Abstract
The molecular interplay between cellular host factors and viral proteins is a continuous process throughout the viral life cycle determining virus host range and pathogenesis. The hepatitis E virus (HEV) is a long-neglected RNA virus and the major causative agent of acute viral hepatitis in humans worldwide. However, the mechanisms of liver pathology and clinical disease remain poorly understood for HEV infection. This review summarizes our current understanding of HEV–host cell interactions and highlights experimental strategies and techniques to identify novel host components required for the viral life cycle as well as restriction factors. Understanding these interactions will provide insight into the viral life cycle of HEV and might further help to devise novel therapeutic strategies and antiviral targets.
Keywords: hepatitis E virus, host factor, drug targets, host interaction, HEV life cycle
Important to Know
At least 20 million HEV infections occur annually, accounting for approximately 3.3 million cases of acute illness and 44 000–70 000 deaths [1]. HEV infections are usually self-limiting and asymptomatic in immunocompetent individuals but can progress to chronicity and cause fulminant hepatitis in immunocompromised patients and other risk groups such as pregnant women [2,3]. Importantly, HEV has also been associated with several extrahepatic manifestations, including neuronal and renal diseases as well as pancreatitis [4]. Current therapeutic options against HEV are constrained to the off-label use of the broad-spectrum antiviral agent ribavirin (RBV) and pegylated interferon-alpha (pegIFNa) [5]. However, RBV therapy is frequently limited due to adverse side-effects, and recently virus isolates have been identified that have lower sensitivity to RBV, leading to higher treatment failure rates [6,7]. Therefore, novel strategies to efficiently and safely target HEV are urgently needed.
HEV is classified as a member of the species Orthohepevirus A within the family Hepeviridae. So far, eight different HEV genotypes (GTs), including five human-pathogenic GTs (GT1/2/3/4/7), have been identified [8]. GTs 1/2 are obligate human pathogens which are present mainly in developing countries and are transmitted via the fecal–oral route, causing waterborne outbreaks [8,9]. By contrast, GTs 3/4/7 are zoonotic pathogens with a broad host range causing sporadic cases of zoonotic hepatitis E in industrialized nations [10]. The genome of HEV encompasses at least three open reading frames (ORFs) which encode: nonstructural proteins (ORF1), comprising a methyltransferase, a papain-like cysteine protease, a helicase, and an RNA-dependent RNA polymerase (RdRp); the capsid protein (ORF2); a small multifunctional protein with key functions in particle assembly and release (ORF3). There is an additional ORF (ORF4) exclusively expressed by HEV GT1 [11,12] (Figure 1 ). Importantly, many facets of the HEV life cycle and, in particular, host–virus interactions that determine the outcome of infection, remain enigmatic. Recent reports on rat HEV, a phylogenetically distinct relative in the Orthohepevirus C species, causing severe hepatitis in immunocompetent patients, make the picture even more puzzling and underline the urge for therapeutic intervention strategies, given its zoonotic potential [13]. Viruses are dependent on the host nucleic acid, protein, and energy metabolism to ensure replication and persistence. Viral proteins frequently interfere with cellular signaling pathways or processes as well as the antiviral defense mechanisms of the infected host cell. This interplay between viral and host factors shapes the course and outcome of infection. Viral host tropism is therefore determined by a combination of susceptibility and permissiveness: a host cell must be both permissive (support viral replication) and susceptible (possess the receptor complement required for viral entry) for a virus to establish an infection. Hence, deciphering the many interactions that occur between HEV and its host cell over the course of infection is essential to understand the mechanisms of pathogenesis and to develop novel antiviral therapies. In particular, alternative therapeutic strategies targeting host factors required during the life cycle of HEV may greatly reduce the emergence of drug-resistant or insensitive variants [6]. Although the broad host range of HEV has led to several new experimental animal models, such as pig models mimicking chronic HEV infection, which offer promising opportunities for future HEV research [14], specific host susceptibility factors have not been identified in animals yet. In the following sections we therefore focus on the current knowledge and recent advances relating to the interactions between HEV and human host cellular factors with respect to different stages of the virus life cycle.
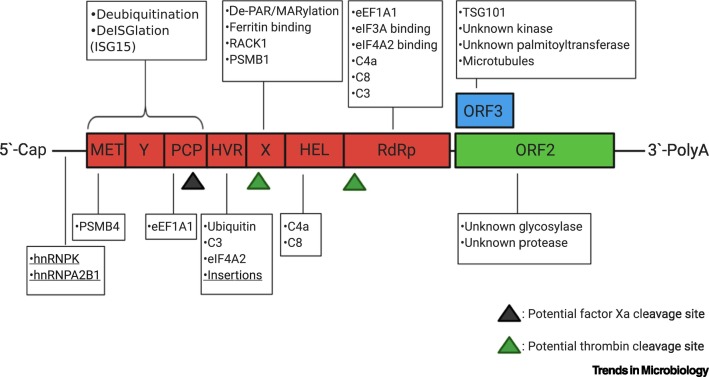
Figure 1.
Host Interactors with Hepatitis E Virus (HEV).
