Abstract
Aims
Here we introduce a wide and complex study comparing effects of growth factors used alone and in combinations on human mesenchymal stem cell (hMSC) proliferation and osteogenic differentiation. Certain ways of cell behaviour can be triggered by specific peptides – growth factors, influencing cell fate through surface cellular receptors.
Methods
In our study transforming growth factor β (TGF-β), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), and vascular endothelial growth factor (VEGF) were used in order to induce osteogenesis and proliferation of hMSCs from bone marrow. These cells are naturally able to differentiate into various mesodermal cell lines. Effect of each factor itself is pretty well known. We designed experimental groups where two and more growth factors were combined. We supposed cumulative effect would appear when more growth factors with the same effect were combined. The cellular metabolism was evaluated using MTS assay and double-stranded DNA (dsDNA) amount using PicoGreen assay. Alkaline phosphatase (ALP) activity, as early osteogenesis marker, was observed. Phase contrast microscopy was used for cell morphology evaluation.
Results
TGF-β and bFGF were shown to significantly enhance cell proliferation. VEGF and IGF-1 supported ALP activity. Light microscopy showed initial extracellular matrix mineralization after VEGF/IGF-1 supply.
Conclusion
A combination of more than two growth factors did not support the cellular metabolism level and ALP activity even though the growth factor itself had a positive effect. This is probably caused by interplay of various messengers shared by more growth factor signalling cascades.
Cite this article: Bone Joint Res 2020;9(7):412–420.
Keywords: Mesenchymal stem cell, Growth factor, Osteogenic differentiation, Regenerative medicine
Article focus
Does combination of more growth factors with a similar role lead to amplification of their effect? We used transforming growth factor β (TGF-β), basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF).
Key messages
A combination of more than two growth factors showing positive effect did not itself lead to either metabolic activity or alkaline phosphatase (ALP) activity enhancement.
Strengths and limitations
Although the effects of certain growth factors are known, we used novel combinations (of up to three growth factors), the effects of which have not been published so far.
In this study we have shown the significant positive effects of certain growth factor combinations; however, the whole phenomenon is probably much more complex and complicated, especially when discussing the in vivo conditions.
Introduction
A general characteristic of mesenchymal stem cells (MSCs) is their ability of self-renewal and multilineage differentiation potential,1 which indicates their main role in an organism-tissue structure and function restoration. MSCs are also of great potential for use in orthopaedics cell therapy once strict criteria are determined.2 Differentiation of MSCs to a certain cell type can be induced by supplementation with specific growth factors, both of synthetic and natural origin, or other biomolecules.3-5 Such substances influence cell metabolism, growth, proliferation, and differentiation by binding unique extracellular surface receptors.
Transforming growth factor β (TGF-β) expression is dependent on the amount of the collagens type I and II, both matrix proteins. It reduces production of osteoclast differentiation factor receptor activator of nuclear factor kappa-B ligand (RANKL) and thus controls the bone mass resorption.6 Additionally, it activates osteoblast precursors and stimulates them to early differentiation and extracellular matrix (ECM) protein synthesis.7 Basic fibroblast growth factor (bFGF) is synthesized by MSCs and mature osteoblasts and stored in the ECM. It regulates genes for osteoprogenitor proliferation and differentiation, and apoptosis of osteoblasts.8 It also decreases noggin expression, which negatively regulates bone morphogenetic protein dependent bone formation.9 Insulin-like growth factor 1 (IGF-1) was shown to influence mature bone cells supporting osterix, osteocalcin, and collagen type I expression. It also modulates chondrocyte functions and is crucial for proper endochondral ossification.10 As an inactive molecule it is stored in bone ECM and released during bone resorption.11 Vascular endothelial growth factor (VEGF) is especially important from the perspective of blood supply during bone development and healing. In the primary osteoblasts VEGF stimulates alkaline phosphatase (ALP) activity and mineralization. The maximal expression level is reached in the terminal phase of differentiation.12 In the osteoclast precursor cells it induces higher receptor activator of nuclear factor kappa-B (RANK) expression, which functions as a RANKL receptor protein and enables precursor activation and activates osteoclast generation.13 Hepatocyte growth factor (HGF) activates the signalling cascade leading to higher expression of RunX2, osterix, osteocalcin, and osteoprotegerin messenger RNA (mRNA), followed by phosphorylation. It also induces the expression of bone morphogenetic protein 4, another factor important for osteogenesis.14,15
In the field of tissue engineering the main aim is to mimic physiological conditions in order to facilitate the development of a new tissue, which would be a fully-fledged substitution of the originally present tissue. With this aim, some bioactive substances and growth factors in certain concentrations can be added to the culture medium. Common osteogenic differentiation protocol includes cultivation of the confluent cell layer with supplementation by synthetic glucocorticoid dexamethasone, β-glycerophosphate as an inorganic phosphate source, and ascorbate as a cofactor for lysine and proline hydroxylation.16
Bone formation is accompanied by ECM mineralization. This process is initiated by the secretion of tiny vesicles containing high concentrations of calcium and phosphates, and these are also the initial sites for hydroxyapatite crystal deposition.17,18 Vesicles usually contain ALP.19 ALP is in the outer membrane of the exocytosed vesicle anchored by the glycosylphosphatidylinositol. This enzyme catalyzes mineral deposition via decreasing extracellular pyrophosphate level and increasing phosphate level. The mineralization always starts at the ends of collagen fibres.
