Abstract
Aims
This study is a prospective, non-randomized trial for the treatment of fractures of the medial malleolus using lean, bioabsorbable, rare-earth element (REE)-free, magnesium (Mg)-based biodegradable screws in the adult skeleton.
Methods
A total of 20 patients with isolated, bimalleolar, or trimalleolar ankle fractures were recruited between July 2018 and October 2019. Fracture reduction was achieved through bioabsorbable Mg-based screws composed of pure Mg alloyed with zinc (Zn) and calcium (Ca) ( Mg-Zn0.45-Ca0.45, in wt.%; ZX00). Visual analogue scale (VAS) and the presence of complications (adverse events) during follow-up (12 weeks) were used to evaluate the clinical outcomes. The functional outcomes were analyzed through the range of motion (ROM) of the ankle joint and the American Orthopaedic Foot and Ankle Society (AOFAS) score. Fracture reduction and gas formation were assessed using several plane radiographs.
Results
The follow-up was performed after at least 12 weeks. The mean difference in ROM of the talocrural joint between the treated and the non-treated sites decreased from 39° (SD 12°) after two weeks to 8° (SD 11°) after 12 weeks (p ≤ 0.05). After 12 weeks, the mean AOFAS score was 92.5 points (SD 4.1). Blood analysis revealed that Mg and Ca were within a physiologically normal range. All ankle fractures were reduced and stabilized sufficiently by two Mg screws. A complete consolidation of all fractures was achieved. No loosening or breakage of screws was observed.
Conclusion
This first prospective clinical investigation of fracture reduction and fixation using lean, bioabsorbable, REE-free ZX00 screws showed excellent clinical and functional outcomes.
Cite this article: Bone Joint Res 2020;9(8):477–483.
Keywords: Lean magnesium screws, Rare-earth elements, Ankle fractures, Functional outcome
Article focus
First prospective clinical evaluation of a lean Magnesium-Zinc-Calcium Alloy ZX00 screw.
Clinical and radiological outcome after fracture stabilization of the medial malleolus with ZX00.
Key messages
Biodegradable magnesium implant without any rare-earth elements.
Unimpeded fracture healing in all patients despite small radiolucency during the first 12 weeks around the screw.
No postoperative adverse events.
Strengths and limitations
Prospective study including 20 patients.
Ethically prohibited control group.
Early results only: the follow-up for all patients was at least 12 weeks.
Introduction
Medial malleolar fractures are very common among the adult population and constitute approximately 9% of all fractures.1 Current literature recommends operative treatment of ankle fractures through open reduction and internal fixation,2 followed by immobilization and rehabilitation.3-6 Many different approaches are described for fixation of medial malleolus fractures, including screw-only fixation and using plates in combination with screws.7 The most commonly employed and accepted clinical technique is the operative fixation of medial malleolar fractures with cancellous screws.8 A recent trial recommended a single screw for fixation of the medial malleolar fracture, with equivalent mid-term patient outcomes, compared to double screw fixation.9 Especially in older patients, conservative treatment with close contact casting is an equivalent therapy in terms of functional outcomes at long-term follow-up, although careful monitoring in the weeks after its application to monitor the maintenance of reduction is required.10
Nevertheless, many patients report postoperative dissatisfaction due to persistent pain. In most cases, the cause of this pain is a soft-tissue irritation resulting from hardware placement, or a shoe. In some circumstances, a patient’s pain might be severe enough to require hardware removal.7
Over the last decade, there has been an increasing demand to overcome these disadvantages associated with conventional implant materials, by developing new, alternative materials and material production approaches for use in trauma care.11,12 Among biodegradable metals, magnesium (Mg)-based implants exhibit good biocompatibility and suitable biomechanical properties, with appropriate mechanical strength and ductility, providing an advantage over biodegradable polymers and ceramics.13-15 Recently, considerable efforts have been made to reduce the Mg degradation rate,16,17 to inhibit excess hydrogen gas evolution and encapsulated gas cavities.18,19 One of these strategies is the use of rare-earth elements (REE), such as yttrium or gadolinium, to decelerate degradation.20,21 Mg-based implants alloyed with REE, however, were observed to induce apoptosis and necrosis with high-concentration extracts. Furthermore, a negative effect on the viability of immune cells in vitro was documented.22
Therefore, in cooperation with ETH Zürich (Laboratory of Metal Physics and Technology, Department of Materials, ETH Zürich, Zürich, Switzerland) we transitioned to lean Mg-based implant materials (ZX00) alloyed with zinc (Zn) and calcium (Ca),23 without any REE, to avoid both short-term and, crucially, long-term adverse effects.