The genome of HEV encodes three open reading frames (ORFs): the nonstructural polyprotein ORF1 (red), the capsid protein ORF2 (green), and the small multifunctional protein with postulated ion-channel activity and involvement in viral egress (ORF3, blue). The ORF1 polyprotein consists of seven functional domains, namely methyltransferase (MET), Y-domain, papain-like-protease (PCP), the hypervariable region (HVR), the X-domain (macro domain), helicase (HEL), and the RNA-dependent RNA polymerase (RdRp). Host factors directly interacting with viral proteins or domains or the viral RNA (underlined) are indicated in boxes and are listed in Table 1 with further information. The HVR tolerates insertions of host RNA sequences, indicating an interaction of host RNAs with the viral genome. Potential host protease cleavage sites are indicated by a black triangle (factor Xa) or green triangles (thrombin). Created with BioRender.com
Who Wants to Play? – Interplay between Host Cell and Viral Factors during HEV Infection
Attachment and Entry
Surface attachment and entry into a host cell are two initial and essential steps in viral infection cycles and are important determinants regarding viral host ranges, tissue tropisms, and pathogenesis. The expression of specific membrane constituents which allow viral attachment to susceptible host cells frequently determines viral tropism, that is, the ability of a virus to infect a limited set of target cells [15]. HEV is a quasi-enveloped virus circulating in a nonenveloped state (neHEV) in bile and feces whereas, in the blood, HEV is cloaked by a layer of host cell membrane covering viral surface proteins [16]. Importantly, available experimental evidence suggests that both forms employ distinct mechanisms for cellular entry [17., 18., 19.]. A number of host factors have been shown to be involved in cell attachment and/or entry of naked HEV (i.e., neHEV) (Figure 2 , Table 1 ). For example, the ectodomain of ASGPR1/2 (asialoglycoprotein receptor 1/2), a cell surface receptor present on the basolateral membrane, has been shown to directly interact with the viral capsid protein (ORF2) in coimmunoprecipitaiton (CoIP) and ELISA experiments. Ectopic expression of ASGPR further increased HEV binding in HeLa cells, whereas depletion of ASGPR in PLC/PRF/5 cells lowered HEV binding but not virion release. Furthermore, anti-ASGPR antibodies and purified ASGPR ectodomain competitively inhibited the binding of neHEV to hepatocytes [20], implying that ASGPR facilitates HEV infection through ORF2 binding. A microarray analysis of (non-)permissive PLC/PRF/5 subclones further suggested integrin α3 (ITGA3) as a potential attachment/entry factor, and a direct interaction with neHEV was observed (Figure 2). Nevertheless, a panel of anti-integrin α3 antibodies could not inhibit the infection of permissive subclones, necessitating further analysis regarding the role of integrin α3 in the cellular entry of neHEV [21]. Various studies using recombinantly expressed capsid protein (ORF2) to generate virus-like particles (VLPs) as a model for neHEV virions further indicated roles for heparan sulfate proteoglycans (HSPGs), ATP5B (ATP synthase subunit 5β), and GRP78 (glucose-regulated protein 78) during virus attachment/entry [11,19,22] (Figure 2 and Table 1). By contrast, the absence of viral proteins on the surface of enveloped particles (eHEVs) implies that these virions use different attachment factors and/or cellular receptors to initiate viral entry. In agreement with this, an overall less efficient surface attachment to host cells, independently of HSPGs or ITGA3, has been observed. Interestingly, the eHEV membrane contains phosphatidylserine (PS) which might bind cell surface receptor TIM-1 (T cell immunoglobulin mucin domain 1) on host cells and thereby serve as a potential attachment factor (Figure 2), a mechanism that has been described for multiple other enveloped viruses with outer envelope leaflets enriched in PS [23,24]. However, whether this dual lifestyle of the HEV virion influences its survival, dissemination, and tissue tropism within the host remains unclear. Nevertheless, given that HEV infection has been associated with various types of extrahepatic manifestation, a less specific cell binding by eHEV compared with neHEV may provide an explanation for the detection of HEV beyond the liver (see Outstanding Questions). Overall, although different host factors have been implicated in cell attachment and/or entry of HEV, little is known about the precise role of the different factors in the context of infection, particularly in primary human hepatocytes and most importantly, the receptor responsible for the entry of neHEV into cells remains unknown [11]. The advent of novel infectious cell culture systems for producing large amounts of infectious HEV particles – in combination with recent advances in conducting genomic screens in various formats and genome coverage (e.g., cDNA, CRISPR, and RNAi libraries) along with deep-sequencing data and -omics – now provide effective tools for identifying the host proteins that serve as viral receptors [25].
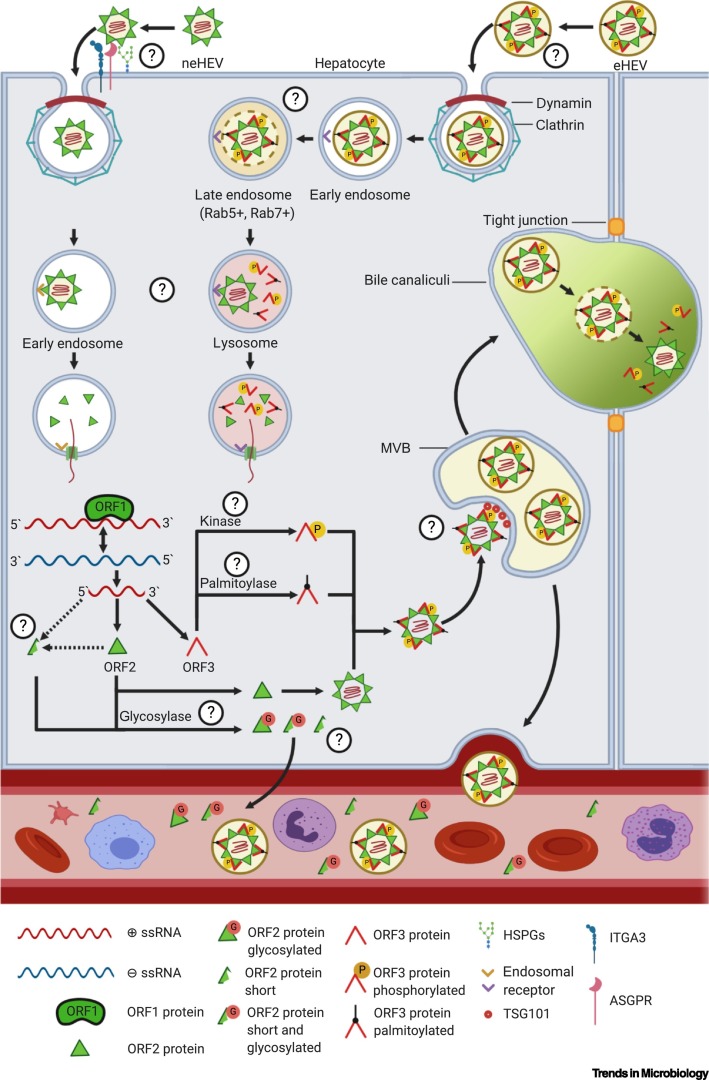
Figure 2.
