Abstract
Ferumoxytol is a promising non-gadolinium-based contrast agent with numerous varied magnetic resonance imaging applications. Previous reviews of vascular applications have focused primarily on cardiac and aortic applications. After considering safety concerns and technical issues, the objective of this paper is to explore peripheral applications for ferumoxytol-enhanced magnetic resonance angiography (MRA) and venography (MRV) in the upper and lower extremities. Separate searches for each of the following keywords were performed in pubmed: “ferumoxytol,” “ultrasmall superparamagnetic iron oxide,” and “USPIO.” All studies pertaining to MRA or MRV in humans are included in this review. Case-based examples of various peripheral applications are used to supplement a relatively scant literature in this space. Ferumoxytol’s unique properties including high T1 relaxivity and prolonged intravascular half-life make it the optimal vascular imaging contrast agent on the market and one whose vast potential has only begun to be tapped.
Introduction
Ferumoxytol (Feraheme, AMAG Pharmaceuticals, Waltham, MA, USA) is a therapeutic agent used in the treatment of iron-deficient anaemia that has also been applied as a non-gadolinium magnetic resonance imaging (MRI) contrast agent with uses throughout the body. Its unique properties allow it to assess degrees of arterial wall inflammation within atherosclerotic plaque,1 detect malignant lymph node involvement in oncology patients,2 and differentiate progression from pseudoprogression after glioblastoma resection.3 In the context of daily clinical imaging practice, however, ferumoxytol is most commonly used for detailed evaluation of cardiovascular anatomy and function. Previous reviews on ferumoxytol’s cardiovascular imaging applications have focused heavily on the heart and aorta4-8; this paper will review ferumoxytol-enhanced (FE) magnetic resonance angiography (MRA) and venography (MRV) in the extremities.
Ferumoxytol background
Ferumoxytol is classified as an ultrasmall superparamagnetic iron oxide (USPIO) nanoparticle. It consists of an iron core that is wrapped in a carbohydrate coat. Its mean diameter is about 30 nm and its molecular weight is 750 kDa. Ferumoxytol has an r1 relaxivity of 15 mM/s at 1.5 T which is responsible for the high intravascular signal generated on T1-weighted images.9 Due to its large size, once it is injected intravenously, ferumoxytol is trapped within the intravascular space producing dramatic enhancement of blood vessels, but virtually no enhancement of extravascular spaces. These properties make it a vascular contrast agent that is highly suited for magnetic resonance imaging (MRI; Fig 1). Eventually, it is cleared from the blood pool by reticuloendothelial system (RES) macrophages predominantly in the spleen and lymph nodes; however, the carbohydrate coating delays this clearance from the blood pool.10 These properties result in a mean intravascular half-life of 15 hours for ferumoxytol, which is an order of magnitude longer than the next closest blood pool agent, gadofosveset (Ablavar, Lantheus Medical Imaging, North Billerica, MA, USA). This means that once the steady state is achieved in approximately 2 minutes, MRI image acquisition can occur anytime within several hours afterward and adequate images can be generated up to a day later. The RES breaks the particle down and incorporates the iron component into the body’s iron stores while the carbohydrate coat is completely excreted. Clearance takes between 3 days and 11 months.10
Figure 1.
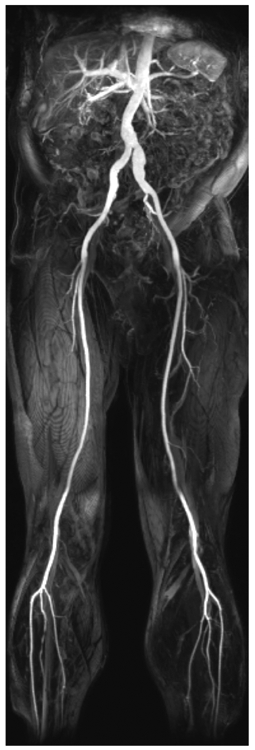
MIP of the abdominal aorta and bilateral lower extremity run-off generated from first-pass acquisition after bolus intravenous injection of ferumoxytol. Note the excellent definition of vascular structures and virtually complete lack of enhancement of non-vascular structures.