Grün et al24 demonstrated the degradation performance of this material termed ZX00 (Mg-Zn0.45-Ca0.45, in wt.%) in a small and large growing-animal model study and reported no negative effects on bone formation and ingrowth. ZX00 showed slow and homogeneous degradation.24
Facing Mg-based implants, the elementary questions of implant loosening caused by gas evolution and long-term result of the radiolucent zones have to be considered.
To date, there are no prospective investigations that elaborate on these findings.
Based on these aspects, we planned this prospective, non-randomized first in man study to evaluate the safety and the early clinical and functional outcomes of lean, REE-free, Mg compression screws (ZX00) in the case of fracture stabilization of the medial malleolus in humans.
Methods
The pilot study was designed as a prospective, non-randomized trial for the treatment of displaced fractures of the medial malleolus with a lean, REE-free, Mg-based biodegradable implant in the adult skeleton. The trial was conducted according to the Good Clinical Practice (ISO 14155:2011) standard and the Declaration of Helsinki.
Patient enrolment
The study was performed at the Department of Orthopaedics and Trauma at our institution and approved by the ethics committee (28 to 071 ex 15/16). A total of 20 subjects were recruited and operated on by three experienced surgeons (PH, FS, MO) in the centre between July 2018 and October 2019. The follow-up timepoint for all patients was at least 12 weeks. Inclusion focused on adults aged between 18 and 65 years presenting with a displaced isolated medial malleolus fracture, a bimalleolar ankle fracture, or a trimalleolar ankle fracture. Fracture displacement was defined as diastasis of the fracture in any direction of 2 mm or more. Exclusion criteria were pathological fractures, underlying diseases (particularly bone diseases, kidney diseases, diabetes mellitus), poly-traumatized patients, and pregnant or breastfeeding women.
Written informed consent was obtained from each patient before the surgery. Standardized electronic case report forms (eCRFs) were used to collect individual patient data during this study. All radiological data were stored in a picture archiving and communication system (PACS). Fractures were classified according to the Herscovici system.25
Bioabsorbable devices
Ultra-high pure Mg (99.999%) was alloyed with Zn and Ca (0.45 wt% Zn and 0.45 wt% Ca) at 750°C under a protective gas atmosphere. This alloy was produced by ETH Zürich in cooperation with de Cavis AG (Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland). The extrusion of the material into 6 mm rods was accomplished by LKR Leichtmetallkompetenzzentrum Ranshofen (Ranshofen, Austria; certified to ISO 9001:2008) and compression screws were produced by Mattig Präzision GmbH (Obertrum am See, Austria; certified to ISO 9001:2008) in Graz. Packaging and sterilization by γ irradiation were performed by Biegler (Mauerbach, Austria) and Mediscan GmbH & Co KG (Kremsmünster, Austria; certified to EN ISO 13485 and ISO 11137, respectively). Prepared screws had a length of 40 mm and a diameter of 3.5 mm. Implants were threaded at the distal part for use as traction screws.