The aim of this study was to find the optimal combination of growth factors supporting human mesenchymal stem cell (hMSC) proliferation and osteogenic differentiation induction. It would be possible to use this combination for the functionalization of scaffolds mimicking the natural 3D cell microenvironment. We designed the groups with the aim to compare the effect of each growth factor itself and in combination with others in order to see if any process enhancement is present when more growth factors with the same effect are used together. We tried to stimulate cell proliferation and early differentiation by bFGF and TGF-β7,8 and subsequently support the differentiation process by HGF, IGF-1, and VEGF, which are supposed to induce osteogenesis.10,12,14 As far as we know a study describing comparison of such a growth factor combination range has not yet been published.
Methods
Cells and culture conditions
We used commercially available hMSCs isolated from bone marrow (Human Bone Marrow-derived Mesenchymal Stem Cells, Catalog #7500; ScienCell, Carlsbad, California, USA). Cells were seeded in the third passage in 96-well culture plates at a density of 5,000 cells/cm2. We performed five biological replicates. The cells were cultivated in Minimum Essential Medium (51,411 C; Merck/MilliporeSigma, St. Louis, Missouri, USA) with 10% Fetal Serum Albumin (F75224; Merck/MilliporeSigma) and 1% penicillin/streptomycin (15140 to 122; Invitrogen, Carlsbad, California, USA). Osteogenic medium was made by enrichment of basal medium with 10 mM β-glycerophosphate (50020; Merck/MilliporeSigma), 100 nM dexamethasone (D4902; Merck/MilliporeSigma), and 100 μM ascorbate-2-phosphate (A8960; Merck/MilliporeSigma). Growth factors were bought from PeproTech (London, UK) with the following catalogue numbers: bFGF–100 to 18 C, TGF-β - 100 to 21, HGF – 100 to 39, IGF-1 – 350 to 10, and VEGF – 100 to 20. These were added in the combinations and concentrations stated in Table I. Culture medium was changed every third day to maintain freshness. The same concentration of growth factors was used for the full duration of the experiments. Cells were cultivated for 21 days at 37 °C and 5% CO2.
Table I.
Composition of culture medium in the experimental groups.
| Number of group | Culture medium composition |
|---|---|
| 1 | negative control - osteogenic medium (OM) |
| 2 | OM+ HGF (20 ng/ml) |
| 3 | OM+ IGF-1 (10 ng/ml) |
| 4 | OM+ TGF-β (10 ng/ml) |
| 5 | OM+ bFGF (10 ng/ml) |
| 6 | OM+ VEGF (1 ng/ml) |
| 7 | OM+ TGF-β (10 ng/ml)+ bFGF (10 ng/ml) |
| 8 | OM+ VEGF (1 ng/ml)+ HGF (10 ng/ml) |
| 9 | OM+ VEGF (1 ng/ml)+ IGF-1 (10 ng/ml) |
| 10 | OM+ HGF (20 ng/ml)+ IGF-1 (10 ng/ml) |
| 11 | OM+ TGF-β (10 ng/ml)+ bFGF (10 ng/ml)+ VEGF (1 ng/ml) |
| 12 | OM+ TGF-β (10 ng/ml)+ bFGF (10 ng/ml)+ HGF (20 ng/ml) |
| 13 | OM+ TGF-β (10 ng/ml)+ bFGF (10 ng/ml)+ IGF-1 (10 ng/ml) |
| 14 | OM+ HGF (20 ng/ml)+ IGF-1 (10 ng/ml)+ VEGF (1 ng/ml) |
bFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
Cell proliferation analysis by MTS test
The MTS test is used to evaluate the level of cellular metabolism and viability. Yellow MTS substrate (G3581; Promega, Madison, Wisconsin, USA) – tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymehoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) is incubated with the cells. It is reduced to insoluble purple formazan by cellular succinate dehydrogenase. To each well 20 μl of MTS substrate and 100 μl of culture medium was added. Samples were further incubated for one hour at 37 °C and 5% CO2. Then an absorbance of 100 μl of product at 490 nm was spectrophotometrically measured, reference wavelength 690 nm. Cells cultivated in a culture medium without osteogenic supplements were used as a negative control.