Schematic Representation of the Hepatitis E Virus (HEV) Replication Cycle and Interaction with Host Factors.
HEV exists in nonenveloped (neHEV) and enveloped (eHEV) forms. Nonenveloped virions attach to heparan sulfate proteoglycans (HSPGs), to the asialoglycoprotein receptor (ASGPR), and the proposed entry receptor integrin alpha 3 (ITGA3), while the enveloped virions are thought to attach to host cells via the interaction of their phosphatidylserine-containing membrane to the T cell immunoglobulin mucin domain 1 (TIM-1) on host cells. Enveloped as well as nonenveloped virions are endocytosed by a clathrin- and dynamin-dependent mechanism. The uncoating is poorly understood, but it is evident that both HEV forms require distinct uncoating mechanisms. The neHEV virions uncoat in early endosomes; the RNA genome is thought to be transferred into the cytoplasm through a pore that forms upon binding of the virion to its receptor. By contrast, eHEV virions need to traffic through late endosomes (Rab5+, Rab7+) and lysosomes in order to uncoat. Lysosomal acidification, the NPC intracellular cholesterol transporter 1 (Niemann–Pick C1 protein or NPC1, not shown), and lysosomal acid lipase (LAL, not shown) are required for uncoating, indicating that the lipid membrane needs to be degraded for this process. The positive-sense RNA genome is translated into the ORF1 polyprotein. The RNA-dependent RNA polymerase (RdRp) produces a negative-sense full-length RNA as template for genome replication and transcription of the subgenomic RNA, encoding for proteins of ORF2 (ORF2) and ORF3 (ORF3). Several states of the ORF2 protein are postulated; they differ in size and glycosylation status. A smaller form of the ORF2 protein is produced either through proteolytic cleavage by an unknown protease (ORF2c – cleaved) or by alternative translation (ORF2S – secreted). Furthermore, both forms can be glycosylated (ORF2g – glycosylated) by a yet to be identified enzyme, leading to three modified forms that are secreted into the bloodstream and are thought to act as immune decoys. Unmodified, infectious ORF2i (infectious) (i.e., ORFC – capsid) can self-assemble to form the capsid. ORF3 is phosphorylated by an unknown kinase, which allows for the binding of the protein to unmodified ORF2 and is thought to mark virions for release. Moreover, phosphoORF3 binds to tumor susceptibility gene 101 (TSG101), which is a part of the endosomal sorting complex required for transport (ESCRT). TSG101 is necessary for efficient viral egress and is required for loading of virions into multivesicular bodies (MVBs). Furthermore, ORF3 is palmitoylated by an unknown palmitoyltransferase, which affects the subcellular localization of ORF3 and virion release. The MVBs fuse with the plasma membrane to release the virions either in the bloodstream (basolateral side), where they keep their envelope (eHEV), or in the bile duct (apical side), where the envelope is removed by bile salts, and neHEV virions are produced. Abbreviation: ssRNA, single stranded RNA. Created with BioRender.com.
Table 1.
Identified Direct Host Factor Interactions with HEV
| Factor | HEV binding partner | Biological functiona | Refs |
|---|---|---|---|
| ASGPR | neHEV virion | Attachment factor | [20] |
| C1-inhibitor (SERPING1) | ORF4 | Altered complement activation or inhibitiona | [39] |
| C3 | RdRp, HVR | Altered complement activation or inhibitiona | [39] |
| C4a | RdRp, HEL | Altered complement activation or inhibitiona | [39] |
| C8 | RdRp, ORF4, HEL | Altered complement activation or inhibitiona | [39] |
| De-MARylation | X-domain (macro domain) | Immune evasiona | [67] |
| De-PARylation | X-domain (macro domain) | Immune evasiona | [67] |
| eEF1A1 | RdRp, PCP, ORF4 | Formation of a translation complexa, increased RdRp activity | [12,39] |
| eIF3A | RdRp, ORF4 | Formation of a translation complexa | [39] |
| eIF4A2 | RdRp, HVR | Formation of a translation complexa | [39] |
| Factor Xa | PCP | Processing of the ORF1 polyproteina | [38] |
| Ferritin | X-domain (macro domain) | [87] | |
| hnRNPA2B1 | Promoter regions in HEV RNA | Structural (re-)arrangementsa | [42] |
| hnRNPK | Promoter regions in HEV RNA | Structural (re-)arrangementsa | [42] |
| HSPGs | neHEV virion | Attachment factor | [16,22] |
| ISG15 | MET-PCP | Invading cellular antiviral pathwaysa | [75] |
| ITGA3 | neHEV virion | Entry receptora | [21] |
| Microtubules | ORF3 | Cytoskeleton rearrangementa | [59,60] |
| PSMB1 | X-domain (macro domain) | Altered processing of MHC-I complexesa | [39] |
| PSMB4 | MET | Altered processing of MHC-I complexesa | [39] |
| RACK1 | X-domain (macro domain) | Part of the viral replication/translation complex | [39] |
| Thrombin | X-domain (macro domain), RdRp | ORF1 polyprotein processinga | [38] |
| TIM-1 | Phosphatidylserine on eHEV virion | Attachment factora | [16] |
| TSG101 | ORF3 | Loading of virions into MVBs | [61] |
| Ubiquitin | HVR, MET-PCP | Altered processing of MHC-I complexesa, invading cellular antiviral pathwaysa | [39,75] |
| Unknown glycosylase | ORF2 | Production of immune decoysa | [47,58] |
| Unknown kinase | ORF3 | Detecting virions ready for releasea | [57,58] |
| Unknown palmitoyltransferase | ORF3 | Viral egress and subcellular localization | [63] |
| Unknown protease | ORF2 | Production of immune decoysa | [47,58] |
Speculated.
