Abstract
Human coronaviruses are RNA viruses that are sensitive to ultraviolet (UV) radiation. Sunlight contains UVA (320–400 nm), UVB (260–320 nm) and UVC (200–260 nm) action spectra. UVC can inactivate coronaviruses, including severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). The incidence and mortality of coronavirus disease 2019 (COVID-19) are considered to be correlated with vitamin D levels. Vitamin D synthesis in human skin is closely related to exposure to UVB radiation. Therefore, the incidence and mortality of COVID-19 are also considered to be correlated with Vitamin D levels. In this study, Spearman and Kendall rank correlation analysis tests were used to analyze the correlation between the average percent positive of five human coronaviruses (SARS-CoV-2, CoVHKU1, CoVNL63, CoVOC43, and CoV229E) in the U.S. and the corresponding sunlight UV radiation dose The results indicated that the monthly average percent positive of four common coronaviruses was significantly negatively correlated with the sunlight UV radiation dose. The weekly percent positive of SARS-CoV-2 during April 17, 2020 to July 10, 2020 showed a significant negative correlation with the sunlight UV radiation dose in census regions 1 and 2 of the U.S. while no statistical significance in the other regions. Additionally, sunlight UV radiation also showed some negative effects with respect to the early SARS-CoV-2 transmission.
Keywords: Human coronaviruses, COVID-19, Sunlight UV radiation, Correlation
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease that has caused a global pandemic. Its causing agent is severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), which belongs to betacoronavirus (Wiersinga et al., 2020). Droplet and aerosol modes are considered the transmission routes of COVID-19 (Shereen et al., 2020; Wiersinga et al., 2020). Seven coronaviruses have been reported to have the ability to cause human diseases, and they show great similarities in species types and transmission patterns (Zhang et al., 2020). Four human coronaviruses, including CoVHKU1, CoVNL63, CoVOC43, and CoV229E, were reported to cause seasonal epidemics commonly in the U.S. and some other countries (Gaunt et al., 2010; Killerby et al., 2018). Although the seasonality of the COVID-19 epidemic remains unclear, the epidemics caused by other four common human coronaviruses have been confirmed to be correlated with season; the incidence rates of the epidemics caused by these four common human coronaviruses are higher in winter and lower in summer (Friedman et al., 2018; Gaunt et al., 2010; Killerby et al., 2018). However, the cause of seasonality is still not fully understood (Fisman, 2012).
The wavelength of ultraviolet (UV) radiation in sunlight ranges from 100 nm to 400 nm, which are largely divided into three parts, including UVA (320–400 nm), UVB (260–320 nm) and UVC (200–260 nm) (D'Orazio et al., 2013). Human coronaviruses, including SARS-CoV and SARS-CoV-2, are sensitive to UV radiation (Duan et al., 2003; Hessling et al., 2020). The UVC is thought to be able to inactivate pathogens in aerosol and skin surface by forming pyrimidine dimers and causing DNA or RNA damage (Welch et al., 2018). Previous studies had suggested that more than 90% of the SARS-CoV-2 virus load will be inactivated after exposure to summer midday sunlight in the summer for 34 min (Sagripanti and Lytle, 2020). Moreover, more evidences have indicated the important role of vitamin D levels in the COVID-19 epidemic. Vitamin D, including vitamins D2 and D3, is a group of nutrients essential for human beings (Lips, 2006). COVID-19 confirmed cases and mortality rate in some European countries were reported to be negatively correlated with the mean vitamin D level (Hastie et al., 2020; Ilie et al., 2020). The same association was also been observed in countries 35 degrees south of latitude (Mansur, 2020; Rhodes et al., 2020). Further, lower vitamin D levels were linked to higher mortality, and vitamin D supplementation could reduce the mortality rate of COVID-19 patients (Grant et al., 2020). Considering that vitamin D3 is mainly synthesized from 7-dehydrocholesterol in the skin under sunlight UVB (Holick et al., 2007; Lips, 2006), the above-mentioned correlations suggested that sunlight UV radiation might affect the COVID-19 epidemic.
This study is aimed to determine the correlation between the percent positive of human coronaviruses, including SARS-CoV-2 in the U.S. and the sunlight UV radiation dose. Since the ongoing COVID-19 epidemic will possibly become a long-term seasonal disease like other common human coronavirus diseases, our research may contribute to the deeper understanding of the mechanism underlying the seasonality and transmission processes in human coronavirus diseases.