Surgery
All patients were operated in a supine position. In case of a bimalleolar or trimalleolar fracture, the fibular and the dorsal tibial fragments were treated before the medial malleolus. Subsequently, two parallel Kirschner wires perpendicular to the medial malleolar fracture line were positioned with fluoroscopic control. A cannulated drill bit with 2.7 mm width was used for preparing the hole. Subsequently, one wire was removed and the ZX00 compression screw was inserted into the hole. Final fixation of the fracture was achieved with the second bioabsorbable Mg screw after removal of the second wire. The insertion torque of the screws was limited at a force of 1.5 newton metre with a torque handle. In all patients, no additional implants other than the Mg screws were used for the fixation of the medial malleolus. Patients with bimalleolar fractures and trimalleolar fractures were fixed with titanium plates and screws. Moreover, patients were immobilized with an under-knee plaster cast four to six weeks postoperatively and encouraged to attempt full weight-bearing as tolerated. Ankle movement exercises were started immediately after removal of the plaster.
Assessments and measurements
For each subject, the study consisted of assessments conducted preoperatively, immediately after operation and postoperative follow-up visits (Table I). During follow-up, complications including vital signs, erythema, swelling, pain, secretions, wound healing disorders, wound infections, or implant infections were recorded. Pain was assessed using visual analogue scale (VAS). Blood analysis and detection of Ca and Mg were recorded. Functional outcomes were evaluated through range of motion (ROM) in dorsal and plantar flexion. Additionally, the American Orthopaedic Foot and Ankle Society (AOFAS) score26 was performed after three months. Anteroposterior and lateral ankle radiographs (X-rays) were used to evaluate the fracture union and loss of reduction during follow-up. Postoperative radiograph evaluation was performed after surgery and after two, six, and 12 weeks. Fracture reduction and persisting gap or step were examined.
Table I.
Patient follow-up, assessments, and visits.
| Study visit per patient | Baseline | Operation | Dismissal | 2 wks (SD 4 days) |
6 wks (SD 4 days) |
12 wks (SD 4 days) |
|---|---|---|---|---|---|---|
| Visit number | V1 | V2 | V3 | V4 | V5 | V6 |
| Inclusion/exclusion criteria | X | |||||
| Signed informed consent | X | |||||
| Demographics | X | |||||
| Relevant medical history | X | |||||
| Laboratory/urinary analysis | X | X | X | |||
| Radiograph (two plane) | X | X | X | X | X | X |
| Range of motion (ROM) (standard goniometer) |
X | X | X | |||
| Clinical examination | X | X | X | X | ||
| Wound (pain, redness, secretion, erythema) |
X | X | X | X | ||
| Visual Analogue Scale (VAS) | X | X | X | X | X | |
| AOFAS | X |
AOFAS, American Orthopaedic Foot and Ankle Society.
Statistical analysis
A descriptive analysis of the data was performed using proportions, frequency distributions, means, and standard deviations. Data were presented as the mean (SD). Comparisons between timepoints were assessed using a paired t-test. A p-value less than or equal to 0.05 was considered statistically significant.
Results
Demographic data
A total of 20 patients were enrolled in the study (11 males and nine females with a mean age of 40.1 years (SD 14.5)) between July 2018 and October 2019. The follow-up timepoint for all patients was at least 12 weeks. Patient demographics are shown in Table II.
Table II.
Patient demographics.
| Demographic | Data |
|---|---|
| Mean age, yrs (SD) | 40.1 (14.5) |
| Mean height, cm (SD) | 171 (14) |
| Mean weight, kg (SD) | 79.05 (9.92) |
| Mean body mass index (BMI), kg/m2 (SD) | 26.25 (2.25) |
During the first screening of the patients, every patient presented with normal vital signs and electrocardiograms (ECGs) were normal.
Aetiology and fracture classification
Ankle sprains caused 45% (nine patients) of all fractures. The rest were attributed to sports injuries (cycling, soccer, climbing, and skiing accidents). Four patients presented with traffic-related injuries (20%): two subjects (10%) had a bicycle accident, while one had a motor bike accident and another a car accident. According to the position of the medial malleolar fracture, 13 patients had a fracture type C Hercovici, six patients had a type B fracture, and one patient had a type A fracture.