Internalization and Uncoating of HEV
Although essential for infection, passage through the cellular membrane barrier is only the initial step to establishing a successful viral infection. While the quasi-envelope of eHEV represents an elegant strategy for evading antibody-mediated immune responses [26,27] it also imposes a need for additional steps during cellular entry prior to uncoating of the genome. The internalization of both (n)eHEV particles involves clathrin- and dynamin-dependent pathways; however, different release points of the viral genome have been suggested (Figure 2). Perturbations of intracellular trafficking by Rab5/7 knockdown and/or lysosomotropic agents did not alter neHEV infectivity, while eHEV infectivity was greatly reduced [16,28]. Trafficking of eHEV towards lysosomal membranes is believed to be required for degradation of its lipid envelope. In agreement with this, perturbation of different enzymes – for example, NPC intracellular cholesterol transporter 1 (Niemann–Pick C1 protein or NPC1), lysosomal acid lipase (LAL) – required for lipid membrane degradation in lysosomes selectively reduced eHEV infectivity. A similar mechanism also accounts for the loss of membrane from quasi-enveloped hepatitis A virus (HAV) [29]. Interestingly, nonspecific extracellular vesicles released from uninfected cells were not observed to traffic towards lysosomes, suggesting the presence (or absence) of a specific targeting signal within the eHAV – and likewise eHEV – membrane which redirects quasi-enveloped virions towards lysosomes [29]. Hence, analysis of the protein composition of eHEV versus exosomes released from uninfected cells using quantitative proteomic approaches could provide more support for this hypothesis. Although, the release of virion contents of (n)eHEV into the interior of the cell is temporally and spatially different, it remains unclear whether this process also occurs in a mechanistically distinct manner and involves binding of the viral capsid to a cellular receptor protein as a trigger. However, if the capsid of eHEV interacts with the same protein (following membrane removal) as neHEV, a potential receptor must be present on both late endosome-lysosomal and early endosomal compartments and would thus be a protein that traffics between these compartments (Figure 2). Antiviral strategies targeting early steps of infection, such as viral uncoating, are appealing, particularly when the probability for successful interference with both forms of HEV due a shared uncoating receptor would be given.
HEV Replication Complex
In the process of host cell invasion, positive-stranded RNA viruses frequently induce the reorganization of intracellular membranes [e.g., endoplasmic reticulum (ER), Golgi, mitochondria, endosomes and/or lysosomes] to establish sites of replication. These replication complexes function as scaffolds to increase the local concentration of viral and cellular cofactors and provide a protected environment which limits the recognition of viral proteins and nucleic acids by the innate immune system [30]. The replicative machinery of HEV is largely encoded by ORF1, which features a methyltransferase, RNA helicase, and an RdRp (Figure 1). Whether the polyprotein is processed into distinct active domains during the HEV life cycle, as observed for various other viruses, remains controversial [31., 32., 33., 34., 35., 36., 37.]. Nevertheless, conserved across all HEV genotypes, two potential cleavage sites for cellular thrombin and factor Xa were recently identified, and serine protease inhibitors, as well as thrombin and Xa-targeting siRNAs, inhibited viral replication [38] (Figure 1). A yeast two-hybrid screen identified 138 protein–protein interactions between HEV and host proteins, indicating roles for HEV proteins in modulating host cell processes such as stress and immune responses, the ubiquitin–proteasome system, energy and iron metabolism, and protein translation. Most importantly, a set of host translation factors (eIF4A, eIF3A, and RACK1), required for HEV replication, was identified [39] (Figure 1 and Table 1). As part of the eIF4F complex, eIF4A drives cap-dependent translation initiation in eukaryotes and has been involved in the replication of various RNA viruses. The natural compound silvestrol, a specific inhibitor of eIF4A, was further identified as a highly potent inhibitor of HEV replication in vitro and in vivo [40,41]. Additional host proteins interacting directly with the HEV genome were identified via affinity chromatography and mass spectrometry. Subsequent in vitro analysis revealed binding of two nuclear ribonucleoproteins – the heterogeneous nuclear ribonucleoprotein K (hnRNPK) and the heterogeneous nuclear ribonucleoproteins A2/B1 (hnRNPA2B1) – to promoter regions in the viral RNA [42]. The involvement of these two proteins in packaging of nascent pre-mRNA, as well as alternative transcript splicing and post-translational modifications (PTMs) by the recruitment of regulatory proteins of the eukaryotic RNA metabolism, may point to structural (re-)arrangements during HEV replication [43]. Candidate HEV replication complexes harboring viral RNA and the multifunctional ORF1 protein were found in cytoplasmic dot-like structures which partially overlap ORF2 and ORF3 proteins as well as exosomal markers [44]. However, the structure and composition of the HEV replication complex, as well as its spatial and temporal organization, remain poorly characterized, in particular due to the limited availability of functional tools to directly study the subcellular localization of viral proteins (see Outstanding Questions). Hence, as a first step, unbiased global approaches via morphological profiling approaches, such as the cell painting assay – a microscopy-based assay to evaluate morphological features of single cells, including shape, texture, size, etc. – could be used to define a multiparametric fingerprint to identify even subtle changes in cellular morphology and thus signatures of virus infection [45]. Importantly, small-molecule compounds frequently deliver similar phenotypic profiles. Hence, using reference compounds with known modes of action, such as RBV, this approach further offers the potential to evaluate compound libraries to identify novel molecules targeting HEV replication. Furthermore, novel RNA proximity labeling techniques, such as APEX-seq, can be applied to probe the spatial microenvironment of viral proteins and thus promise new insights into the composition of the HEV replication complex [46].