2. Materials and methods
2.1. Data collection
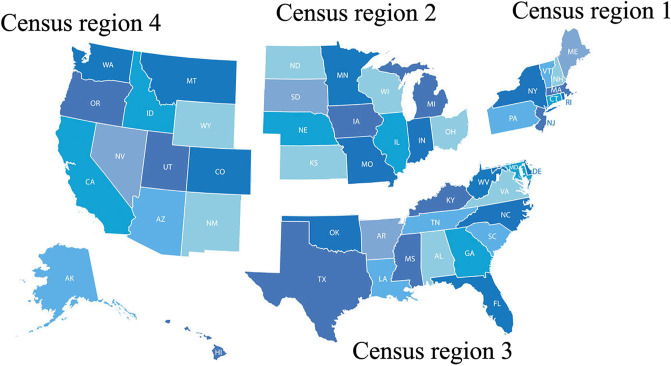
To better understand the incidence of human coronavirus diseases in different areas of the U.S., the U.S. was divided into four census regions, as suggested by the U.S. Census Bureau suggested (Fig. 1 ). The percent positive of four common human coronaviruses (CoVHKU1, CoVNL63, CoVOC43, and CoV229E) from July 2018 to June 2020 in each region was obtained from The National Respiratory and Enteric Virus Surveillance System (NREVSS) of the U.S. Centers for Disease Control and Prevention (CDC) (https://www.cdc.gov/surveillance/nrevss/coronavirus/index.html). The number of tests and confirmed cases of COVID-19 in the U.S. from April 17, 2020 to July 10, 2020 were obtained from Johns Hopkins University Center for Systems Science and Engineering (JHU CSSE) on GitHub (https://github.com/CSSEGISandData/COVID-19).
Fig. 1.
Division of the U.S. into different census regions, as suggested by the U.S. Census Bureau.
Daily sunlight UV radiation dose integrated throughout the day is the effective UV irradiance (kJ/m2) that reaches the Earth's surface and longer sunshine duration leads to higher sunlight UV radiation dose under the same sunshine intensity. Data on the daily sunlight UV radiation dose data, including that on three different UV action spectrum doses (erythemal, vitamin-D and DNA-damage UV doses, which were computed depending on their different biological effects) were obtained from the operational Tropospheric Emission Monitoring Internet Service (TEMIS) ozone data archive (http://www.temis.nl/uv-radiation/UVdose.html). The data on the sunlight UV dose from TEMIS was compiled from surface atmospheric observation stations and Meteosat Second Generation (MSG) observations. The sunlight UV radiation dose at the mean latitude and longitude in the selected areas was used to approximate the UV dose in the whole area.
2.2. Statistical analysis
To analyze the correlation between the sunlight UV radiation dose and the human coronavirus diseases, Spearman and Kendall rank correlation tests were used to examine and determine the nonlinear relationships between the percent positive of the human coronaviruses and sunlight UV radiation dose. Spearman and Kendall rank correlation tests are non-parametric tests, which are not depending on the concrete values and distributions of the data (Dalmay et al., 2003; Kahraman et al., 2004). Spearman rank correlation is mainly used to measure the strength of the association between two variables, while Kendall rank correlation is mainly used to measure the ordinal association between two measured variables (Bonett and Wright, 2000; Menebo, 2020; Pani et al., 2020). Both those tests are sensitive to the rank and consistency, instead of the precise value of the variables (Bonett and Wright, 2000; Dalmay et al., 2003).
The percent positive is the proportion of positive individuals in the total number of tested individuals. The monthly average percent positive of four common human coronaviruses was calculated from the weekly data obtained from NREVSS while the weekly average percent positive of SARS-CoV-2 was estimated from the weekly total tests and confirmed cases obtained from JHU CSSE.
Due to the data of total COVID-19 test cases were absence in early transmission period in the U.S., percent positive of SARS-CoV-2 in the U.S. during that period was unavailable. We approximated the infectivity of COVID-19 during the early transmission period as follows (Wallinga and Lipsitch, 2007)
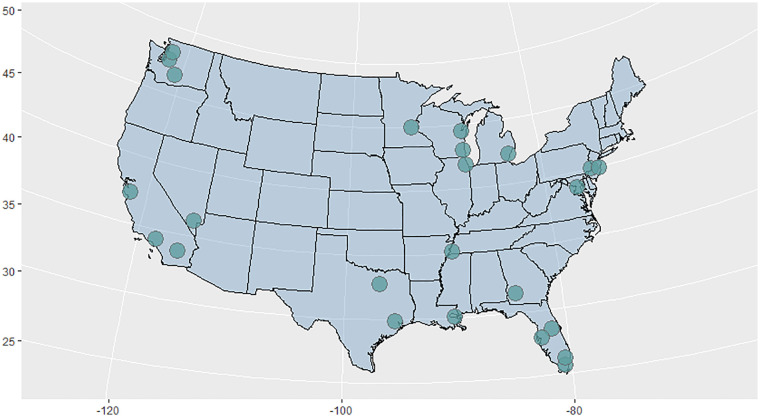
where C(t) represents the number of confirmed cases at time point t, Δg represents the generation interval, and Raverage represents the approximation of infectivity. We set the generation interval as 5, 6, and 7 days, according to previous studies on the incubation period of COVID-19 (Guan et al., 2020; Wiersinga et al., 2020). In this study, we selected 24 counties (one county with the highest number of confirmed cases up to April 30, 2020 on each latitude ranging from 24°N to 48°N was chosen; see Supplementary materials, Table S1) outside the State of New York in the U.S. to study the relationship between the sunlight UV radiation dose and COVID-19 early transmission (Fig. 2).