Surgery and intraoperative outcomes
During surgery, all Mg-based screws were fixed successfully (Figure 1) and no intraoperative complications (including screw fracture) were observed. Most of the patients (60%, 12 patients) had an anatomical intraoperative reduction without any diastasis or gap. In total, 35% of the patients had a persisting gap of 1 mm and one patient had a fracture reduction with a persisting diastasis of more than 1 mm. Moreover, after surgery one unplanned CT scan analysis, performed because of debatable reduction, showed a persisting gap of 2 mm. None of the patients included in this study required unscheduled revision.
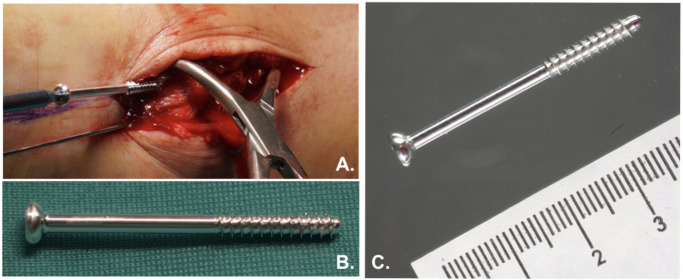
Fig. 1.
a) Placement of a ZX00 screw in the medial malleolar during surgery. b) and c) External appearance of ZX00 screw.
Clinical and functional outcomes
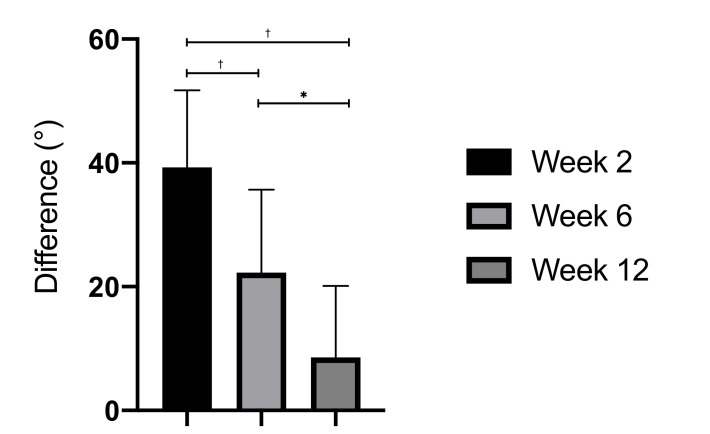
After surgery, the mean VAS was 1.6 points (SD 0.6) postoperative and 1.3 points (SD 0.5) after two weeks. Six weeks after surgery, all patients reported no pain (VAS 1) regarding the medial malleolus. Blood analysis and specifically the dosage of Mg and Ca were normal at all follow-up visits in the 20 patients with a Mg concentration between 0.700 mmol/l and 1.100 mmol/l and a Ca concentration between 2.20 mmol/l and 2.65 mmol/l. Moreover, renal function remained stable. The mean difference of the total movement of the talocrural joint (dorsal flexion/plantar flexion) between the operated site and the nonoperated site was 39° (SD 12°) after two weeks, 22° (SD 13°) after six weeks, and decreased to 8° (SD 11°) after 12 weeks (Figure 2). There was a statistically significant difference between weeks 2 and 6 (p = 0.002) and between weeks 6 and 12 (p = 0.001) concerning the difference of the total movement of the talocrural joint. At the final follow-up, the mean AOFAS score was 92.5 points (SD 4.1), which represents an excellent result for all patients. None of the patients showed wound healing disorders, and none showed erythema, swelling, deep wound infections, or implant site infections. There was no loosening of the implant as a result of gas evolution.
Fig. 2.
Difference of the total movement of the talocrural joint between the operated site and the nonoperated site. *p < 0.05. †p < 0.001.