Ferumoxytol was initially developed as a treatment option for iron-deficient anaemia and was investigated as a contrast agent for MRI in the 1990s. Anzai et al. first reported use of ferumoxytol for MRA in 1997 with improved visualisation of vascular anatomy due to T1 shortening and long intravascular half-life.11 Additionally, a potential for equilibrium-phase MRI of both arterial and venous structures was first described. Prince et al. evaluated escalating doses of ferumoxytol from 0.4 mg/kg to 4 mg/kg for MRA at the tibial trifurcation in five patients and at 4 mg/kg for cine cardiac MRI in five patients for a pilot study published in 2003.12 The highest signal-to-noise ratio (SNR) at the tibial trifurcation was found with the 4 mg/kg dose. The authors noted that although enhancement improved visualisation of luminal details for large structures, there was difficulty evaluating the trifurcation on equilibrium-phase images due to surrounding venous structures. Ersoy et al. published a paper on the use of ferumoxytol for endoleak detection after EVAR in 2004.13; however, AMAG’s clinical trials using ferumoxytol were designed to test efficacy for treating iron-deficient anaemia in patients with chronic kidney disease, rather than as an MRI contrast agent. Approval from the United States Food and Drug Administration (FDA) was granted in 2009 for the anaemia indication. This indication called for a 510-mg bolus of Feraheme to be injected in 17 seconds with the potential for a second dose 3–8 days later.14
Ferumoxytol’s powerful potential as a blood pool contrast agent for MRA may not have been re-visited were it not for the description of nephrogenic systemic fibrosis in the mid-2000s. Kuo et al. reported the rare, but potentially morbid and possibly even fatal condition, in 2007 that has been closely linked to high doses of gadolinium-based contrast agents (GBCAs) in patients with renal failure.15 Neuwelt et al. postulated that ferumoxytol could be the answer to the NSF conundrum for patients with renal failure needing MRA in 2009,16 but the MRI community did not produce studies of ferumoxytol’s use in clinical applications until Sigovan et al. compared FE-MRA to non-contrast time-of-flight (NCTOF) MRA for evaluation of dialysis fistulas in 2012.17 Between 2012 and 2015, numerous clinical studies appeared in the literature (several of which will be discussed in more detail later in this review), and enthusiasm for ferumoxytol gained momentum. Reports of gadolinium deposition in the brains of patients with normal renal function that underwent GBCA-enhanced MRI in 2015 further stimulated interest in the use of ferumoxytol.18
Safety concerns
In 2015, the FDA issued a boxed warning for ferumoxytol after 79 severe anaphylactoid hypersensitivity reactions including 18 deaths were reported.19 The vast majority of these serious reactions occurred during the rapid injection of Feraheme at outpatient dialysis centres. No deaths have been linked to use of ferumoxytol as an imaging agent, but understandably the attitude toward its use has shifted to a more cautious approach. Among the FDA recommendations for safe use was a change in the way ferumoxytol should be administered. The rapid bolus injection was identified as a possible contributor to the adverse reactions. A slow infusion of dilute ferumoxytol is now recommended to take place over 15 minutes outside of the scanner in an environment where vital signs can be monitored by nursing staff. Since 2015, scattered reports of safety during use of ferumoxytol as an imaging agent have started to appear in the literature supporting its safety, but patient numbers are small and larger studies remain necessary.20-23 The largest report to date includes 671 patients that received ferumoxytol for central nervous system (CNS) MRI with zero anaphylactic, life threatening, or fatal events.24 Furthermore, a paper published in 2017 evaluated hypersensitivity reactions encountered during ferumoxytol infusion compared to the those encountered during infusion of other iron-containing products (iron sucrose, sodium ferric gluconate, or iron dextran); and ferumoxytol did not show a significant difference in adverse reactions, including deaths, when compared to the other agents.25 It should also be noted that reaction rates (including severe reactions) for patients receiving a bolus injection of ferumoxytol decreased significantly after clinical practice shift to routine premedication with 8 mg of intravenous dexamethasone at one institution.26
Technical considerations
Conventional GBCA MRI of the peripheral vasculature typically involves a multi-bed-position bolus-chase acquisition. Both a pre-contrast mask and dynamic images are acquired as the GBCA is administered, starting in the abdomen, and then in the thighs and calves. Oftentimes a dynamic acquisition is acquired in the feet using a small bolus before the bolus-chase acquisition in order to see the calf and foot vessels.27 Due to the 15 minute infusion of ferumoxytol outside the scanner, pre-contrast masks and dynamic first-pass imaging are not possible. This is both a negative and a benefit of ferumoxytol imaging. The negative is primarily in the small vessels in the calf where early dynamic imaging is important to prevent venous contamination to small adjacent arteries. In the abdomen and thighs, steady-state imaging is beneficial. It allows much longer acquisitions where the spatial resolution can be pushed without concern for missing the bolus on the subsequent bed position. Moreover, one can repeat a bed position if there is motion artefact from a patient moving their leg or breathing. In the abdomen, one can use respiratory-gated acquisitions in order to compensate for patient motion during the longer acquisitions and one is no longer limited by the patient’s ability to hold their breath.
For vascular imaging, the most commonly used dose of ferumoxytol is 2-5 mg/kg diluted in two to four parts of saline. The original goal of the dilution was to limit T2* effects during first-pass imaging, but has been maintained in practice to slow the injection rate in order to limit allergic reactions based on FDA recommendations. Once the agent is administered, the patient can be brought into the scanner at any time for imaging. Imaging sequences used for imaging ferumoxytol typically involve conventional T1-weighted sequences used for post-GBCA imaging, mainly spoiled gradient-echo based pulse sequences (FLASH on Siemens or SPGR on GE MRI systems). Adjustments to the pulse sequence should be made to increase scan time thereby permitting increased resolution, which is particularly beneficial in the thighs and calves. In the abdomen, respiratory artefact can cause some blurring so incorporating either cardiac and/or respiratory gating can significantly improve image quality. One aspect that can be disconcerting is the absence of pre-contrast masks, and therefore, an inability to perform subtraction imaging; however, the improved spatial resolution should allow straightforward interpretation of lesions that would be difficult to evaluate on bolus-chase GBCA acquisitions.