Alkaline phosphatase activity evaluation
ALP activity was measured spectrophotometrically using a substrate for this enzyme (N7653; Merck/MilliporeSigma) p-nitrophenyl phosphate (pNPP). ALP catalyzes the conversion of a colourless substrate to the yellow product p-nitrophenol. Cells were washed twice with PBS and then 100 μl of ALP substrate was added. Samples were shielded from direct light and incubated for 15 minutes at room temperature. Following this, the whole volume of the solution was transferred to a new well and 50 μl of 2 M NaOH was added to stop the reaction. Finally, the absorbance of the product was measured at 405 nm. Cells cultivated in culture medium without osteogenic supplements were used as a negative control.
Cell proliferation assay
After incubation with the ALP substrate (N7653; Merck/MilliporeSigma) cells were washed twice with PBS and 200 μl of a lysis buffer was added. The lysis buffer consists of 10 mM Tris (T1503; Merck/MilliporeSigma), 1 mM ethylenediaminetetraacetic acid (EDTA) (EDS; Merck/MilliporeSigma), and 0.0004% Triton X-100 (T8787; Merck/MilliporeSigma). Cells were then frozen in wells. After thawing, cells were scrubbed from the bottom of the well by a pipette, transferred to a 1.5 ml tube, and vortexed. Samples were further frozen, thawed, and vortexed in two more cycles. Finally, 200 μl of working solution Quant-iT PicoGreenR dsDNA Assay Reagent (Q33120; Invitrogen) was transferred to a black 96-well plate with a transparent bottom, then 10 μl of sample, respectively DNA standard from the assay, was added. There is a fluorescently labelled probe in the working solution which starts to emit a signal after binding. The fluorescence was measured at excitation wavelength 485 nm and emission 528 nm.
Light microscopy
On the 21st day of the experiment samples were visualized using phase contrast microscopy on Olympus IX51, 100× magnification.
Statistical analysis
The obtained data were analyzed with one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test with the level of significance p ≤ 0.001 (marked with the number of the group plus an asterisk (*)) and p ≤ 0.05 (marked with the number of the group only). The number in the graph description marks in comparison with which group reached a certain group of significantly higher value. Use of “significant/significantly” in text always refers to statistical significance.
Results
Supplementation with TGF-β/bFGF supports cellular metabolic activity
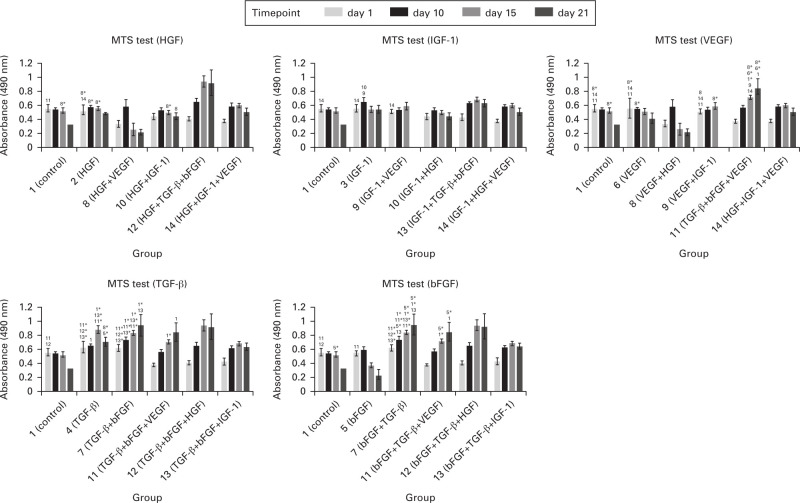
The cell metabolic activity in each experimental group was examined using MTS test on days 1, 10, 15, and 21 (Figure 1). As apparent, the additions of TGF-β or TGF-β combined with bFGF stimulated cells to increase metabolic activity. On the other hand, supplementation with VEGF, HGF, and IGF-1 led to lower metabolic activity than in the control group (no. 1), even on day 1. The metabolism level was growing continuously in groups containing the combination of TGF-β and bFGF regardless of which growth factor they were supplied with, except for IGF-1. In the experimental groups treated with HGF, IGF-1, and VEGF alone or in combination, the metabolic activity remained on approximately the same level during the whole experiment, and we also observed a decrease on days 15 and 21. Changes in metabolic activity are shown in Figure 1. In the graph there is a stated comparison of certain growth factor itself versus the same growth factor combined with others. We have presented the data in five different graphs in order to make the differences easier to see.