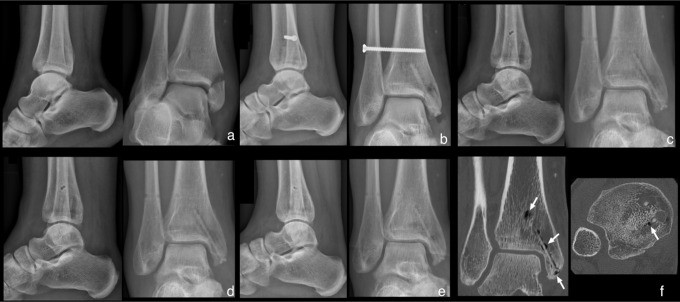
After anteroposterior and lateral radiograph analysis, the ankle fracture was healed in 18 patients (90%) after six weeks. After 12 weeks, all patients showed complete consolidation of the ankle fractures. Serial radiographs displayed small radiolucent zones around the screws, increasing up to week six, with a constant period to week 12 followed by a decrease to week 24. Despite this gas formation around the screws, no encapsulation was detected. In one patient, a CT scan was performed after 52 weeks because of uncertainty of appropriate distal tibiofibular reduction after evaluation of the radiographs. Here, a screw volume loss of about 50% was displayed. Additionally an increased endosteal bone mass at the screw head was detected (Figure 3).
Fig. 3.
a) Anteroposterior and lateral ankle radiographs of a 49-year-old female patient; medial malleolar fracture combined with distal tibiofibular joint disruption (Maisonneuve fracture). b) Two-week timepoint radiographs, anatomical reduction with two magnesium (Mg)-based screws and one titanium screw; visible fracture line at the medial malleolus, small signs of radiolucent zones within the bone surrounding the screws. c) Six-week timepoint, plane radiographs with complete fracture consolidation, increase of radiolucent zones within the bone surrounding the screws, removed titanium screw. d) Timepoint radiograph images at 12 weeks with constant radiolucent zones. e) Timepoint radiographs at 24 weeks. f) CT scan at 52 weeks, decrease of radiolucent zones, increased endosteal bone mass, and periosteal bone ingrowth at the screw head (white arrow).
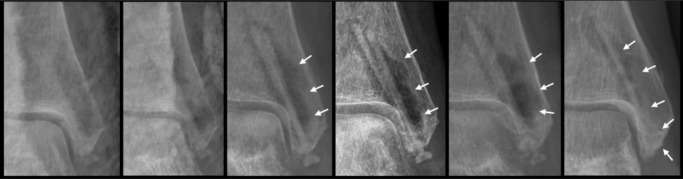
In one patient, a manifest osteoporosis was noticed before surgery. Compared to the other patients, an increased gas formation was seen around the screws (Figure 4). Nevertheless, bone healing was accomplished after six weeks. At this timepoint, a small sclerotic line could be detected at the border of the radiolucent zone to the surrounding tissue. Regardless, an increase in density in this area due to higher endosteal bone mass at the region of expressed gas at the screw could be presented in the radiographs taken after 24 and 52 weeks of follow-up (Figures 3-4).
Fig. 4.
Anteroposterior ankle radiographs of a 61-year-old female patient with pre-existing osteoporosis, bimalleolar fracture with a posterior malleolar fragment. From left to right, anteroposterior ankle radiograph after surgery, after two weeks (reduction of the fractures with two magnesium (Mg)-based screws and a titanium plate and screws), after six weeks (obvious radiolucent zones fracture consolidation), after 12 weeks (steady radiolucency, small sclerotic line at the border of the radiolucent zone to the surrounding tissue), after 24 weeks, and after 52 weeks (steady sclerotic line, increase in density at former radiolucent area). The white arrows show the gas formation arround the screw.
Discussion
To date, 16 clinical studies and case reports have been conducted for the clinical implementation of Mg implants alloyed with REE.27–42 The earliest clinical trials on metatarsal osteotomy fixation for the correction of hallux valgus deformities31–33 showed similar complications compared to titanium implants. Subsequently, good results were achieved for ankle fracture fixation and intra-articular fragment fixation.29
Apart from these promising results, poor outcomes have been reported in two studies with extensive gas formation, loosening, and cystic formation within carpal bones after scapho-trapezio-trapezoid (STT) fusion27 and secondarily after acute scaphoid fracture fixation.28
In our opinion, the size of the bone and its capacity to distribute the evolving gas plays an important role. Therefore, we expect a higher risk of failure in small bones, caused by the accumulation of entrapped gas, as illustrated in recent literature.27,28
This is the first implementation of the lean, REE-free Mg implant ZX00 in humans. Evaluation was performed by a prospective clinical investigation on functional and radiological results of medial malleolus fracture fixation with these implants. The promising results of this study demonstrate an adequate fixation of medial malleolus fractures using REE-free Mg screws ZX00. Despite the radiolucent areas around the screws at the first 12 weeks after implantation, all fractures healed without secondary displacement or implant breakage.
