Alterations in blood pressure, both hypertension (Liu et al., 2020) and severe hypotension in critically patients (Goyal et al., 2020), are highly prevalent in COVID-19. Furthermore, it has been suggested that involvement of brainstem respiratory nuclei may contribute to the respiratory failure (Li et al., 2020), but no alterations in respiratory centers or chemoreceptors (carotid bodies) was observed at autopsy in a patient with respiratory dysregulation (Kantonen et al., 2020). To date, evidence of central autonomic involvement is lacking.
What are the long-term neurological and neuropsychiatric consequences of COVID-19? Respiratory virus infections are associated with neurological and psychiatric sequelae, including Parkinsonism, dementia, depression, post-traumatic stress disorder, and anxiety (Limphaibool et al., 2019; Rogers et al., 2020). Brain infection is not required for these long-term effects. Inflammation and cytokine elevation in sepsis survivors are linked to subsequent hippocampal atrophy and cognitive impairment (Iwashyna et al., 2010). Experimental studies suggest a link between activation of the NLRP3 inflammasome, which may occur in COVID-19, and Alzheimer pathology (Ising et al., 2019). ARDS survivors also exhibit increased incidence of long-term depression, anxiety, and cognitive impairment (Hopkins et al., 2005). Whether these late manifestations are related to non-resolving inflammation or a low-grade immune process driven by molecular mimicry or dysregulated adaptive immunity remains to be established. Chronic damage to systemic organs can also harm the brain through chronic hypoxia, metabolic dysfunction, and hormonal dysregulation. Based on these considerations, significant long-term neurological and psychiatric sequelae have to be anticipated in COVID-19, especially in survivors of severe disease.
Experimental Models
Models would help address these outstanding questions and facilitate therapeutic development. Unfortunately, mice, the most popular laboratory animals, are not susceptible to SARS-CoV-2 due to differences between mouse and human ACE2 (Lakdawala and Menachery, 2020). Mice expressing human ACE2 have been developed and show evidence of brain infection, but only minimal symptoms of disease (Song et al., 2020). Hamsters, ferrets, cats, and non-human primates could be more viable models (Lakdawala and Menachery, 2020). Reproducing the systemic effects of the disease would be critical for studying the neurological aspects of COVID-19. In vitro approaches involving human pluripotent stem cells organoids and co-cultures are useful to examine infectious mechanisms in brain cells (Song et al., 2020; Yang et al., 2020) but do not provide insight into the harmful systemic effects. Therefore, there is a pressing need to develop animal models that are amenable to investigate not only the effects of SARS-CoV-2 on brain cells, but also the systemic effects of the infection and the long-term neuropsychiatric consequences.
Therapeutic Considerations
Until safe and effective vaccines are developed, therapeutic efforts have to focus on antiviral agents and on how to best manage respiratory insufficiency, organ failure, hypercoagulable state, and immune dysregulation. There is no specific treatment for the neurological manifestations, which are managed according to standard protocols. However, because the neurological complications emerge mainly in severe systemic disease, minimizing hypoxia and protecting the brain from cytokines, DAMPs, PAMPs, and thromboembolic complications are important therapeutic goals. Immunosuppression with steroids improves mortality in patients with severe disease, but not in those with milder forms (Horby et al., 2020). Furthermore, more nuanced approaches to counteract the immune dysregulation, such as targeting specific cytokines or inflammatory pathways are also being tested (Vabret et al., 2020). Whether these interventions reduce the short- and long-term neurological and psychiatric complications remain to be established.
In conclusion, the neurological manifestations of COVID-19 constitute a major public health challenge not only for the acute effects on the brain, but also for the long-term harm to brain health that may ensue. These delayed manifestations are anticipated to be significant, because they are likely to also affect patients who did not show neurological symptoms in the acute phase. Therefore, clinical and laboratory efforts aiming to elucidate the mechanisms of the acute effects on the brain of SARS-CoV-2 need to be coupled with investigations on the deleterious delayed neuropsychiatric sequelae of the infection. These efforts should be driven by a close cooperation between clinical and basic scientists and take advantage of the wealth of clinical-epidemiological data and biological specimens that are accumulating worldwide. Considering that COVID-19 is still raging in many countries, including the United States, and there might be a seasonal resurgence of infection, it is imperative that a concerted effort is implemented swiftly and on a large scale.