Although there does not appear to be an imminent change in the FDA’s position on ferumoxytol infusion parameters, the steadily increasing literature touting ferumoxytol’s safety in clinical imaging could revive the possibility of bolus injection in the future. Should the recommendation against bolus injection change in the future, ferumoxytol’s true potential as the most powerful and versatile vascular imaging agent in practice could be realised. A single bolus injection could once again be used to acquire first-pass MRA images eliminating confusion due to venous contamination in the calves; and a few minutes later, whole-body high-resolution steady-state MRA/MRV images could be obtained generating a truly comprehensive vascular examination.
Materials and methods
Separate individual PUBMED searches were performed using the search terms “ferumoxytol,” “USPIO,” and “ultrasmall superparamagnetic iron oxide.” Each citation was evaluated for relevance to MRI of the peripheral vasculature, peripheral to the aortic bifurcation in the legs and peripheral to the thoracic inlet in the arms. Both MRA and MRV imaging studies in humans were included. Animal studies were excluded. As the existing literature in this space is relatively scant, case-based examples are used to demonstrate many of the possible uses for ferumoxytol outside of the thorax and abdomen.
Peripheral applications
Peripheral vascular disease
As with many vascular conditions, patients with peripheral arterial disease (PAD) often have concurrent renal insufficiency. Patients with PAD and renal insufficiency may require endovascular intervention requiring use of iodinated contrast during angiography. These patients can benefit from precise detailed knowledge of atherosclerotic plaque location and morphology prior to any planned intervention (Fig 2). Additionally, if more complex and extensive PAD with dissection (Fig 3) and occlusion (Fig 4) can be mapped out, the patient with borderline renal function can be spared an angiogram and taken directly for bypass surgery. Similarly, precise measurements and detailed evaluation of mural thrombus allow for patient-specific intervention for enlarging lower extremity aneurysms (Fig 5). Finally, in our experience, ferumoxytol serves as an excellent option for evaluating graft patency after bypass (Electronic Supplementary Material Fig S1).
Figure 2.

Atherosclerotic plaque resulting in right superficial femoral artery (SFA) stenoses. (a) High-resolution steady-state FE-MRA curved multiplanar reformatted (CMPR) image of the right SFA demonstrating significant atherosclerotic plaque diffusely distributed in the proximal segment (straight line) and focally distributed in the distal segment (dashed line) with relatively minimal disease in the intervening segment (dotted line). (b–d). Orthogonal axial reformatted images showing the cross-sectional patency of the SFA at each location from part (a). (e) MIP from the first-pass acquisition during the same examination appears to overestimate the degree of disease proximally (white arrow) and underestimate it distally (dashed arrow). (f,g) Pre-and post-stent angiograms in the proximal SFA respectively.
Figure 3.

Mid-to-distal SFA with multifocal dissection and focal occlusion. (a) CMPR image from a steady-state FE-MRA of the SFA (arrowheads), demonstrating multifocal dissections (white line and dotted line) as well as a complex lesion distally (dashed line) with a combination of dissection and occlusion. (b–d) Orthogonal axial reformatted images at each level showing the dissection flaps (solid arrow and dotted arrow) as well as the occluded true lumen and surrounding false lumen of the complex lesion (dashed arrow).
Figure 4.
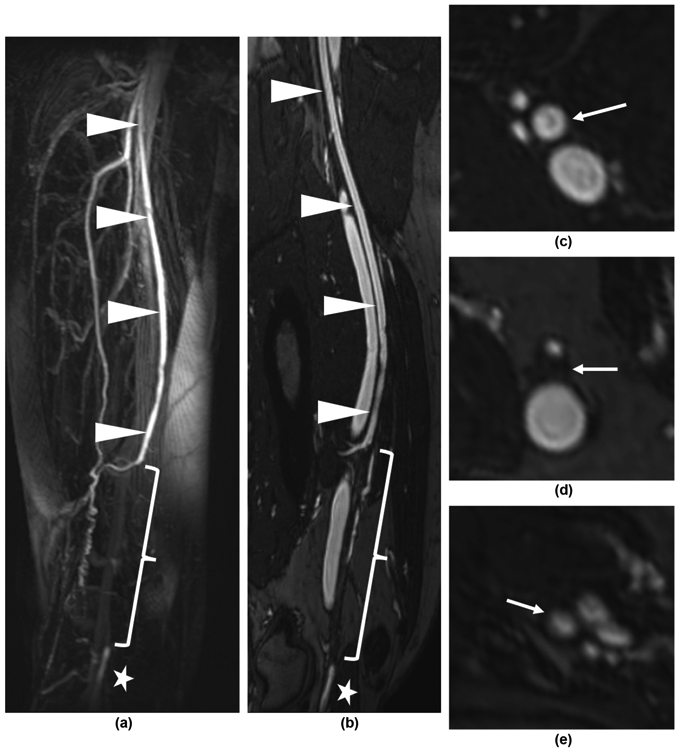
Distal right SFA occlusion. (a) First-pass FE-MRA MIP demonstrates patent proximal SFA (arrowheads) and the occlusion (bracket) with distal reconstitution of the popliteal artery (star) by profunda femoris artery collaterals. (b) Steady-state CMPR image shows the patent proximal SFA (arrowheads) as well as the low signal intensity occlusion (bracket) and reconstituted popliteal artery (star). The orthogonal axial reformatted images show patent SFA (c), occluded SFA (d) and patent reconstituted popliteal artery (e), respectively (arrows).