Increased gas formation was seen around the screws in one patient with a manifest osteoporosis. To our knowledge, metabolic and immunological changes due to osteoporotic disease can cause an increased degradation of Mg implants with expanded gas volume, seen as radiolucent areas. However, further investigations should focus on this highly sensitive elderly patient collective to understand the importance and underlying mechanism of osteoporosis in combination with Mg implants. Nevertheless, bone healing was accomplished in this patient after six weeks. Astonishingly, an obvious increase in density at the radiolucent area within the sclerotic line was observed in the CT scan after 52 weeks. In our opinion, this finding of newly formed endosteal bone mass demonstrates the osteoinductive effect of the Mg screws, which compensates for the negative impact of gas expression in early stages.
Finally, this study will be extended for up to two years to visualize the expected complete degradation of the material and a full conversion of the radiolucent zones formed by expressed gas, into newly formed endosteal bone mass. Additionally, measurements of long-term functional outcomes will be performed.
There are some limitations to this study. Since this is the first implementation of this new, REE-free Mg material (ZX00) in humans, a control group was ethically not permissible. However, 20 patients were included in the study and visited at least until bone healing was achieved.
In conclusion, this investigation aimed to study the performance and safety of a new, REE-free material. Therefore, vital parameters as well as laboratory parameters in combination with early clinical outcome and radiological monitoring were examined. It can be stated that lean, REE-free bioabsorbable Mg screw fixation for medial malleolus fractures provided unimpeded fracture healing. All vital and laboratory parameters were normal and the patients presented very good functional outcomes.
In our opinion, resorbable implants such as Mg are the next step in a long history of orthopaedic implants. From this point of view, it is of utmost importance to clarify the way Mg implants work. Small radiolucency around the implants at early postoperative stages should not be misinterpreted as osteolysis. Despite this phenomenon, fracture healing was not adversely affected.
Author contributions
P. Holweg: Planned the study, Performed the surgeries, Wrote the manuscript.
V. Herber: Wrote the manuscript, Managed and analyzed the data.
M. Ornig: Performed the surgeries.
G. Hohenberger: Managed the data.
N. Donohue: Wrote the manuscript.
P. Puchwein: Managed the data.
A. Leithner: Supervised the study.
F. Seibert: Performed the surgeries, Supervised the study.
Funding statement
This study is supported by a LORENZ BÖHLER Fund.Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non- profit organization with which one or more of the authors are associated.
Ethical review statement
The study was performed at the Department of Orthopaedics and Trauma at our institution and approved by the ethics committee (28 to 071 ex 15/16).