HEV Assembly and Release
The processes of virus assembly usually involve interactions between viral capsid and nonstructural proteins and the coordinated help of host factors. Intriguingly, only a minor fraction of the ORF2 capsid protein is assembled into infectious particles (ORF2i). By contrast, large amounts of non-virion-associated ORF2 variants, with potential immunomodulatory function, are secreted through the exosomal pathway (Figure 2). Two heavily secreted glycoproteins were described by Montpellier et al. – an ORF2g and a smaller, cleaved form, ORF2c [47]. While these two forms arise from PTMs, another study found evidence for alternative translational regulation resulting in an ORF2C, the regular capsid form, and a secreted form ORF2S [48]. To what extent these findings overlap is still unclear, and, as for the ORF2S, no glycosylation was verified. Although various studies using VLPs have provided some insights into the mechanical aspects of HEV genome packaging into viral particles [49., 50., 51., 52., 53.], many fundamental questions regarding the assembly of HEV virions remain open (see Outstanding Questions). Most importantly, the subcellular compartment of virus assembly, the participating host factors, and how these steps are orchestrated in space and time remain unknown. A recently established elegant imaging system allowed visualization of the dynamics and subcellular structures of the Hepatitis C virus (HCV) virus, providing a topological map of how HCV might coordinate the steps of viral replication and virion assembly [54]. Likewise, the combination of confocal and electron microscopy, together with tagged HEV genomes, provides all possible means to study subcellular localization of ORF2 and to identify the potential viral assembly site. Combined with proteomic and genetic approaches, the host factors involved could be further identified; this could not only deepen the understanding of HEV assembly but also indicate attractive drug targets. Following viral particle assembly, progeny virions are released to initiate another round of infection, a process relying on the multifunctional phosphoprotein encoded by ORF3 [55., 56., 57.]. Phosphorylation of ORF3 through a yet unknown host kinase promotes its interaction with ORF2, which has been suggested as a possible mechanism for ORF3 to recognize the viral particles for release [57,58]. Furthermore, ORF3 was shown to interact with different components of the intracellular transport machinery, including microtubules [59,60] and TSG101, a component of the endosomal sorting complex, required for transport (ESCRT) [61], which loads virions into multivesicular bodies (MVBs) for egress [62]. Interestingly, to fulfill this multifunctional role, ORF3 relies not only on phosphorylation but also on palmitoylation through an as yet unknown palmitoyltransferase, required for its subcellular localization in order to mediate virion release [63] (Figure 1, Figure 2). Hence, both host proteins provide attractive strategies to perturb the viral life cycle. Within hepatocytes, polarized trafficking and secretion from MVBs results in the egress of eHEV particles from basolateral membranes to spread within the host, and from the apical membranes into the bile, where high concentrations of human bile acids and salts convert eHEV to neHEV virions. In this context, novel HEV-permissive polarized cell models will allow further study of the mechanisms and determinants of directional HEV secretion [64,65].
Host Factor Modifications Mediated by HEV Proteins
PTMs of HEV viral proteins (e.g., ORF2/3) realized by host molecules play an essential role in modulating their functions. Furthermore, recent studies also illustrate how enzymes encoded by pathogens modify target host cell proteins to shape an optimized environment for their replication and to facilitate evasion from the immune system [66]. For example, the different domains of the polyprotein encoded by ORF1 have been suggested to alter PTMs of host cell proteins. The X-domain of ORF1, also known as macro domain, functions as a de-MARylation and de-PARylation enzyme [67] (Figure 1, Table 1). PARylation and MARylation regulate key biological and pathological processes, and ADP-ribosylation of viral RNA has been suggested to serve as a signal to initiate a cellular immune response as observed for Venezuelan equine encephalitis virus (VEEV) and severe acute respiratory syndrome coronavirus (SARS-CoV) [68., 69., 70., 71., 72., 73.]. Interestingly, enzymes encoded by VEEV and SARS-CoV also contain domains with ADP-ribosylhydrolase activity which support viral replication in host cells [74]. Accordingly, the X-domain of HEV might perturb the antiviral response of a given host cell and likewise the Met-PCP domain is able to remove ubiquitin residues and interferon-stimulated gene 15 (ISG15) residues from proteins [75]. ISG15 is one of the most upregulated genes upon viral infection and is a ubiquitin-like modifier that plays a role in resistance to various viruses – for example, influenza viruses A and B (IAV, IBV), herpes simplex virus I (HSV-1), murine gamma herpesvirus 68 (MHV68), and Sindbis virus (SINV) [76]. Therefore, the ability to remove ISG residues (deISGylating) could provide an immune-evasive advantage for HEV. In this context it has been further observed that HEV can block the expression of different ISGs in response to IFN types IFN I–III; however, the mechanism remains unclear [77].
Genetic Exchange between Host Cell and HEV
Due to the error-prone nature of the viral RdRp, RNA viruses, such as HEV, diversify into populations with high intrahost variability, providing a potential benefit to the virus population across changing environments (e.g., immune response, antiviral therapy). Moreover, ORF1 of the HEV genome contains a hypervariable region (HVR) (Figure 1) which displays considerable sequence divergence even between isolates of the same virus genotype [78]. Recent reports identified several HEV strains harboring genomic rearrangements in patients at the acute phase of infection and further indicated that enhanced population heterogeneity is associated with HEV persistence and possible RBV insensitivity [7,79., 80., 81., 82.]. Furthermore, two HEV strains (recovered from the feces of chronically infected patients) containing insertions from human ribosomal subunits (S17 and S19) showed increased replicative capacity in cell culture and an expanded host range [83,84]. Given that sequences which are not required for virus infectivity or replication are normally rapidly lost in small RNA viruses such as HEV, a potential biological role of the HVR during HEV replication and/or pathogenesis can be inferred [85]. In general, two types of recombination-promoting insertion into viral genomes have been described for different viruses: the replicative 'copy-choice' mechanism and the nonreplicative 'breakage–ligation' mechanism [86]. Since insertions into the HVR potentially lead to the emergence of more pathogenic forms of recombinant HEV, a deeper understanding of the mechanisms governing genetic exchange and plasticity is of great interest – in particular, if insertions are responsible for treatment failures in chronically infected patients or provide a potential determinant for chronicity [6,80].