Figure 5.

Large SFA aneurysm. (a) First-pass FE-MRA MIP demonstrating the flow channel of the aneurysm. (b) Steady-state CMPR image demonstrates the flow channel and mural thrombus of the aneurysm. (c) Orthogonal axial reformatted images show the true diameter of the aneurysm (arrows); (d) a section of relatively normal artery that could accept a stent graft (dotted arrow); and (e) an incidental irregular eccentric plaque (dashed arrow).
Of 12 patients evaluated by Li et al. in 2005, four focused on the lower extremity vessels. Three of these were looking at arteriovenous malformations and one was done for foot pain.28 First-pass and steady-state acquisitions both produced comparable SNR. Seven of the examinations were graded excellent for first-pass image quality and five were graded good. The authors concluded that adequate arterial enhancement had been obtained with ferumoxytol in line with other prior vascular studies undergone by the patients.
In 2015, Walker et al. published a feasibility study for FE-MRA in the lower extremities for clinical decision-making prior to revascularisation.29 Five patients underwent FE-MRA and another five patients underwent gadolinium-enhanced (GE)-MRA. Qualitative and semi-quantitative (degree of stenosis) evaluations were made at the iliac, femoral, popliteal, and tibial segments by four blinded vascular surgeons. The surgeons were also asked if they would make a revascularisation decision based on the provided imaging in each case. The readers used maximum intensity projections (MIPs), coronal source images, and axial reformatted images for evaluation. FE-MRA performed significantly better in the iliac segments whereas the GE-MRA performed significantly better in the tibial segment. Comfort with basing revascularisation decisions on the MRA was identical at 89% for both contrast agents.
Hope et al. reported a single-centre experience with FE-MRA in 102 patients in 2015.5 Twenty-seven of these patients with renal insufficiency received lower extremity run-off examinations with both first-pass and steady-state acquisitions. The authors concluded that the high-resolution steady-state images were beneficial for fine detail of individual vascular lesions, but that the close proximity of arteries and veins could be problematic for interpretation below the knee, particularly in the setting of extensive pathology and numerous arterial collaterals.
Transcatheter aortic valve replacement access vessel evaluation
The high spatial resolution of computed tomography angiography (CTA) makes it the current reference standard for preoperative planning prior to transcatheter aortic valve replacement (TAVR) for patients with aortic stenosis (AS). Given the large size and stiffness of the endovascular transcatheter devices to be delivered through the iliofemoral arteries, vessel lumen diameter and tortuosity become important predictors for TAVR feasibility (Electronic Supplementary Material Fig S2). If the vessels are too small, the artery can be avulsed and pulled out of the body with the sheath when it is removed (so-called “iliac artery on a stick”), a potentially catastrophic complication.30 In patients with renal failure, however, the ability to obtain a high-quality CTA or GE-MRA is limited.
Kallianos et al. retrospectively evaluated seven patients with renal failure and AS that underwent FE-MRA prior to TAVR.31 Although the precise measurement of the anatomy at the aortic root is outside of the scope of this review, the evaluation of the access vessels is important to consider. In the study, the access recommendations based on the FE-MRA examinations were followed and no complications were encountered at the access sites. In one case, bilateral iliac artery stenting was required prior to TAVR based on the FE-MRA images.
Additionally, although MRA may be limited in identification of calcified plaque in TAVR access vessels, noncontrast CT can be a useful adjunctive examination and may even be fused with the FE-MRA.32
Kidney transplant: pre- and postoperative evaluation
A critical step in determining a patient’s candidacy for kidney transplant remains anatomically defining the vascular structures that will be involved in the anastomoses, usually the external iliac artery and vein. Indwelling tunnelled dialysis catheter placement, which occurs in a significant percentage of patients that go onto kidney transplant, can increase the risk of venous stenosis or occlusion.
A case report by Mukundan et al. demonstrates the necessity for good-quality high-resolution imaging of the arteries and veins of the pelvis (Electronic Supplementary Material Fig S3).33 The right external iliac vein is unremarkable but the IVC is completely occluded. The FE-MRV shows adequate venous outflow through markedly enlarged gonadal veins so the patient was able to receive a transplant.
Stoumpos etal. published a cohort of 20 potential kidney transplant recipients that underwent FE-MRA/MRV to evaluate iliac anatomy for determination of transplant suitability.34 The authors found FE-MRA/MRV to be feasible for evaluation of kidney transplant candidates with the benefits of not having potential artefact from calcified plaque as seen with CTA and superior evaluation of the venous structures compared to CT venography. Of the 20 patients, FE-MRA/MRV findings led to aorto-bifemoral stent graft placement and left renal artery stent in one patient, renal cell carcinoma resection in two patients, and definition of duplicated IVC and retro-aortic left renal vein in two other patients.
In the post-transplant setting, patients with transplant renal artery stenosis (TRAS) often have graft dysfunction and are poor candidates for CTA or GE-MRA. Doppler evaluation is sensitive for picking up the condition, but anatomical delineation of the lesion remains poor and there is a high false-positive rate.35 In patients with TRAS that need to undergo an endovascular intervention, precise knowledge of the anatomy of the stenotic lesion is critical for minimising contrast load, which can limit contrast-induced nephropathy in the graft. Furthermore, long segment stenoses or stenoses involving a bifurcation may not be amenable to endovascular intervention and surgical revision may be the most appropriate course of treatment in these patients.