Fig. 1.
Metabolic activity of cells evaluated by MTS test. Absorbance measured at 490 nm. Each graph shows a comparison of the growth factor effect itself (stated in the graph heading) and in combination with others (stated on the x-axis). Statistically significant differences are marked with the number of certain groups. Significant increase was observed in groups treated with transforming growth factor β (TGF-β) only or TGF-β with basic fibroblast growth factor (bFGF). p < 0.05 marked with the number of the group; p < 0.001 marked with the number of the group plus an asterisk (*). HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; VEGF, vascular endothelial growth factor.
Double-stranded DNA (quantification)
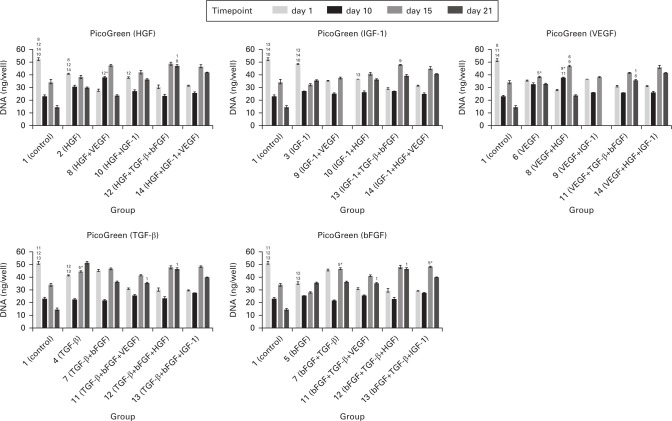
For evaluation of the cellular proliferation level, we used PicoGreen assay with fluorescent probe. We obtained data indicating that in the groups 1, 2, 3, 4, 5, and 10, significantly more cells than in the remaining experimental groups were detected 24 hours after seeding (Figure 2). We did not observe a continuous double-stranded DNA (dsDNA) amount increase and despite the high SDs we observed significant differences. On day 10 there was a decrease of cellular dsDNA in all groups except group 8. A statistically significant difference was measured in group 3 in comparison with groups 7 and 12. Cells started to proliferate on day 15 in all groups. Significant differences were detected in groups 4, 6, 7, 8, and 13. The highest amount of DNA was present in groups 7, 8, and 13. There was no increase of cell proliferation shown on day 21. However, on the last experimental day we could see an increase of dsDNA in some samples in comparison with the first day. In the graph there is a stated comparison of a certain growth factor itself versus the same growth factor combined with others. We have presented the data in five different graphs in order to make the differences easier to see.
Fig. 2.
Quantification of cellular double stranded DNA (dsDNA). Each graph shows a comparison of the growth factor effect itself (stated in the graph heading) and in combination with others (stated on the x-axis). As visible from the day 1 data, the initial adhesion was not equally successful in all groups. Therefore, the number of cells varied among groups. Some statistically significant differences were measured on day 15. In general, we obtained data with high SDs. Statistically significant differences are marked with the number of a certain group. p < 0.05 marked with the number of the group; p < 0.001 marked with the number of the group plus an asterisk (*). bFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
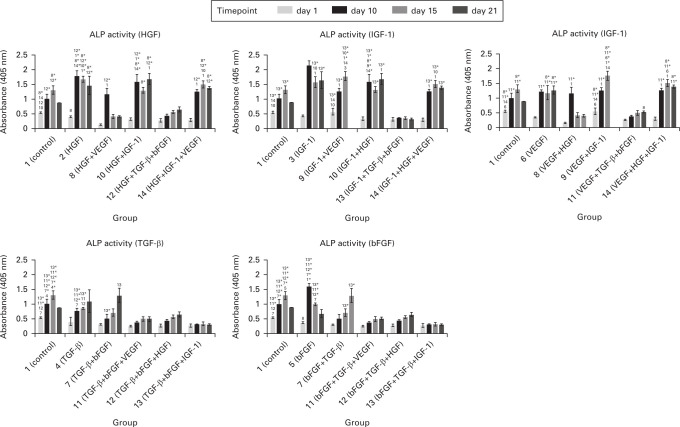
Supplementation with TGF-β/bFGF decreased alkaline phosphatase activity while HGF/IGF-1 caused an increase
The activity of ALP was measured in order to evaluate the level of stimulation of osteogenic differentiation by growth factors. In groups supplemented with the combination TGF-β/bFGF we measured significantly lower levels of ALP activity. In cases where these two growth factors were not used, the ALP activity rose steeply between days 1 and 10. As an early marker of osteogenic differentiation, ALP reached its maximal activity on day 10 in most of the experimental groups. When the cells were supplemented with TGF-β, activity of ALP was growing continuously during the whole experiment lasting for 21 days. This indicates that the whole process of osteogenic differentiation was delayed. Changes in ALP activity are shown in Figure 3. In the graph there is a stated comparison of a certain growth factor itself versus the same growth factor combined with others. We have presented the data in five different graphs in order to make the differences easier to see.