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691–697. [DOI] [PubMed] [Google Scholar]
- 2.Tile M. Fractures of the Ankle. In: Schatzker J, Tile M, eds The Rationale of Operative Fracture Care. 2nd ed Berlin: Springer, 1996:523–561. [Google Scholar]
- 3.Donatto KC. Ankle fractures and syndesmosis injuries. Orthop Clin North Am. 2001;32(1):79–90. [DOI] [PubMed] [Google Scholar]
- 4.Shaffer MA, Okereke E, Esterhai JL, et al. Effects of immobilization on plantar-flexion torque, fatigue resistance, and functional ability following an ankle fracture. Phys Ther. 2000;80(8):769–780. [PubMed] [Google Scholar]
- 5.Keene DJ, Willett K. Implications of the ankle injury management (AIM) trial: close contact casting or surgery for older adults with an unstable ankle fracture? Bone Joint J. 2019;101-B(12):1472–1475. [DOI] [PubMed] [Google Scholar]
- 6.Vandenborne K, Elliott MA, Walter GA, et al. Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle Nerve. 1998;21(8):1006–1012. [DOI] [PubMed] [Google Scholar]
- 7.Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365–1367. [DOI] [PubMed] [Google Scholar]
- 8.Femino JE, Gruber BF, Karunakar MA. Safe zone for the placement of medial malleolar screws. J Bone Joint Surg Am. 2007;89-A(1):133–138. [DOI] [PubMed] [Google Scholar]
- 9.Buckley R, Kwek E, Duffy P, et al. Single-Screw fixation compared with double screw fixation for treatment of medial Malleolar fractures: a prospective randomized trial. J Orthop Trauma. 2018;32(11):548–553. [DOI] [PubMed] [Google Scholar]
- 10.Keene DJ, Willett K. Implications of the Ankle Injury Management (AIM) trial: close contact casting or surgery for older adults with an unstable ankle fracture? Bone Joint J. 2019;101-B(12):1472–1475. [DOI] [PubMed] [Google Scholar]
- 11.Walsh WR, Cotton NJ, Stephens P, et al. Comparison of poly-L-lactide and polylactide carbonate interference screws in an ovine anterior cruciate ligament reconstruction model. Arthroscopy. 2007;23(7):757–765. [DOI] [PubMed] [Google Scholar]
- 12.Thormann U, Alt V, Heimann L, et al. The biocompatibility of degradable magnesium interference screws: an experimental study with sheep. Biomed Res Int. 2015;2015:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Feyerabend F, Schilling AF, Willumeit-Römer R, Luthringer BJC. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015;27:294–304. [DOI] [PubMed] [Google Scholar]
- 14.Jähn K, Saito H, Taipaleenmäki H, et al. Intramedullary Mg2Ag nails augment callus formation during fracture healing in mice. Acta Biomater. 2016;36:350–360. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Xu J, Ruan YC, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichler K, Kraus T, Martinelli E, et al. Cellular reactions to biodegradable magnesium alloys on human growth plate chondrocytes and osteoblasts. Int Orthop. 2014;38(4):881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myrissa A, Bräuer S, Martinelli E, et al. Gadolinium accumulation in organs of Sprague–Dawley® rats after implantation of a biodegradable magnesium-gadolinium alloy. Acta Biomater. 2017;48:521–529. [DOI] [PubMed] [Google Scholar]
- 18.Cheng M, Wahafu T, Jiang G-feng, et al. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci Rep. 2016;6:24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makkar P, Sarkar SK, Padalhin AR, et al. In vitro and in vivo assessment of biomedical Mg–Ca alloys for bone implant applications. J Appl Biomater Funct Mater. 2018;16(3):126–136. [DOI] [PubMed] [Google Scholar]
- 20.Myrissa A, Agha NA, Lu Y, et al. In vitro and in vivo comparison of binary Mg alloys and pure Mg. Mater Sci Eng C. 2016;61:865–874. [DOI] [PubMed] [Google Scholar]
- 21.Amerstorfer F, Fischerauer S, Fischer L, et al. Long-term in vivo degradation behavior and near-implant distribution of resorbed elements for magnesium alloys WZ21 and ZX50. Acta Biomater. 2016;42:440–450. [DOI] [PubMed] [Google Scholar]
- 22.Jin L, Wu J, Yuan G, Chen T. In vitro study of the inflammatory cells response to biodegradable Mg-based alloy extract. PLoS One. 2018;13(3):e0193276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofstetter J, Becker M, Martinelli E, et al. High-strength low-alloy (HSLA) Mg–Zn–Ca alloys with excellent biodegradation performance. JOM. 2014;66(4):566–572. [Google Scholar]