Bashir et al. first reported the feasibility of using FE-MRA to evaluate kidney transplant vasculature in 2013.36 FE-MRA was performed in 16 patients with acute kidney injury in the post-transplant setting that had either nondiagnostic or equivocal Doppler evaluations. Of the 16 patients, one had a completely occluded renal artery, two had significant renal artery stenoses, and the remaining 13 had widely patent renal arteries. In the two patients that had significant TRAS, endovascular intervention resulted in improved graft function.
Corwin et al. compared first-pass and steady-state acquisitions in 15 patients that underwent FE-MRA to confirm ultrasound findings suspicious of TRAS and help plan for endovascular intervention.37 There was no statistically significant difference in the SNR between the two acquisitions, but the steady-state acquisition images demonstrated significantly higher “edge sharpness.” Edge sharpness was determined by measuring the distance required for the signal to increase from vessel wall (low) to peak intravascular signal (high). Shorter distances correspond to increased resolution at the edge of the vessel. Since acquisition times of several minutes can be used during free-breathing in this location, images with small voxel size and much higher spatial resolution can be generated. This is an important concept that can be applied to other anatomical locations in the peripheral vasculature.
Fananapazir et al. retrospectively evaluated 33 patients that underwent both a FE-MRA and a digital subtraction angiogram (DSA) in the setting of clinical concern for TRAS or Doppler findings suggesting TRAS.38 Comparison of the degree of stenosis between the reference standard DSA with steady-state high-resolution FE-MRA images was performed with three independent MRA readers (Electronic Supplementary Material Fig S4). Sensitivity was 100% for all readers and specificity ranged from 75–87.5%. Positive predictive value was 94.4–97.1% with a negative predictive value of 100%. Based on these results, if the FE-MRA does not show a stenosis, the patient does not need to undergo the DSA. Degree of stenosis was slightly over-estimated by MRA relative to DSA, which is a recognised potential pitfall, but image quality down to the intraparenchymal arteries was uniformly graded as excellent. The authors concluded that perhaps FE-MRA will evolve into the next sequential step in the workup of TRAS after a positive Doppler exam.
Preoperative evaluation prior to fibular flap
As discussed previously, evaluating the tibial vessels on steady-state acquisition images can be challenging due to identical signal in the very small adjacent arteries and veins in the calf; however, when there is a lack of pathology in the tibial vessels (i.e., three vessel run-off to the foot), the authors contend that this can be confidently read as normal (Fig 6). This particular scenario is of clinical importance for patients with head and neck cancer that need fibular flap reconstructive surgery. Preoperative imaging, such as DSA, CTA, or MRA, needs to determine patency of all three tibial vessels and presence of any anatomical variation.39 In the presence of renal insufficiency, FE-MRA represents a feasible option. Of course, an alternate imaging technique may need to be performed for further evaluation if the FE-MRA is equivocal.
Figure 6.
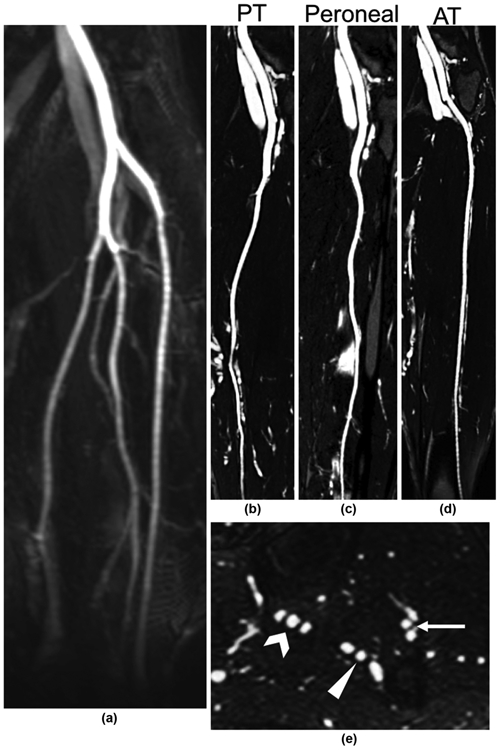
FE-MRA evaluation of the tibial vessels prior to fibular flap reconstructive surgery. (a) First-pass MIP showing normal arterial anatomy and patent vessels. (b–d) Steady-state CMPR images through each of the tibial arteries confirming patency. (e) Steady-state axial reformatted image in the calf showing a typical configuration of each tibial artery between two adjacent veins: posterior tibial artery (carat), peroneal artery (arrowhead), and anterior tibial artery (arrow).
Deep vein thrombosis
Ferumoxytol’s intravascular distribution at steady state makes it an excellent technique for high-resolution venographic imaging as well. The deep and superficial systems of the legs can be evaluated in great detail, with depiction of the vein walls and normal valves (Fig 7) as well as filling defects representing deep vein thrombosis (DVT; Fig 8 and Electronic Supplementary Material Fig S5). An additional benefit, of FE-MRV is the potential for detection of pulmonary embolus in addition to DVT in the same examination with minimal additional scanner time.5
Figure 7.

Femoral vein normal valve leaflets visualised in axial (a) and oblique coronal (b) planes (arrows).
Figure 8.