Fig. 3.
Cellular alkaline phosphatase activity. Absorbance measured at 405 nm. Each graph shows comparison of the growth factor effect itself (stated in the graph heading) and in combination with others (stated on the x-axis). Significantly higher alkaline phosphatase (ALP) activity was observed in groups treated with hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1) only, or in combination even with vascular endothelial growth factor (VEGF). Low ALP activity was observed in groups treated with basic fibroblast growth factor (bFGF) and transforming growth factor β (TGF-β) used separately or in combination even with other growth factors. Statistically significant differences are marked with the number of a certain group. p < 0.05 marked with the number of the group; p < 0.001 marked with the number of the group plus an asterisk.
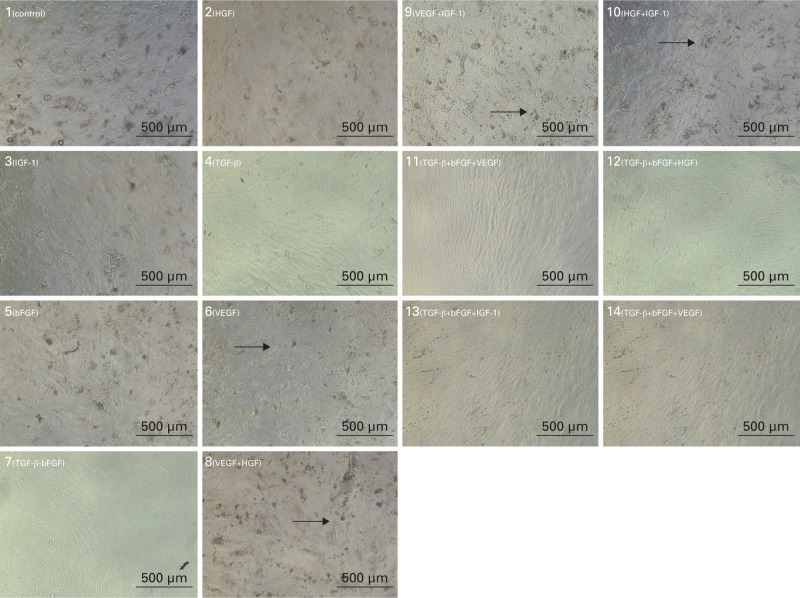
On the last day of the experiment, we used a light microscope to visualize the cells and see their morphology (Figure 4). In groups where the cells were cultured in osteogenic medium alone or with additions of HGF, IGF-1, and VEGF, tiny 3D structures, equally on the whole surface of the cell culture, were visible. Cells in all groups were confluent by this experimental day.
Fig. 4.
Photographs from light microscope, 21st experimental day. Scale bar 500 μm. Photos showing initial phases of extracellular matrix (ECM) mineralization in groups treated with hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF-1) alone or in combination even with other growth factors. Arrows point to the mineralization sites. bFGF, basic fibroblast growth factor; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
Discussion
The osteogenic differentiation of MSCs in vitro can be induced by the addition of growth factors and other bioactive substances to the culture medium. Common osteogenic medium with defined supplements was used for this purpose. In this study, the osteogenic medium was used as a basic culture medium with the aim to provide a sufficient inflow of mineral substances to the cells. Therefore, the cells can deposit mineral substances in their ECM or use them as a source of inorganic phosphate – ALP substrate, or ascorbate as a collagen synthesis cofactor. We assume that the use of the osteogenic medium approximated culture conditions to the in vivo situation in the manner of spectrum of biomolecules influencing cells composition. In this case MSCs would have been influenced by growth factors in the basal medium, ALP would be activated, but without follow-up calcium and other mineral substance accumulation or osteogenic marker expression.20 In studies examining osteogenic differentiation of MSCs, growth factors were added to the culture medium separately or in combinations.14,21-24 In our study we were investigating the effects of TGF-β, bFGF, VEGF, IGF-1, and HGF.