- 24.Grün NG, Holweg P, Tangl S, et al. Comparison of a resorbable magnesium implant in small and large growing-animal models. Acta Biomater. 2018;78:378–386. [DOI] [PubMed] [Google Scholar]
- 25.Herscovici D, Scaduto JM, Infante A. Conservative treatment of isolated fractures of the medial malleolus. J Bone Joint Surg Br. 2007;89-B(1):89–93. [DOI] [PubMed] [Google Scholar]
- 26.Kitaoka HB, Alexander IJ, Adelaar RS, et al. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349–353. [DOI] [PubMed] [Google Scholar]
- 27.Wichelhaus A, Emmerich J, Mittlmeier T. A case of implant failure in partial wrist fusion applying Magnesium-Based headless bone screws. Case Rep Orthop. 2016;2016(2016):7049130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier G, Panzica R. [First results with a resorbable MgYREZr compression screw in unstable scaphoid fractures show extensive bone cysts]. Handchir Mikrochir Plast Chir. 2017;49(1):37–41. (Article in German) [DOI] [PubMed] [Google Scholar]
- 29.Biber R, Pauser J, Geßlein M, Bail HJ. Magnesium-Based absorbable metal screws for intra-articular fracture fixation. Case Rep Orthop. 2016;2016:9673174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turan A, Kati YA, Acar B, Kose O. Magnesium bioabsorbable screw fixation of radial styloid fractures: case report. J Wrist Surg. 2020;9(2):150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaass C, von Falck C, Ettinger S, et al. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies - 3 year results of a randomized clinical trial. J Orthop Sci. 2018;23(2):321–327. [DOI] [PubMed] [Google Scholar]
- 32.Windhagen H, Radtke K, Weizbauer A, et al. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online. 2013;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plaass C, Ettinger S, Sonnow L, et al. Early results using biodegradable magnesium screw for modified chevron osteotomies. J Orthop Res. 2016;34(12):2207–2214. [DOI] [PubMed] [Google Scholar]
- 34.Leonhardt H, Franke A, McLeod NMH, Lauer G, Nowak A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br J Oral Maxillofac Surg. 2017;55(6):623–625. [DOI] [PubMed] [Google Scholar]
- 35.Biber R, Pauser J, Brem M, Bail HJ. Bioabsorbable metal screws in traumatology: A promising innovation. Trauma Case Reports. 2017;8:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kose O, Turan A, Unal M, Acar B, Guler F. Fixation of medial malleolar fractures with magnesium bioabsorbable headless compression screws: short-term clinical and radiological outcomes in eleven patients. Arch Orthop Trauma Surg. 2018;138(8):1069–1075. [DOI] [PubMed] [Google Scholar]
- 37.Acar B, Kose O, Turan A, et al. Comparison of bioabsorbable magnesium versus titanium screw fixation for modified distal chevron osteotomy in hallux valgus. Biomed Res Int. 2018;2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choo JT, Lai SHS, Tang CQY, Thevendran G. Magnesium-based bioabsorbable screw fixation for hallux valgus surgery - A suitable alternative to metallic implants. Foot Ankle Surg. 2019;25(6):727–732. [DOI] [PubMed] [Google Scholar]
- 39.Klauser H. Internal fixation of three-dimensional distal metatarsal I osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws. Foot Ankle Surg. 2019;25(3):398–405. [DOI] [PubMed] [Google Scholar]
- 40.Gigante A, Setaro N, Rotini M, Finzi SS, Marinelli M. Intercondylar eminence fracture treated by resorbable magnesium screws osteosynthesis: A case series. Injury. 2018;49(Suppl 3):S48–S53. [DOI] [PubMed] [Google Scholar]
- 41.Acar B, Unal M, Turan A, Kose O. Isolated lateral Malleolar fracture treated with a bioabsorbable magnesium compression screw. Cureus. 2018;10(4):e2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aktan C, Ertan MB, Turan A, Kose O. Fixation of small osteochondral fragments in a comminuted distal humerus fracture with magnesium bioabsorbable screws: a case report. Cureus. 2018;10(12):e3752. [DOI] [PMC free article] [PubMed] [Google Scholar]