Chronic femoropopliteal DVT. (a) Steady-state FE-MRV CMPR image shows chronic retracted eccentric thrombus (short arrows). (b,c) Orthogonal axial reformatted images at the levels indicated in (a) showing web formation (dashed arrow) (b), and non-occlusive eccentric thrombus (dotted arrow) (c). (d) Extent of residual clot is more completely demonstrated than on corresponding longitudinal greyscale ultrasound image from 1 day prior.
Li et al. published the first report of ferumoxytol for imaging of DVT in the lower extremities in 2007 using a dual-contrast approach in nine patients with previous positive Doppler studies.40 The MRV examinations consisted of a NCTOF acquisition followed bright-blood first-pass and steady-state acquisitions after ferumoxytol injection, followed by a black-blood steady-state acquisition. Doppler findings were confirmed by the FE-MRVs in nine of 10 cases, but also picked up two additional sites of thrombi in patients that had only undergone unilateral Doppler. TOF depicted thrombus in seven of nine patients but artefacts made the readers less confident of the findings and interfered with extent of thrombus measurements. Interestingly, two additional thrombi were detected using the black-blood acquisition, where the thrombi demonstrated high signal intensity in small veins where there was no blood surrounding the clot.
In 2014, Bashir et al. compared MRV in the abdomen and lower extremities using either ferumoxytol or gadofosveset in 34 patients.41 The authors found no significant difference in the enhancement of venous segments and visualisation of thrombus between the two contrast agents.
Dialysis access fistula
Dialysis access planning and evaluation of dysfunctional fistulas represents another potential niche for ferumoxytol-based imaging (Electronic Supplementary Material Fig S6). A paper by Sigovan et al. in 2012 comparing NCTOF MRI with first-pass FE-MRA represents the first paper after several years without much interest in ferumoxytol as an MRI contrast agent.17 The authors demonstrated the feasibility of using FE-MRA/V to evaluate haemodialysis access and noted superior image quality compared to NCTOF.
Venous stenosis and occlusion
Central venous stenoses and occlusions resulting from central venous catheters, especially in dialysis dependent patients, remain a critical clinical dilemma. These patients are often referred to interventional radiology for central-line placement, but many require central venous recanalisation first, which can be difficult and risky. Pre-procedural imaging is paramount to a successful procedure. Steady-state FE-MRV represents the ideal technique for evaluating potential venous access options in patients with occlusion or stenosis because the high-resolution acquisition can be reformatted into multiple planes, MIPs and three-dimensional reconstructions, which are not possible with CT venography (Fig 9). Armed with this information the interventionalist can plan out multiple access sites and consider the possible necessity of sharp (needle based) recanalisation.
Figure 9.
A 23-year-old women with multiple abdominal surgeries for short gut syndrome requiring central venous access for total parenteral nutrition and difficulty with prior attempts at venous access. (a) Volume-rendered and (b) coronal MIP FE-MRV, which demonstrates bilateral subclavian vein and internal jugular vein occlusion with collateralisation (dashed arrows) via anterior chest wall and paravertebral plexus and a 6 mm non-occlusive thrombus in SVC (solid arrow). (c) A Hickman catheter was placed in the right external jugular vein.
Nayak et al. demonstrated the successful role of FE-MRV in paediatric patients with chronic kidney disease requiring vascular access and/or pre/post-kidney transplantation.42 In five of the 10 patients included in the paper, FE-MRV was used specifically to evaluate venous access placement. Image quality was scored as diagnostic with excellent definition of the vascular borders in all cases.
Luhar et al. retrospectively evaluated FE-MRVs performed in 20 paediatric patients for varied indications including venous patency for line access, pre/posttransplantation evaluation, DVT, and haemorrhage.43 The two reviewing paediatric radiologists were confident of diagnosis in all 20 patients and 12 of the 20 patients had other imaging or invasive procedures, all which were concordant with the FE-MRV.
Pelvic congestion syndrome
As the collective experience with ferumoxytol as an MRI contrast agent continues to accrue, various additional MRA and MRV applications will continue to be demonstrated. Recently, the authors used FE-MRV to beautifully demonstrate the classic anatomical findings in a patient with suspected pelvic congestion syndrome (Fig 10). The anatomy in such cases can be complex with contributions from both internal iliac and ovarian veins. With high-resolution definition of anatomy, endovascular embolisation procedures can be planned with more precision, potentially leading to greater clinical success rates.
Figure 10.

FE-MRV in a patient with pelvic congestion syndrome. (a) Coronal MIP shows numerous collaterals throughout the pelvis (arrowheads). Connection is identified to both internal iliac veins (white arrows). (b) Coronal MIP shows enlarged complex bilateral ovarian vein networks bilaterally (dashed white arrows and prominent vulvar varices (carats).
Conclusion
As evidenced by the wide variety of vascular conditions represented in this review, the vascular imaging community has merely started to scratch the surface of ferumoxytol’s potential as a contrast agent. The level of spatial resolution and detail encountered at the interface between flowing blood and vessel wall or pathologic lesion is unsurpassed and creates new insight into some of the disease processes that affect the vascular system.