Concluding Remarks
The development of novel HEV cell culture systems has provided important advances in the study of HEV infection biology; however, many steps of the viral life cycle remain elusive. For example, although several entry and/or attachment factors have been described, little is known about the precise role of the different adaptors and, most importantly, the receptor responsible for viral entry into the host cell remains unknown. Likewise, endosomal escape mechanisms were shown to differ between eHEV and neHEV, but only a few of the required proteins have been described so far. Moreover, modifications of the ORF3 have been demonstrated to be crucial for viral egress, yet the modifying proteins need to be identified. Finally, recent studies have reported that transcription factors, as well as translation factors, are required for HEV replication.
Antiviral therapies are focused on disturbing virus propagation, mainly by directly targeting the viral genome/proteins or host interaction partners. All the proteins described, and many more host proteins not yet identified, are potential targets for pharmaceutical intervention strategies (Table 1). It is of the utmost importance to shed further light on the life cycle of HEV in order to understand its manipulation and hijacking of host cells and to identify new drug targets.
Outstanding Questions.
How does eHEV bind to cells, and how is its cell tropism determined? Do eHEV and neHEV differ in their tissue tropism?
Which host factors are essential for HEV RNA replication? In particular, which host factors contribute to the formation of intracellular replication complexes?
Is there a specific signal that directs the endocytosed eHEV virion to lysosomes?
What is the role of the HVR? How do insertions in this region confer cell culture adaptation and potentially therapy resistance?
What is the subcellular site of HEV virion assembly? What is the mechanism to switch from replication to assembly? Which proteins mediate the intracellular trafficking of the assembled virions to promote particle release?
Alt-text: Outstanding Questions
Acknowledgments
We thank all members of the Department for Molecular and Medical Virology of the Ruhr University Bochum for critically reading the manuscript and helpful input, especially Thomas Burkard, Volker Kinast, Toni Luise Meister, and Jil Schrader. E.S. was supported by an Exploration Grant from the Boehringer Ingelheim Foundation, by the Deutsche Forschungsgemeinschaft (398066876/GRK 2485/1), and by grants from the German Federal Ministry of Health (ZMVI1-2518FSB705) and the German Federal Ministry of Education and Research (16GW0202).
References
- 1.Rein D.B., et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology (Baltimore) 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 2.Bose P.D., et al. High viral load and deregulation of the progesterone receptor signaling pathway: association with hepatitis E-related poor pregnancy outcome. J. Hepatol. 2011;54:1107–1113. doi: 10.1016/j.jhep.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Wedemeyer H., et al. Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology. 2012;142:1388–1397.e1. doi: 10.1053/j.gastro.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Kamar N., et al. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016;36:467–472. doi: 10.1111/liv.13037. [DOI] [PubMed] [Google Scholar]
- 5.Kinast V., et al. Hepatitis E virus drug development. Viruses. 2019;11:485. doi: 10.3390/v11060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todt D., et al. Hepatitis E virus treatment and ribavirin therapy: viral mechanisms of nonresponse. Curr. Opin. Virol. 2018;32:80–87. doi: 10.1016/j.coviro.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Todt D., et al. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut. 2016;65:1733–1743. doi: 10.1136/gutjnl-2015-311000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimgaonkar I., et al. Hepatitis E virus: advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2017;15:96–110. doi: 10.1038/nrgastro.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenney S.P. The current host range of hepatitis E viruses. Viruses. 2019;11:452. doi: 10.3390/v11050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamar N., et al. Hepatitis E virus infection. Nat. Rev. Dis. Primers. 2017;3:17086. doi: 10.1038/nrdp.2017.86. [DOI] [PubMed] [Google Scholar]
- 11.Yin X., Feng Z. Hepatitis E virus entry. Viruses. 2019;11:883. doi: 10.3390/v11100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair V.P., et al. Endoplasmic reticulum stress induced synthesis of a novel viral factor mediates efficient replication of genotype-1 hepatitis E virus. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andonov A., et al. Rat hepatitis E virus linked to severe acute hepatitis in an immunocompetent patient. J. Infect. Dis. 2019;220:951–955. doi: 10.1093/infdis/jiz025. [DOI] [PubMed] [Google Scholar]
- 14.Cao D., et al. Pig model mimicking chronic hepatitis E virus infection in immunocompromised patients to assess immune correlates during chronicity. Proc. Natl. Acad. Sci. U. S. A. 2017;114:6914–6923. doi: 10.1073/pnas.1705446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grove J., Marsh M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin X., et al. Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses. J. Virol. 2016;90:4232–4242. doi: 10.1128/JVI.02804-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagashima S., et al. Characterization of the quasi-enveloped hepatitis E virus particles released by the cellular exosomal pathway. J. Virol. 2017;91 doi: 10.1128/JVI.00822-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapuy-Regaud S., et al. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie. 2017;141:70–79. doi: 10.1016/j.biochi.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Yin X., et al. Role of envelopment in the HEV life cycle. Viruses. 2016;8:229. doi: 10.3390/v8080229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., et al. Asialoglycoprotein receptor facilitates infection of PLC/PRF/5 cells by HEV through interaction with ORF2. J. Med. Virol. 2016;88:2186–2195. doi: 10.1002/jmv.24570. [DOI] [PubMed] [Google Scholar]