Acknowledgments
The authors are supported by NIH (R01-NS34179, R01-NS100447, R37-NS089323, R01-NS095441, R01-NS/HL37853 to C.I.; R01NS097443 to H.K.; and NS094507 and NS081179 to J.A.
Declaration of Interests
C.I. serves on the Scientific Advisory Board of Broadview Ventures. H.K. serves as co-PI for the NIH-funded ARCADIA trial (NINDS U01NS095869) that receives in-kind study drug from the BMS-Pfizer Alliance for Eliquis and ancillary study support from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. J.A. has no conflict of interests to declare.
References
- Bergmann C.C., Lane T.E., Stohlman S.A. Coronavirus infection of the central nervous system: host-virus stand-off. Nat. Rev. Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Valnet R., Pizzarotti B., Anichini A., Demars Y., Russo E., Schmidhauser M., Cerutti-Sola J., Rossetti A.O., Du Pasquier R. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur. J. Neurol. 2020 doi: 10.1111/ene.14298. Published online May 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E., Blecher-Gonen R., Cohen M., Medaglia C., Li H., et al. Host-Viral Infection Maps Reveal Signatures of Severe COVID-19 Patients. Cell. 2020;181:1475–1488. doi: 10.1016/j.cell.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.-j., Fletcher R.B., et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;382:eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C., Grimes Z., Pujadas E., Ahuja S., Beasley M.B., Albrecht R., Hernandez T., Stock A., Zhao Z., Al Rasheed M., et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020 doi: 10.1101/2020.05.18.20099960. [DOI] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., Kallio K., Kaya T., Anastasina M., Smura T., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. bioRxiv. 2020 doi: 10.1101/2020.06.07.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30434-5. Published online June 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Feng Z., Diao B., Wang R., Wang G., Wang C., Tang Y., Liu L., Wang C., Liu Y., et al. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. MedRxiv. 2020 doi: 10.1101/2020.03.27.20045427. [DOI] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y., Wei J., Luo H., Zhu H., Huang L., et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020;368:1–3. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Yu J., Wang K., Howard D., French L., Chen Z., Wen C., Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. bioRxiv. 2020 doi: 10.1101/2020.04.07.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow E.J., Doyle J.D., Uyeki T.M. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit. Care. 2019;23:214. doi: 10.1186/s13054-019-2491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen T., Lolli V., Sadeghi N., Rovaï A., Trotta N., Taccone F.S., Creteur J., Henrard S., Goffard J.C., De Witte O., et al. Early postmortem brain MRI findings in COVID-19 non-survivors. medRxiv. 2020 doi: 10.1101/2020.05.04.20090316. [DOI] [PubMed] [Google Scholar]
- Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalager-Pedersen M., Søgaard M., Schønheyder H.C., Nielsen H., Thomsen R.W. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014;129:1387–1396. doi: 10.1161/CIRCULATIONAHA.113.006699. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018;98:477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J. Virol. 2018;92:e00404-18. doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe I.E., Aydin O.U., Alabulut A., Celik O., Aydin K. COVID-19-Associated Encephalitis Mimicking Glial Tumor. World Neurosurg. 2020;140:46–48. doi: 10.1016/j.wneu.2020.05.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely E.W., Inouye S.K., Bernard G.R., Gordon S., Francis J., May L., Truman B., Speroff T., Gautam S., Margolin R., et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Vajkoczy P., Weller R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- Erickson M.A., Banks W.A. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol. Rev. 2018;70:278–314. doi: 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian S., Glick L.R., Vogels C.B.F., Thomas J., Chiarella J., Casanovas-Massana A., Zhou J., Odio C., Vijayakumar P., Geng B., et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20:248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges Harb J., Noureldine H.A., Chedid G., Eldine M.N., Abdallah D.A., Chedid N.F., Nour-Eldine W. SARS, MERS and COVID-19: clinical manifestations and organ-system complications: a mini review. Pathog. Dis. 2020;78:ftaa033. doi: 10.1093/femspd/ftaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., Satlin M.J., Campion T.R., Jr., Nahid M., Ringel J.B., et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. COVID-19 Lombardy ICU Network Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1578. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., de Aragón-Gómez F., Benito-León J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Hachim M.Y., Al Heialy S., Hachim I.Y., Halwani R., Senok A.C., Maghazachi A.A., Hamid Q. Interferon-Induced Transmembrane Protein (IFITM3) Is Upregulated Explicitly in SARS-CoV-2 Infected Lung Epithelial Cells. Front. Immunol. 2020;11:1372. doi: 10.3389/fimmu.2020.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.H., Hwang S.-I., Roy D.B., Lundgren D.H., Price J.V., Ousman S.S., Fernald G.H., Gerlitz B., Robinson W.H., Baranzini S.E., et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- He L., Mäe M.A., Muhl L., Sun Y., Pietilä R., Nahar K., Liébanas E.V., Fagerlund M.J., Oldner A., Liu J., et al. Pericyte-specific vascular expression of SARS-CoV-2 receptor ACE2-implications for microvascular inflammation and hypercoagulopathy in COVID-19 patients. bioRxiv. 2020 doi: 10.1101/2020.05.11.088500. [DOI] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge R.D., Bakken T.E., Miller J.A., Smith K.A., Barkan E.R., Graybuck L.T., Close J.L., Long B., Johansen N., Penn O., et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573:61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R.O., Weaver L.K., Collingridge D., Parkinson R.B., Chan K.J., Orme J.F., Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. RECOVERY Collaborative Group Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report. medRxiv. 2020 doi: 10.1101/2020.06.22.20137273. [DOI] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Buckwalter M.S., Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J. Clin. Invest. 2020;130:2777–2788. doi: 10.1172/JCI135530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., Italia L., Zaccone G., Tedino C., Fabbricatore D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S.V., Vieira-Saecker A., Schwartz S., Albasset S., McManus R.M., Tejera D., et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–673. doi: 10.1038/s41586-019-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., Kaya Y., Yildirim D., Tuzuner F., Yildirim M.S., et al. Brain MRI Findings in Patients in the Intensive Care Unit with COVID-19 Infection. Radiology. 2020 doi: 10.1148/radiol.2020201697. Published online May 8 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantonen J., Mahzabin S., Mäyränpää M.I., Tynninen O., Paetau A., Andersson N., Sajantila A., Vapalahti O., Carpén O., Kekäläinen E., et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020 doi: 10.1111/bpa.12889. Published online August 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Ling E.-A. The circumventricular organs. Histol. Histopathol. 2017;32:879–892. doi: 10.14670/HH-11-881. [DOI] [PubMed] [Google Scholar]
- Lakdawala S.S., Menachery V.D. The search for a COVID-19 animal model. Science. 2020;368:942–943. doi: 10.1126/science.abc6141. [DOI] [PubMed] [Google Scholar]
- Lake B.B., Chen S., Sos B.C., Fan J., Kaeser G.E., Yung Y.C., Duong T.E., Gao D., Chun J., Kharchenko P.V., Zhang K. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 2018;36:70–80. doi: 10.1038/nbt.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limphaibool N., Iwanowski P., Holstad M.J.V., Kobylarek D., Kozubski W. Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications. Front. Neurol. 2019;10:652. doi: 10.3389/fneur.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Alexia B., et al. Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas M.B. Critical Medical Illness and the Nervous System. Continuum (Minneap. Minn.) 2020;26:675–694. doi: 10.1212/CON.0000000000000869. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E., Lantos J., Schenck E.J., Goyal P., Bruce S.S., et al. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2730. Published online July 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., et al. Neutrophil Extracellular Traps (NETs) Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood. 2020 doi: 10.1182/blood.2020007008. Published online June 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampoothiri S., Sauve F., Ternier G., Fernandois D., Coelho C., Imbernon M., Deligia E., Perbet R., Florent V., Baroncini M., et al. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. bioRxiv. 2020 doi: 10.1101/2020.06.08.139329. [DOI] [Google Scholar]