For some of the growth factors used, an ability to induce even other types of differentiation has been shown. Both TGF-β and bFGF were used for cartilage and tendon formation induction.25,26 The cells showed morphological characteristics, specific tissue mRNA expression, and protein synthesis.26,27 These are all connective tissues. They are especially functionally related, and besides that fact, both cartilage and tendons temporarily occur in spots of new bone formation. This could explain the heterogenous effect of particular growth factors on MSC differentiation. During the process of intramembranous ossification, a ligamentous tissue occurs. MSCs originating from the neural crest differentiate into osteoblasts and start to produce bone ECM components – collagen and proteoglycans which bind calcium salts. Osteoblasts subsequently migrate to the calcified areas, so-called ossification centres, and are gradually closed in newly forming ECM. Entrapped cells transform into osteocytes. Finally, ligamentous periosteum is developed on the flat bone surface.28 On the contrary, endochondral ossification involves hyaline cartilage, which is replaced by bone in the later phases. MSCs have activated transcription factors Pax1 and scleraxis leading to chondrogenic gene expression. Chondrocytes then condense through n-cadherins and Sox9 transcription is triggered. They consequently proliferate, synthetize cartilaginous matrix, and become hypertrophic. Fibronectin and collagen type X are synthesized, which allows calcium deposition. Simultaneously, the hypertrophic chondrocytes secrete vesicles containing enzymes generating calcium and phosphorus ions which initiate matrix mineralization in the primary ossification centres. In the final stages some chondrocytes die by apoptosis and others become osteoblasts and change their anaerobic metabolism to aerobic, following which the cartilage is replaced by bone.29
A positive effect on cellular metabolic activity measured by MTS test was observed for bFGF and TGF-β (Figure 1). If these two molecules are combined, they work synergistically and potentiate each other, which is the same conclusion as in studies dealing with their mutual interactions.30 A common molecule in their signalling cascades is Ras GTPase, whose activation leads to proliferative gene transcription. Due to the fact that this GTPase is also part of signalling pathways of distinct receptor types, there are most likely some other molecules ensuring TGF-β and bFGF summation. As apparent from Figure 1, a more significant increase of cellular metabolism was observed after TGF-β addition. This growth factor supports cell viability and its positive effect on MSC proliferation has also been proven.31 The principle of its action is the promotion of osteoprogenitor cell proliferation by extracellular signal-regulated kinase–mitogen-activated protein kinase (ERK-MAP-kinase) cascade stimulation.32 This fact should manifest as an increase of the amount of cellular DNA detectable by PicoGreen. According to some other studies, TGF-β has an opposite effect – inducing senescence of the cells by increasing reactive oxygen species production in mitochondria.33 The biomolecule bFGF is also supposed to have a mitogenic effect.34 This claim was not validated with any significant differences, yet the graphs documenting cellular metabolic activity development show this trend in certain experimental groups. Synergistic function of TGF-β and bFGF was so strong that even the significant decrease of metabolic activity caused by the addition of some negatively acting growth factors was not big enough to decrease the effect of certain combinations to the level of the control group.
Cell metabolism was significantly decreased by combination of VEGF and HGF. If added separately, the same level of cellular metabolism as in the control group was measured. The data for VEGF obtained in this study by MTS test were in conflict with other published studies, where the positive effect on metabolic activity and proliferation of the cells was shown.35,36 On the contrary, HGF is supposed to inhibit MSC proliferation.37 In this case MSCs were treated by both growth factors, in some part of the cascades there was the most likely growth factors cooperation and effect modulation. The point of contact and signal amplification described by Sulpice et al38 is in synergistic activation of the same set of ERK1/2 and p38 kinases. The resulting decrease of cellular metabolism can be a consequence of the higher number of activated molecules or of different kinetics of kinase stimulation.
A decrease of the amount of dsDNA measured in some groups in the last days of cultivation could be caused by the complexity of insufficient surroundings. As cells were cultivated on plastics, they were not sufficiently stimulated by physical interactions of the 3D microenvironment, which would significantly improve cell viability. In addition, using a 3D microcarrier system can possess advantageous gas diffusion properties.39
ALP activity, as an early osteogenesis marker, should grow until day 14 of cultivation. During the experiments we measured significantly higher ALP activity in groups with VEGF, IGF-1, and HGF in comparison with the untreated group. Stronger stimulation was observed for IGF-1 and VEGF. These data match with already published results.22,40-42 On the other hand studies showing an opposite effect have also been published.21,41,43 A decrease of ALP activity was observed in groups treated with combination TGF-β and bFGF, which is in agreement with results of other studies dealing with MSC osteogenic differentiation.21,30 ALP activity is stimulated by Smad3, a TGF-β signalling cascade molecule. Thus, for ALP activity reduction another cascade that does not include Smad3 has to be used. Sowa et al44 showed that ALP activity can also be regulated by cascades through JNK and ERK1/2 kinases. As apparent from Figure 2, which compares cultivation with and without certain growth factors, an effect of the same molecule was not always the same. It depended on the remaining growth factors added in the culture medium. A combination of IGF-1, which supported ALP activity by itself, with TGF-β or bFGF caused a statistically significant decrease in comparison with TGF-β/bFGF alone. Also, the combinations HGF+ VEGF and HGF+ IGF-1 did not have the expected effect. We predicted these two to strongly support ALP activity due to the common signalling molecules in their cascades, however lower values were measured. When the combination of HGF+ IGF-1+ VEGF was used, the values were comparable with HGF in combination with IGF-1 alone.