- 21.Shiota T., et al. Integrin α3 is involved in non-enveloped hepatitis E virus infection. Virology. 2019;536:119–124. doi: 10.1016/j.virol.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Kalia M., et al. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J. Virol. 2009;83:12714–12724. doi: 10.1128/JVI.00717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jemielity S., et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A., et al. TIM1 (HAVCR1): an essential 'receptor' or an 'accessory attachment factor' for hepatitis A virus? J. Virol. 2019;93 doi: 10.1128/JVI.01793-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Todt D., et al. Robust hepatitis E virus infection and transcriptional response in human hepatocytes. Proc. Natl. Acad. Sci. U. S. A. 2020;117:1731–1741. doi: 10.1073/pnas.1912307117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi M., et al. Hepatitis E virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J. Clin. Microbiol. 2010;48:1112. doi: 10.1128/JCM.02002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Z., Lemon S.M. Peek-a-boo: membrane hijacking and the pathogenesis of viral hepatitis. Trends Microbiol. 2013;22:59–64. doi: 10.1016/j.tim.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapur N., et al. Hepatitis E virus enters liver cells through receptor-dependent clathrin-mediated endocytosis. J. Viral Hepatitis. 2012;19:436–448. doi: 10.1111/j.1365-2893.2011.01559.x. [DOI] [PubMed] [Google Scholar]
- 29.Rivera-Serrano E.E., et al. Cellular entry and uncoating of naked and quasi-enveloped human hepatoviruses. eLife. 2019;8 doi: 10.7554/eLife.43983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heaton N.S., Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ropp S.L., et al. Expression of the hepatitis E virus ORF1. Arch. Virol. 2000;145:1321–1337. doi: 10.1007/s007050070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehgal D., et al. Expression and processing of the hepatitis E virus ORF1 nonstructural polyprotein. Virology J. 2006;3:38. doi: 10.1186/1743-422X-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suppiah S., et al. Lack of processing of the expressed ORF1 gene product of hepatitis E virus. Virol. J. 2011;8:245. doi: 10.1186/1743-422X-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parvez M.K. Molecular characterization of hepatitis E virus ORF1 gene supports a papain-like cysteine protease (PCP)-domain activity. Virus Res. 2013;178:553–556. doi: 10.1016/j.virusres.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perttilä J., et al. Early secretory pathway localization and lack of processing for hepatitis E virus replication protein pORF1. J. Gen. Virol. 2013;94:807–816. doi: 10.1099/vir.0.049577-0. [DOI] [PubMed] [Google Scholar]
- 36.Paliwal D., et al. Hepatitis E virus (HEV) protease: a chymotrypsin-like enzyme that processes both non-structural (pORF1) and capsid (pORF2) protein. J. Gen. Virol. 2014;95:1689–1700. doi: 10.1099/vir.0.066142-0. [DOI] [PubMed] [Google Scholar]
- 37.Spall V.E., et al. Polyprotein processing as a strategy for gene expression in RNA viruses. Sem. Virol. 1997;8:15–23. [Google Scholar]
- 38.Kanade G.D., et al. Activities of thrombin and factor Xa are essential for replication of hepatitis E virus and are possibly implicated in ORF1 polyprotein processing. J. Virol. 2018;92 doi: 10.1128/JVI.01853-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramani C., et al. Host–virus protein interaction network reveals the involvement of multiple host processes in the life cycle of hepatitis E virus. mSystems. 2018;3 doi: 10.1128/mSystems.00135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todt D., et al. The natural compound silvestrol inhibits hepatitis E virus (HEV) replication in vitro and in vivo. Antivir. Res. 2018;157:151–158. doi: 10.1016/j.antiviral.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glitscher M., et al. Inhibition of hepatitis E virus spread by the natural compound silvestrol. Viruses. 2018;10:301. doi: 10.3390/v10060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanade G.D., et al. Protein interactions network of hepatitis E virus RNA and polymerase with host proteins. Front. Microbiol. 2019;10:2501. doi: 10.3389/fmicb.2019.02501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Y., Smith R. Nuclear functions of heterogeneous nuclear ribonucleoproteins A/B. Cell. Mol. Life Sci. 2009;66:1239–1256. doi: 10.1007/s00018-008-8532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szkolnicka D., et al. Recombinant hepatitis E viruses harboring tags in the ORF1 protein. J. Virol. 2019;93 doi: 10.1128/JVI.00459-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bray M.-A., et al. Cell painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protocols. 2016;11:1757–1774. doi: 10.1038/nprot.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazal F.M., et al. Atlas of subcellular RNA localization revealed by APEX-Seq. Cell. 2019;178:473–490.e26. doi: 10.1016/j.cell.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montpellier C., et al. Hepatitis E virus lifecycle and identification of 3 forms of the ORF2 capsid protein. Gastroenterology. 2018;154:211–223.e8. doi: 10.1053/j.gastro.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Yin X., et al. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. U. S. A. 2018;115:4773–4778. doi: 10.1073/pnas.1721345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li T.C., et al. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J. Virol. 1997;71:7207–7213. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xing L., et al. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T = 1 particle presenting native virus epitopes. Virology. 1999;265:35–45. doi: 10.1006/viro.1999.0005. [DOI] [PubMed] [Google Scholar]
- 51.Li T.-C., et al. Essential elements of the capsid protein for self-assembly into empty virus-like particles of hepatitis E virus. J. Virol. 2005;79:12999–13006. doi: 10.1128/JVI.79.20.12999-13006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guu T.S.Y., et al. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assemblyand receptor binding. PNAS. 2009;106:12992–12997. doi: 10.1073/pnas.0904848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita T., et al. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. PNAS. 2009;106:12986–12991. doi: 10.1073/pnas.0903699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J.-Y., et al. Spatiotemporal coupling of the hepatitis C virus replication cycle by creating a lipid droplet-proximal membranous replication compartment. Cell Rep. 2019;27:3602–3617.e5. doi: 10.1016/j.celrep.2019.05.063. [DOI] [PubMed] [Google Scholar]