Fig. 2.
Geographical locations of the 24 selected counties in the U.S.
3. Results
3.1. Correlation between four common human coronaviruses and sunlight UV radiation
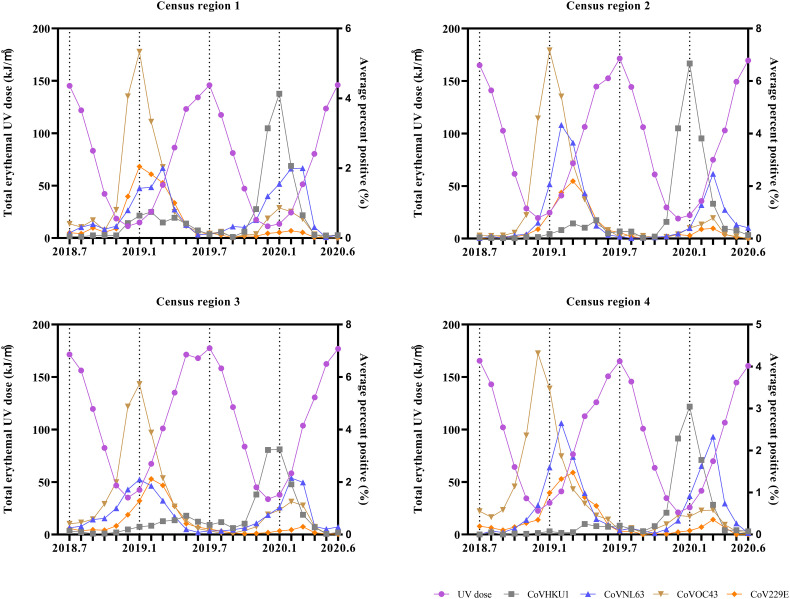
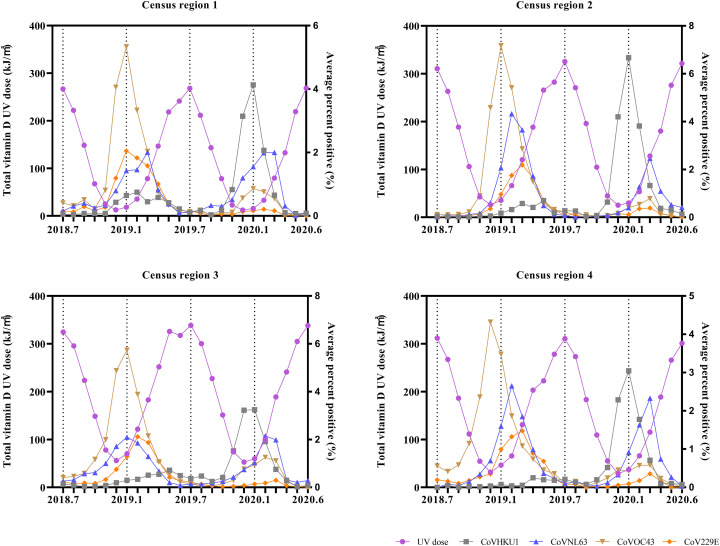
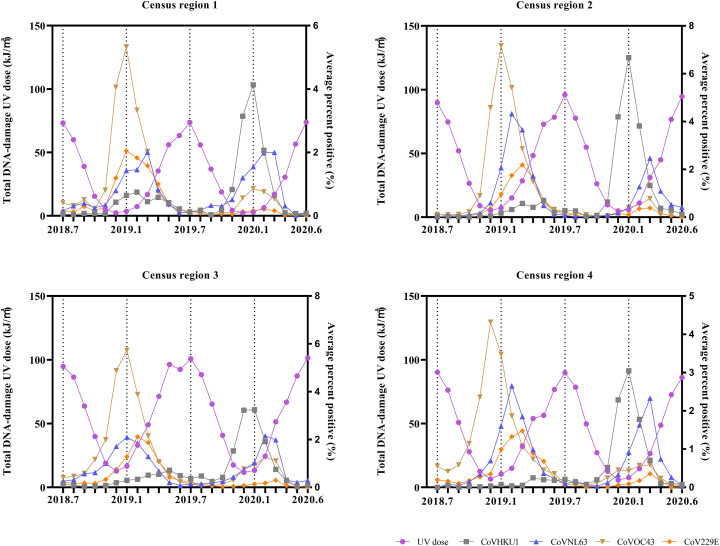
The monthly average percent positive of four common human coronaviruses (CoVHKU1, CoVNL63, CoVOC43, and CoV229E) and the corresponding monthly total erythemal UV dose are shown in Fig. 3. The erythemal UV dose includes UVA and UVB doses, and it is considered the most commonly used standard for UV radiation dose (Fioletov et al., 2009; Webb et al., 2011). The monthly total erythemal UV dose from July 2018 to June 2020 in the U.S. largely ranged from 11.223 kJ/m2 (in December 2018, census region 1) to 176.855 kJ/m2 (in June 2020, census region 3). The results of the Spearman and Kendall rank correlation tests indicated that the monthly average percent positive of human coronaviruses and the corresponding erythemal UV dose showed significant negative association, as shown in Table 1 (the same association was also indicated from vitamin D UV dose and DNA-damage UV dose, as shown in Supplementary materials, Fig. S1, Fig. S2, Table S2, Table S3).