After ALP activity increases, ECM mineralization then occurs. ALP hydrolyzes pyrophosphate and inorganic phosphate, which forms hydroxyapatite with the production of calcium ions. Hydroxyapatite crystals are deposited in the collagen fibre net from approximately the 14th day of cultivation.29 Microscopic structures are visible on the photographs from the light microscope on day 21. These appear as grains among and on cells, whereas cells in the remaining experimental groups seem to be smooth. These structures are present in the same experimental groups where higher ALP activity was measured. Therefore, we assume them to be early signs of ECM mineralization. The cells were also present in a confluent layer, which is a crucial step foregoing mineralization.
In conclusion, our study showed a positive effect of HGF/IGF-1 on ALP activity and a positive effect of bFGF/TGF-β on metabolic activity of hMSCs. However, it is necessary to note that the entire study was done using hMSCs from bone marrow only. Thus, a possible limitation of the study is an MSC-specific reaction to the growth factors. There is always certain variability in cell behaviour associated with the cell type that needs to be considered.
The field of tissue engineering is evolving rapidly. In five years, we will probably be working with non-cellular carriers enriched with specific biomolecules influencing cell fate. The bioactive molecules will stimulate patients’ own cells to proliferate and heal the defect completely with structurally and functionally restored tissue. This will enable fast and safe treatment even of complicated defects. The materials used will be especially synthetic in order to prevent disease transfer and immune system reaction.
Author contributions
V. Hefka Blahnova: Conceptualized the study, Performed the methodology and experimental work, Wrote the manuscript.
J. Dankova: Conceptualized the study, Performed the methodology.
M. Rampichova: Supervised the study, Reviewed the manuscript.
E. Filova: Edited and reviewed the manuscript.
Funding statement
This work was supported by: the Grant Agency of Charles University, Grant number 448218; the Ministry of Education, Youth and Sports of the Czech Republic within National Sustainability Programme I: project number RP NPUI:LO1508 and RP NPUI:LO1309, CZ.2.16/3.1.00/24006; the Czech Science Foundation, project number 18 to 09306 S; and the Internal Grant Agency of the Ministry of Health of the Czech Republic project number NV18-05-00379, 16-28637A, 17-32285A.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical review statement
This study did not require ethical approval.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Pittenger MF , et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 2.Rodeo SA. Cell therapy in orthopaedics: where are we in 2019? Bone Joint J. 2019;101-B(4):361–364. [DOI] [PubMed] [Google Scholar]
- 3.Osagie-Clouard L, Sanghani-Kerai A, Coathup M, et al. . The influence of parathyroid hormone 1-34 on the osteogenic characteristics of adipose- and bone-marrow-derived mesenchymal stem cells from juvenile and ovarectomized rats. Bone Joint Res. 2019;8(8):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Liu M, Meng Q. Angelica polysaccharide promotes proliferation and osteoblast differentiation of mesenchymal stem cells by regulation of long non-coding RNA H19. Bone Joint Res. 2019;8(7):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshikawa M, Nakasa T, Ishikawa M, Adachi N, Ochi M. Evaluation of autologous skeletal muscle-derived factors for regenerative medicine applications. Bone Joint Res. 2017;6(5):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan S, Baylink DJ. Bone growth factors. Clin Orthop Relat Res. 1991;263:30–48. [PubMed] [Google Scholar]
- 7.Meyers EA, Kessler JA. TGF-β Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb Perspect Biol. 2017;9(8):a022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marie PJ. Fibroblast growth factor signaling controlling bone formation: an update. Gene. 2012;498(1):1–4. [DOI] [PubMed] [Google Scholar]
- 9.Fei Y, Xiao L, Doetschman T, Coffin DJ, Hurley MM. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J Biol Chem. 2011;286(47):40575–40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locatelli V, Bianchi VE. Effect of GH/IGF-1 on bone metabolism and Osteoporsosis. Int J Endocrinol. 2014;2014(12):235060–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long MW. Osteogenesis and bone-marrow-derived cells. Blood Cells, Molecules, and Diseases. 2001;27(3):677–690. [DOI] [PubMed] [Google Scholar]
- 12.Hu K, Olsen BR. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev Dyn. 2017;246(4):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosso A, Burger MG, Lunger A, et al. . It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol.. 2017;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aenlle KK, Curtis KM, Roos BA, Howard GA. Hepatocyte growth factor and p38 promote osteogenic differentiation of human mesenchymal stem cells. Molecular Endocrinology. 2014;28(5):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO Journal. 2007;53(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kielty CM, Grant ME. The Collagen Family: Structure, Assembly, and Organization in the Extracellular Matrix. In: Royce PM, Steinmann B, eds Connective Tissue and Its Heritable Disorders. Second ed. New York, New York: Wiley-Liss, 2002:159–221. [Google Scholar]