- 55.Emerson S.U., et al. ORF3 protein of hepatitis E virus is not required for replication, virion assembly, or infection of hepatoma cells in vitro. J. Virol. 2006;80:10457–10464. doi: 10.1128/JVI.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada K., et al. ORF3 protein of hepatitis E virus is essential for virion release from infected cells. J. Gen. Virol. 2009;90:1880–1891. doi: 10.1099/vir.0.010561-0. [DOI] [PubMed] [Google Scholar]
- 57.Ju X., Ding Q. Hepatitis E virus assembly and release. Viruses. 2019;11:539. doi: 10.3390/v11060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyagi S., et al. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its non-glycosylated form of the major capsid protein, ORF2. J. Biol. Chem. 2002;277:22759–22767. doi: 10.1074/jbc.M200185200. [DOI] [PubMed] [Google Scholar]
- 59.Zafrullah M., et al. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 1997;71:9045–9053. doi: 10.1128/jvi.71.12.9045-9053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kannan H., et al. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009;83:6375–6382. doi: 10.1128/JVI.02571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagashima S., et al. Tumour susceptibility gene 101 and the vacuolar protein sorting pathway are required for the release of hepatitis E virions. J. Gen. Virol. 2011;92:2838–2848. doi: 10.1099/vir.0.035378-0. [DOI] [PubMed] [Google Scholar]
- 62.Nagashima S., et al. The membrane on the surface of hepatitis E virus particles is derived from the intracellular membrane and contains trans-Golgi network protein 2. Arch. Virol. 2014;159:979–991. doi: 10.1007/s00705-013-1912-3. [DOI] [PubMed] [Google Scholar]
- 63.Gouttenoire J., et al. Palmitoylation mediates membrane association of hepatitis E virus ORF3 protein and is required for infectious particle secretion. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dao Thi V.L., et al. Stem cell-derived culture models of hepatitis E virus infection. Cold Spring Harbor Persp. Med. 2019;9 doi: 10.1101/cshperspect.a031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dao Thi V.L., et al. Stem cell-derived polarized hepatocytes. Nat. Commun. 2020;11:1677. doi: 10.1038/s41467-020-15337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ribet D., Cossart P. Pathogen-mediated posttranslational modifications: A re-emerging field. Cell. 2010;143:694–702. doi: 10.1016/j.cell.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C., et al. Viral macro domains reverse protein ADP-ribosylation. J. Virol. 2016;90:8478–8486. doi: 10.1128/JVI.00705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atasheva S., et al. Interferon-stimulated poly(ADP-ribose) polymerases are potent inhibitors of cellular translation and virus replication. J. Virol. 2014;88:2116–2130. doi: 10.1128/JVI.03443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durkacz B.W., et al. (ADP-ribose)n participates in DNA excision repair. Nature. 1980;283:593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- 70.Rouleau M., et al. Poly(ADP-ribosyl)ated chromatin domains: access granted. J. Cell Sci. 2004;117:815. doi: 10.1242/jcs.01080. [DOI] [PubMed] [Google Scholar]
- 71.Karras G.I., et al. Themacrodomain is an ADP-ribose binding module. EMBO J. 2005;24:1911–1920. doi: 10.1038/sj.emboj.7600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeDesma R., et al. Hepatitis E virus replication. Viruses. 2019;11:719. doi: 10.3390/v11080719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosado M.M., et al. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology. 2013;139:428–437. doi: 10.1111/imm.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munnur D., et al. Reversible ADP-ribosylation of RNA. Nucleic Acids Res. 2019;47:5658–5669. doi: 10.1093/nar/gkz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karpe Y.A., Lole K.S. Deubiquitination activity associated with hepatitis E virus putative papain-like cysteine protease. J. Gen. Virol. 2011;92:2088–2092. doi: 10.1099/vir.0.033738-0. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D., Zhang D.-E. Interferon-stimulated gene 15 and the protein ISGylation system. J. Interf. Cytokine Res. 2011;31:119–130. doi: 10.1089/jir.2010.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Todt D., et al. Antiviral activities of different interferon types and subtypes against hepatitis E virus replication. Antimicrob. Agents Chemother. 2016;60:2132–2139. doi: 10.1128/AAC.02427-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith D.B., et al. Evolution of the hepatitis E virus hypervariable region. J. Gen. Virol. 2012;93:2408–2418. doi: 10.1099/vir.0.045351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Debing Y., et al. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology. 2014;147:1008–1011.e7. doi: 10.1053/j.gastro.2014.08.040. quiz e15–e16. [DOI] [PubMed] [Google Scholar]
- 80.Debing Y., et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J. Hepatol. 2016;65:499–508. doi: 10.1016/j.jhep.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Todt D., et al. Mutagenic effects of ribavirin on hepatitis E Virus – viral extinction versus selection of fitness-enhancing mutations. Viruses. 2016;8:283. doi: 10.3390/v8100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lhomme S., et al. Insertions and duplications in the polyproline region of the hepatitis E virus. Front. Microbiol. 2020;11:1. doi: 10.3389/fmicb.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shukla P., et al. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depends on an inserted human gene segment acquired by recombination. J. Virol. 2012;86:5697–5707. doi: 10.1128/JVI.00146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen H.T., et al. A naturally occurring human/hepatitis E recombinant virus predominates in serum but not in faeces of a chronic hepatitis E patient and has a growth advantage in cell culture. J. Gen. Virol. 2012;93:526–530. doi: 10.1099/vir.0.037259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pudupakam R.S., et al. Deletions of the hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: evidence for attenuation of HVR deletion mutants in vivo. J. Virol. 2009;83:384–395. doi: 10.1128/JVI.01854-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muslin C., et al. Recombination in enteroviruses, a multi-step modular evolutionary process. Viruses. 2019;11:859. doi: 10.3390/v11090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ojha N.K., Lole K.S. Hepatitis E virus ORF1 encoded macro domain protein interacts with light chain subunit of human ferritin and inhibits its secretion. Mol. Cell. Biochem. 2016;417:75–85. doi: 10.1007/s11010-016-2715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]