Exciting new applications that go beyond anatomical definition are on the horizon. Ferumoxytol’s uptake by macrophages will facilitate combined MRA with delayed assessment of inflammation in the wall of aneurysms and in arterial plaque located in critical territories (e.g., carotid arteries).1 FE-MRA also can be combined with other advanced MRI capabilities, such as flow assessment and perfusion/ viability.44 Additionally, dialysis patients with iron-deficiency anaemia derive therapeutic benefit from the iron-containing contrast agent, making ferumoxytol a true theranostic agent.
Certainly, however, many challenges remain present to the widespread adoption of ferumoxytol and FE-MRA/FE-MRV. The safety concerns are at the centre of this controversy, and rightly so. Continued accrual of literature demonstrating safety during use of ferumoxytol creates hope that the bolus injection can return to routine administration protocols. Although the 15-minute infusion in a monitored setting has certain benefits, such as significantly decreased total scanner time, the addition of first-pass acquisitions would further solidify ferumoxytol as the premiere vascular imaging agents. Cost also inhibits more prevalent usage of ferumoxytol. A single vial of Feraheme costs several times the amount of a single vial of Omniscan, which can be difficult to justify in patients that are able to receive both agents. Another potential deterrent involves the incompletely understood changes to normal MRI for patients that have previously received ferumoxytol, where normal structures, such as liver, spleen, lymphatic structures, and blood vessels, can have altered signal characteristics.45
Further prospective trials demonstrating safety and efficacy are also needed. Although difficult to perform, comparison of GE-MRA and subsequent FE-MRA (as performed in one study in brain MRI) would confirm our innate understanding of ferumoxytol’s powerful potential.45
Supplementary Material
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.crad.2018.02.021.
References
- 1.Smits LP, Tiessens F, Zheng KH, et al. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 2017;263:211–8. [DOI] [PubMed] [Google Scholar]
- 2.Bravo SM, Myers C, Bravo T, et al. Ferumoxytol as a lymph node contrast agent in patients with metastatic prostate carcinoma. Pract Radiat Oncol 2013;3(2 Suppl. 1):S11. [DOI] [PubMed] [Google Scholar]
- 3.Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology 2013;266(3):842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashir MR, Bhatti L, Marin D, et al. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging: JMRI 2015;41(4):884–98. [DOI] [PubMed] [Google Scholar]
- 5.Hope MD, Hope TA, Zhu C, et al. Vascular imaging with ferumoxytol as a contrast agent. AJR Am J Roentgenol 2015;205(3):W366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn JP, Nguyen KL, Han F, et al. Cardiovascular MRI with ferumoxytol. Clin Radiol 2016;71(8):796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toth GB, Varallyay CG, Horvath A, et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 2017;92(1):47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn JP, Nguyen KL, Hu P. Ferumoxytol vs. Gadolinium agents for contrast-enhanced MRI: thoughts on evolving indications, risks, and benefits. J Magn Reson Imaging: JMRI 2017;46(3):919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corot C, Robert P, Idee JM, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 2006;58(14):1471–504. [DOI] [PubMed] [Google Scholar]
- 10.Storey P, Lim RP, Chandarana H, et al. MRI assessment of hepatic iron clearance rates after USPIO administration in healthy adults. Invest Radiol 2012;47(12):717–24. [DOI] [PubMed] [Google Scholar]
- 11.Anzai Y, Prince MR, Chenevert TL, et al. MR angiography with an ultrasmall superparamagnetic iron oxide blood pool agent. J Magn Reson Imaging: JMRI 1997;7(1):209–14. [DOI] [PubMed] [Google Scholar]
- 12.Prince MR, Zhang HL, Chabra SG, et al. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J X-ray Sci Technol 2003;11(4):231–40. [PubMed] [Google Scholar]
- 13.Ersoy H, Jacobs P, Kent CK, et al. Blood pool MR angiography of aortic stent-graft endoleak. AJR Am J Roentgenol 2004;182(5):1181–6. [DOI] [PubMed] [Google Scholar]
- 14.Lu M, Cohen MH, Rieves D, et al. FDA report: ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol 2010;85(5):315–9. [DOI] [PubMed] [Google Scholar]
- 15.Kuo PH, Kanal E, Abu-Alfa AK, et al. Gadolinium-based MRcontrast agents and nephrogenic systemic fibrosis. Radiology 2007;242(3):647–9. [DOI] [PubMed] [Google Scholar]
- 16.Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int 2009;75(5):465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigovan M, Gasper W, Alley HF, et al. USPIO-enhanced MR angiography of arteriovenous fistulas in patients with renal failure. Radiology 2012;265(2):584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanal E, Tweedle MF. Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 2015;275(3):630–4. [DOI] [PubMed] [Google Scholar]
- 19.FDA Drug Safety Communication. FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). U S Food and Drug Administration Home Page; 30 March 2015. www.fda.gov/Drugs/DrugSafety/ucm440138.htm. [Google Scholar]
