Abstract
Background
As countermeasures against the COVID-19 outbreak, sports and entertainment events were canceled (VEC) in Japan for two weeks from 26 February through 13 March. Moreover, most schools were closed (SC).
Objective
For this study, we estimated the basic reproduction number (R0) and SC and VEC effects.
Method
After constructing a susceptible–infected–recovered model with three age classes, we used data of symptomatic patients in Japan for 14 January through 24 March. The SC and VEC effects were incorporated into the model through changes in contact patterns and contact frequencies among age classes.
Results
Results suggest R0 as 2.56, with 95% CI of [2.51, 2.96] before SC and VEC. The respective effects of SC and VEC were estimated as 0.4 (95% CI [0.3, 0.5]) and 0.5 (95% CI [0.3, 0.7]).
Conclusion
The estimated R0 is similar to those found from other studies of China and Japan. Significant reduction of contact frequency has been achieved by SC and VEC. Nevertheless, its magnitude was insufficient to contain the outbreak.
Keywords: COVID-19, School closure, SIR model, Three age class, Voluntary event cancellation
1. Introduction
The initial case of COVID-19 in Japan was that of a patient returning from Wuhan, China who showed symptoms on 3 January, 2020. Subsequently, as of 24 March, 2020, 883 people had been reported as infected while in the community. Their onset dates were reported with the exclusion of asymptomatic cases, those infected abroad, and those infected on a large cruise ship: the Diamond Princess [1].
In Japan, school closure (SC) and voluntary event cancellation (VEC) policies were introduced on 27 February. The former was intended to decrease contact among children. The latter presumably decreased contact among adults. We estimated the effects of these policies using a susceptible–infected–recovered (SIR) model [2,3] incorporating three age classes: children, adults, and elderly people. An earlier study [2] did not examine age classes. For that reason, they did not measure SC and VEC, separately. Moreover, protection among children [4] was not incorporated into the model. Because we extend the model to have three age classes, we incorporated protection among children into the model and measured SC and VEC, separately.
2. Method
2.1. Model setting
We applied a simple SIR model [2,3] with three age classes: children and adolescents 20 years old and younger, adults older than 20 but younger than 60, and elderly people 60 and older. We assumed that some children were protected initially from COVID-19 [4]. The incubation periods were assumed to be equal for the three age classes. We followed the empirical distribution through the outbreak in Japan. We assumed the ratio of asymptomatic cases over symptomatic cases as 4/19 [2].
Assuming that the degrees of infectivity among severely affected patients and mild patients were equal among asymptomatic cases, symptomatic cases can be inferred as half of the total number. This relation of infectiousness for asymptomatic cases and symptomatic cases has been used for many influenza simulation studies [[5], [6], [7], [8], [9]].
Contact patterns among children, adults, and elderly people were estimated similarly to an earlier study [10]. We presumed that SC and VEC reduced contact somewhat.
2.2. Data
We used community outbreak data of patients with COVID-19 who showed any symptom in Japan for 14 January–24 March, 2020. We excluded some patients who had returned from abroad, e.g. from China, and some patients who were presumably infected persons from the Diamond Princess. They were presumed not to have been community-infected in Japan.
For COVID-19 patients with symptoms, information published by the Ministry of Health, Labour and Welfare (MHLW) shows that Japan was adversely affected by some uncertainty-related delay related to visiting a doctor or a delay in the timing of a physician's suspicion of COVID-19. Therefore, published data of patients must be adjusted by at least a few days. To adjust the data, we applied the same regression as that used in an earlier study [2]. We used this adjusted number of patients in the latest few days including those after VEC was adopted. In all, 410 data were used for the study period in 14 January–24 March, 2020 [1].
2.3. Estimation procedure
We estimated the SC and VEC effects and the respective proportions of the protected children using a 10% point grid. Simultaneously, we sought R0 to fit the number of patients by age class during 14 January – 24 March and to minimize the sum of squared residuals between the reported numbers and the fitted values.
First, the joint distribution of R0 and the protection rate for children in Japan were estimated as fitted to the community outbreak data before SC and VEC were introduced on 27 February. Using the estimated R0 and the protection rate for children, we simulated the SC and VEC effects assuming a hypothetical change in the contact matrix.
Simulation incorporating both SC and VEC requires the assumption that the contact matrix changes to reflect the combined effects of SC and VEC. To evaluate SC and VEC, we compared the base case and intervention case total numbers of patients with any symptom. Then we calculated their 95% confidence interval (CI) was calculated using 10,000 iterations of bootstrapping for the empirical distribution of the epidemic curve.