- Ouimet S., Kavanagh B.P., Gottfried S.B., Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J. Endocrinol. Invest. 2020;43:1027–1031. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., Li P., Hu B., Wang J., Hu C., et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panesar N.S., Lam C.W.K., Chan M.H.M., Wong C.K., Sung J.J.Y. Lymphopenia and neutrophilia in SARS are related to the prevailing serum cortisol. Eur. J. Clin. Invest. 2004;34:382–384. doi: 10.1111/j.1365-2362.2004.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh N.S., Merkler A.E., Iadecola C. Inflammation, Autoimmunity, Infection, and Stroke: Epidemiology and Lessons From Therapeutic Intervention. Stroke. 2020;51:711–718. doi: 10.1161/STROKEAHA.119.024157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020 doi: 10.1007/s00415-020-09951-9. Published online May 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender S., Mar K.B., Michailidis E., Kratzel A., Hirt D., V’Kovski P., Fan W., Ebert N., Stalder H., Kleine-Weber H., et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. bioRxiv. 2020 doi: 10.1101/2020.03.05.979260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Masciocchi S., Comelli A., Volonghi I., Gazzina S., Nocivelli S., Pezzini A., Focà E., Caruso A., et al. Steroid-Responsive Encephalitis in Coronavirus Disease 2019. Ann. Neurol. 2020;88:423–427. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure S.J., Green A.J., Josephson S.A. The Spectrum of Neurologic Disease in the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic Infection: Neurologists Move to the Frontlines. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1065. Published online April 10, 2020. [DOI] [PubMed] [Google Scholar]
- Politi L.S., Salsano E., Grimaldi M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2125. Published online May 29, 2020. [DOI] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlall V., Thangaraj P.M., Meydan C., Foox J., Butler D., Kim J., May B., De Freitas J.K., Glicksberg B.S., Mason C.E., et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020 doi: 10.1038/s41591-020-1021-2. Published online August 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2010;2:14–19. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B., Krämer B., Krammer T., Brumhard S., Bonaguro L., et al. Suppressive myeloid cells are a hallmark of severe COVID-19. medRxiv. 2020 doi: 10.1101/2020.06.03.20119818. [DOI] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin A., Chapuis N., Dunsmore G., Goubet A.-G., Dubuisson A., Derosa L., Almire C., Hénon C., Kosmider O., Droin N., et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1–18. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Jr., Sabeti P. Neuropathological Features of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2019373. Published online June 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu P., Weizman O.-E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., et al. Neuroinvasive potential of SARS-CoV-2 revealed in a human brain organoid model. bioRxiv. 2020 doi: 10.1101/2020.06.25.169946. [DOI] [Google Scholar]
- Su H., Yang M., Wan C., Yi L.-X., Tang F., Zhu H.-Y., Yi F., Yang H.-C., Fogo A.B., Nie X., Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees R., DeSousa C. Influenza virus associated encephalopathy. Arch. Dis. Child. 2006;91:455–456. doi: 10.1136/adc.2005.092890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., et al. Guillain-Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., et al. Sinai Immunology Review Project Immunology of COVID-19: Current State of the Science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A., Stahl J.P., Mailles A., Drebot M., Rupprecht C.E., et al. International Encephalitis Consortium Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin. Infect. Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P., Gong L., Zhang Y., Cui H.-Y., Geng J.-J., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner Moyer M. 2020. Can Covid Damage the Brain? The New York Times.https://www.nytimes.com/2020/06/26/opinion/coronavirus-brain-damage-dementia.html?smid=em-share June 26, 2020. [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K., Henninger N., Trivedi T., Lillemoe K., Alam S., et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X., Tang X., Zhu J., Zhao Z., Jaffre F., et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27:125–136. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zheng S., Chen D., Zheng M., Li X., Li G., Lin H., Chang J., Zeng H., Guo J.T. LY6E Restricts the Entry of Human Coronaviruses, Including the Currently Pandemic SARS-CoV-2. J. Virol. 2020 doi: 10.1128/JVI.00562-20. Published online July 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe. 2020;27:883–890. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]