- 20.Nguyen KL, Yoshida T, Han F, et al. MRI with ferumoxytol: a single center experience of safety across the age spectrum. J Magn Reson Imaging: JMRI 2017;45(3):804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasanawala SS, Nguyen KL, Hope MD, et al. Safety and technique of ferumoxytol administration for MRI. Magn Resonan Med 2016;75(5):2107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muehe AM, Feng D, von Eyben R, et al. Safety report of ferumoxytol for magnetic resonance imaging in children and young adults. Invest Radiol 2016;51(4):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning P, Zucker EJ, Wong P, et al. Hemodynamic safety and efficacy of ferumoxytol as an intravenous contrast agents in pediatric patients and young adults. Magn Reson Imaging 2016;34(2):152–8. [DOI] [PubMed] [Google Scholar]
- 24.Varallyay CG, Toth GB, Fu R, et al. What does the boxed warning tell us? Safe practice of using ferumoxytol as an MRI contrast agent. AJNR Am J Neuroradiol 2017;38(7):1297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetmore JB, Weinhandl ED, Zhou J, et al. Relative incidence of acute adverse events with ferumoxytol compared to other intravenous iron compounds: a matched cohort study. PLoS One 2017;12(1):e0171098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braaten K, Holcombe RF, Kim SS. Premedicationwith IVsteroids effectively prevented anaphylactic reactions following ferumoxytol given as IV push in hematology and oncology patients. Am J Hematol 2015;90(10):E207. [DOI] [PubMed] [Google Scholar]
- 27.Ho KY, Leiner T, van Engelshoven JM. MR angiography of run-off vessels. Eur Radiol 1999:1285–9. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Tutton S, Vu AT, et al. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging: JMRI 2005;21(1):46–52. [DOI] [PubMed] [Google Scholar]
- 29.Walker JP, Nosova E, Sigovan M, et al. Ferumoxytol-enhanced magnetic resonance angiography is a feasible method for the clinical evaluation of lower extremity arterial disease. Ann Vasc Surg 2015;29(1):63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obon-Dent M, Reul RM, Mortazavi A. Endovascular iliac rescue technique for complete arterial avulsion after transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2014;84(2):306–10. [DOI] [PubMed] [Google Scholar]
- 31.Kallianos K, Henry TS, Yeghiazarians Y, et al. Ferumoxytol MRA for transcatheter aortic valve replacement planning with renal insufficiency. Int J Cardiol 2017;231:255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida THF, Zhou Z, Aksoy O, et al. Ferumoxytol MRA and non-contrast CT fusion in TAVR candidates with renal failure. Society for Cardiovascular Magnetic Resonance Annual Meeting. 2016. Los Angeles, California, USA. [Google Scholar]
- 33.Mukundan S, Steigner ML, Hsiao LL, et al. Ferumoxytol-enhanced magnetic resonance imaging in late-stage CKD. Am J Kidney Dis: Off J Natl Kidney Found 2016;67(6):984–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoumpos S, Hennessy M, Vesey AT, et al. Ferumoxytol-enhanced magnetic resonance angiography for the assessment of potential kidney transplant recipients. Eur Radiol 2018:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granata A, Clementi S, Londrino F, et al. Renal transplant vascular complications: the role of Doppler ultrasound. J Ultrasound 2015; 18(2):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bashir MR, Jaffe TA, Brennan TV, et al. Renal transplant imaging using magnetic resonance angiography with a nonnephrotoxic contrast agent. Transplantation 2013;96(1):91–6. [DOI] [PubMed] [Google Scholar]
- 37.Corwin MT, Fananapazir G, Chaudhari AJ. MR angiography of renal transplant vasculature with ferumoxytol: comparison of high-resolution steady-state and first-pass acquisitions. Acad Radiol 2016;23(3):368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fananapazir G, Bashir MR, Corwin MT, et al. Comparison of ferumoxytol-enhanced MRA with conventional angiography for assessment of severity of transplant renal artery stenosis. J Magn Reson Imaging: JMRI 2017;45(3):779–85. [DOI] [PubMed] [Google Scholar]
- 39.Holzle F, Ristow O, Rau A, et al. Evaluation of the vessels of the lower leg before microsurgical fibular transfer. Part II: magnetic resonance angiography for standard preoperative assessment. Br J Oral Maxillofac Surg 2011;49(4):275–80. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Salanitri J, Tutton S, et al. Lower extremity deep venous thrombosis: evaluation with ferumoxytol-enhanced MR imaging and dual-contrast mechanism–preliminary experience. Radiology 2007;242(3):873–81. [DOI] [PubMed] [Google Scholar]
- 41.Bashir MR, Mody R, Neville A, et al. Retrospective assessment of the utility of an iron-based agent for contrast-enhanced magnetic resonance venography in patients with endstage renal diseases. J Magn Reson Imaging: JMRI 2014;40(1):113–8. [DOI] [PubMed] [Google Scholar]
- 42.Nayak AB, Luhar A, Hanudel M, et al. High-resolution, whole-body vascular imaging with ferumoxytol as an alternative to gadolinium agents in a pediatric chronic kidney disease cohort. Pediatr Nephrol 2015;30(3):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luhar A, Khan S, Finn JP, et al. Contrast-enhanced magnetic resonance venography in pediatric patients with chronic kidney disease: initial experience with ferumoxytol. Pediatr Radiol 2016;46(9):1332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z, Han F, Rapacchi S, et al. Accelerated ferumoxytol-enhanced 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) cardiovascular MRI: validation in pediatric congenital heart disease. NMR Biomed 2017;30(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCullough BJ, Kolokythas O, Maki JH, et al. Ferumoxytol in clinical practice: implications for MRI. J Magn Reson Imaging: JMRI 2013;37(6):1476–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