- 17.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5(3):222–226. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Khong ML, ELH L, et al. . Long-Chain polyphosphate in osteoblast matrix vesicles: enrichment and inhibition of mineralization. Biochim Biophys Acta Gen Subj. 1863;2019(1):199–209. [DOI] [PubMed] [Google Scholar]
- 19.Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta. 1790;2009(12):1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7(2):463–477. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Ren P-G, Ma T, Smith RL, Goodman SB. Modulating osteogenesis of mesenchymal stem cells by modifying growth factor availability. Cytokine. 2010;51(3):305–310. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Mu J, Fan Z, et al. . Insulin-Like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res. 2012;8(3):346–356. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Bai Y, Yin G, et al. . Synergistic and sequential effects of BMP-2, bFGF and VEGF on osteogenic differentiation of rat osteoblasts. J Bone Miner Metab. 2014;32(6):627–635. [DOI] [PubMed] [Google Scholar]
- 24.Gurkan UA, Gargac J, Akkus O. The sequential production profiles of growth factors and their relations to bone volume in ossifying bone marrow explants. Tissue Eng Part A. 2010;16(7):2295–2306. [DOI] [PubMed] [Google Scholar]
- 25.Havlas V, Kos P, Jendelová P, et al. . [Comparison of chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells with cultured chondrocytes and bone marrow mesenchymal stem cells]. Acta Chir Orthop Traumatol Cech. 2011;78(2):138–144. (Article in Czech). [PubMed] [Google Scholar]
- 26.Hankemeier S, Keus M, Zeichen J, et al. . Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11(1-2):41–49. [DOI] [PubMed] [Google Scholar]
- 27.Shu C, Smith SM, Little CB, Melrose J. Use of FGF-2 and FGF-18 to direct bone marrow stromal stem cells to chondrogenic and osteogenic lineages. Future Sci OA. 2016;2(4):FSO142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010;77(1):4–12. [DOI] [PubMed] [Google Scholar]
- 30.Bosetti M, Boccafoschi F, Leigheb M, Cannas MF. Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomol Eng. 2007;24(6):613–618. [DOI] [PubMed] [Google Scholar]
- 31.Jian H, Shen X, Liu I. Smad3-Dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20(6):666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Deng C, Li Y-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Niu J, Li X, et al. . TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014;14(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benavente CA, Sierralta WD, Conget PA, Minguell JJ. Subcellular distribution and mitogenic effect of basic fibroblast growth factor in mesenchymal uncommitted stem cells. Growth Factors. 2003;21(2):87–94. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1(4):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong X, Zheng F, Guo LY, et al. . [VEGF promotes the proliferation of bone marrow derived mesenchymal stem cells through ERK1/2 signal pathway]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(5):1292–1296. (Article in Chinese). [PubMed] [Google Scholar]
- 37.Frisch RN, Curtis KM, Aenlle KK, Howard GA. Hepatocyte growth factor and alternative splice variants - expression, regulation and implications in osteogenesis and bone health and repair. Expert Opin Ther Targets. 2016;20(9):1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulpice E, Ding S, Muscatelli-Groux B, et al. . Cross-Talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 2009;101(9):525–539. [DOI] [PubMed] [Google Scholar]
- 39.Meng X, Leslie P, Zhang Y, Dong J. Stem cells in a three-dimensional scaffold environment. Springerplus. 2014;3(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen Q, Zhou L, Zhou C, et al. . Change in hepatocyte growth factor concentration promote mesenchymal stem cell-mediated osteogenic regeneration. J Cell Mol Med. 2012;16(6):1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J-H, Um S, Jang J-H, Seo BM, Effects of V. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012;348(3):475–484. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C, Zhang X, Xu L, et al. . Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids. 2014;46(7):1673–1680. [DOI] [PubMed] [Google Scholar]
- 43.Standal T, Abildgaard N, Fagerli U-M, et al. . Hgf inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma. Blood. 2007;109(7):3024–3030. [DOI] [PubMed] [Google Scholar]
- 44.Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Activations of ERK1/2 and JNK by transforming growth factor β negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J Biol Chem. 2002;277(39):36024–36031. [DOI] [PubMed] [Google Scholar]