3. Results
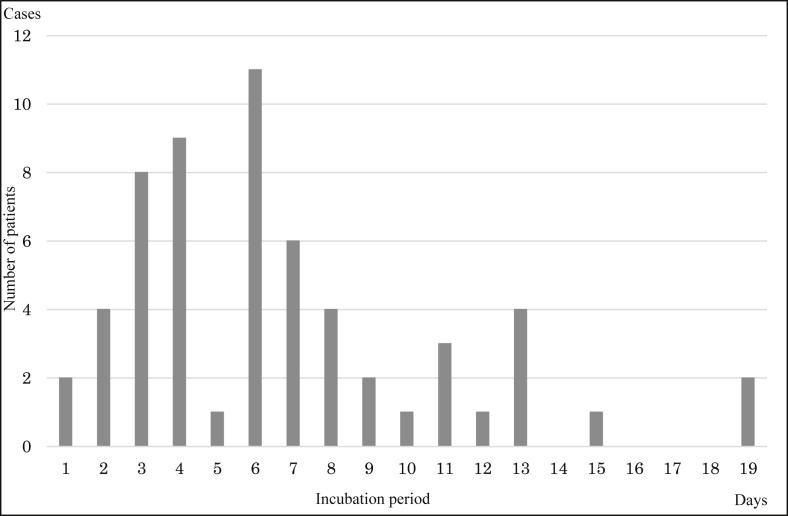
During 14 January–24 March in Japan, 16 children, 510 adults, and 418 elderly people were identified as having community-acquired COVID-19 for whom the onset date was published. Fig. 1 presents the empirical distribution of the incubation period among 62 cases for whom the exposed date and onset date were published by MHLW. The mode and median of that distribution were six days; the average was 6.74 days.
Fig. 1.
Empirical distribution of incubation period published by the Ministry of Health, Labour and Welfare, Japan. Notes: Bars represent the number of patients diagnosed by incubation period among 62 cases for which the exposed date and onset date were published by the Ministry of Health, Labour and Welfare, Japan.
The value of R0 was estimated as 2.56 with 95% CI of [2.51, 2.96] before SC and VEC. The proportion of the protected child initially was estimated as 0.4 (95% CI [0.2, 0.7]). The SC and VEC effects were estimated respectively as 0.4 (95% CI [0.3, 0.5]) and 0.5 (95% CI [0.3, 0.7]). Results indicate Re for children as 1.75. For adults it was 1.84. For elderly people, it was 2.19.
4. Discussion
We applied a simple SIR model with three age classes including asymptomatic cases and knowing that some proportion of children were protected. An earlier study [[11], [12], [13]] estimated R0 for COVID-19 as 2.24–3.58 in Wuhan. The R0 estimated in the present study was comparable with those found in other studies.
Moreover, results demonstrate that 40% of children were protected. Another study found that 50% of adults were protected [14]. However, no report of the relevant literature to date has described a similar study of cross-protection in children. Cross-protection among children might exceed 40%.
Additionally, results show that SC and VEC have significant effects for reducing contact frequencies for the same age classes. However, the effect on R0 was approximately 0.5. Because SC and VEC do not affect contacts between the other age classes, Re under SC and VEC was probably greater than one. Therefore, the outbreak itself might not be contained even if SC and VEC were to continue until the outbreak ceases.
5. Conclusion
Results revealed that significant reduction of contact frequency was achieved by SC and VEC. Nevertheless, magnitude of the reduction was insufficient to contain the outbreak.
Ethical considerations
All information used for this study was published earlier. Therefore, no ethical issue is presented.
Author's contribution
Contributors YO was responsible for the organization and coordination of the study. JK was the chief investigator and responsible for the data setting. TS developed the model and illustration the results. All authors contributed to the writing of the final manuscript.
Declaration of Competing Interest
No author has any conflict of interest, financial or otherwise, to declare in relation to this study.
Acknowledgments
We acknowledge the great efforts of all staff at public health centers, medical institutions, and other facilities fighting the spread and destruction associated with COVID-19.
This report represents the author's opinion, but does not reflect any stance of affiliated organizations.
References
- 1.Japan Ministry of Health, Labour and Welfare Press Releases of Domestic Situation (in Japanese) https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00086.html
- 2.Sugishita Y., Kurita J., Sugawara T., Ohkusa Y. Preliminary evaluation for as voluntary event cancellation as counter measure to COVID-19 outbreak in Japan as of 10 March, 2020. [DOI] [PMC free article] [PubMed]
- 3.Ohkusa Y., Sugawara T., Taniguchi K., Okabe N. Real-time estimation and prediction for pandemic A/H1N1(2009) in Japan. J Infect Chemother. 2011;17:468–472. doi: 10.1007/s10156-010-0200-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson N.M., Cummings D.A., Cauchemez S., Fraser C., Riley S., Meeyai A., et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 6.Longini I.M., Jr., Nizam A., Xu S., Ungchusak K., Hanshaoworakul W., Cummings D.A., et al. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 7.Germann T.C., Kadau L., Longini I.M., Jr., Macken C.A. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA. 2006;103:5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson N.M., Cummings D.A., Fraser C., Cajka J.C., Cooley P.C., Burke D.S. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkusa Y., Sugawara T. Simulation model of pandemic influenza in the whole of Japan. Jpn J Infect Dis. 2009;62:98–106. [PubMed] [Google Scholar]
- 10.Ibuka Y., Ohkusa Y., Sugawara T., Chapman G.B., Yamin D., Atkins K.E., et al. Social contacts, vaccination decisions and influenza in Japan. J Epidemiol Community Health. 2016;70:162–167. doi: 10.1136/jech-2015-205777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W., et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Gayle A.A., Wilder-Smith A., Rockly J. The reproductive number of COVID-19 is higher than SARS coronavirus. J Trav Med. 2020 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020 doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]