Fig. 3.
The monthly average percent positive of four common human coronaviruses (CoVHKU1, CoVNL63, CoVOC43, and CoV229E) and the corresponding monthly total sunlight erythemal UV dose in the four census regions of the U.S.
Table 1.
The results of Spearman and Kendall rank correlation tests between the monthly average percent positive of four common human (CoVHKU1, CoVNL63, CoVOC43, CoV229E) and the corresponding monthly total sunlight erythemal UV dose.
| Virus species | Spearman rank correlation |
Kendall rank correlation |
|||
|---|---|---|---|---|---|
| rs | p | τ | p | ||
| Census region 1 | CoVHKU1 | −0.611⁎⁎ | 0.002 | −0.435⁎⁎ | 0.003 |
| CoVNL63 | −0.702⁎⁎ | 0 | −0.493⁎⁎ | 0.001 | |
| CoVOC43 | −0.602⁎⁎ | 0.002 | −0.439⁎⁎ | 0.003 | |
| CoV229E | −0.406⁎ | 0.049 | −0.279 | 0.056 | |
| Census region 2 | CoVHKU1 | −0.243 | 0.253 | −0.174 | 0.234 |
| CoVNL63 | −0.322 | 0.125 | −0.239 | 0.102 | |
| CoVOC43 | −0.497⁎ | 0.014 | −0.355⁎ | 0.015 | |
| CoV229E | −0.288 | 0.172 | −0.207 | 0.157 | |
| Census region 3 | CoVHKU1 | −0.311 | 0.139 | −0.21 | 0.15 |
| CoVNL63 | −0.738⁎⁎ | 0 | −0.514⁎⁎ | 0 | |
| CoVOC43 | −0.657⁎⁎ | 0 | −0.478⁎⁎ | 0.001 | |
| CoV229E | −0.15 | 0.483 | −0.116 | 0.427 | |
| Census region 4 | CoVHKU1 | −0.164 | 0.445 | −0.098 | 0.503 |
| CoVNL63 | −0.482⁎ | 0.017 | −0.333⁎ | 0.022 | |
| CoVOC43 | −0.543⁎⁎ | 0.006 | −0.413⁎⁎ | 0.005 | |
| CoV229E | −0.19 | 0.375 | −0.128 | 0.385 | |
p significance level of the two-tailed test.
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
Fig. S1.
The monthly average percent positive of four common human coronaviruses (CoVHKU1, CoVNL63, CoVOC43, and CoV229E) and the corresponding monthly total sunlight vitamin D UV dose in four census regions of the U.S.
Fig. S2.
The monthly average percent positive of four common human coronaviruses (CoVHKU1, CoVNL63, CoVOC43, and CoV229E) and the corresponding monthly total sunlight DNA-damage UV dose in four census regions of the U.S.
3.2. Correlation between SARS-CoV-2 and sunlight UV radiation
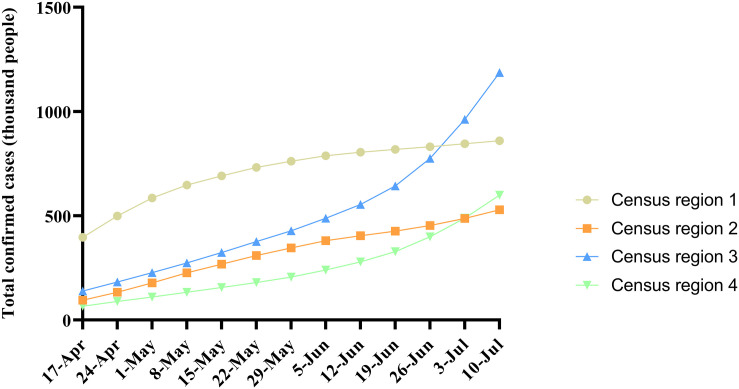
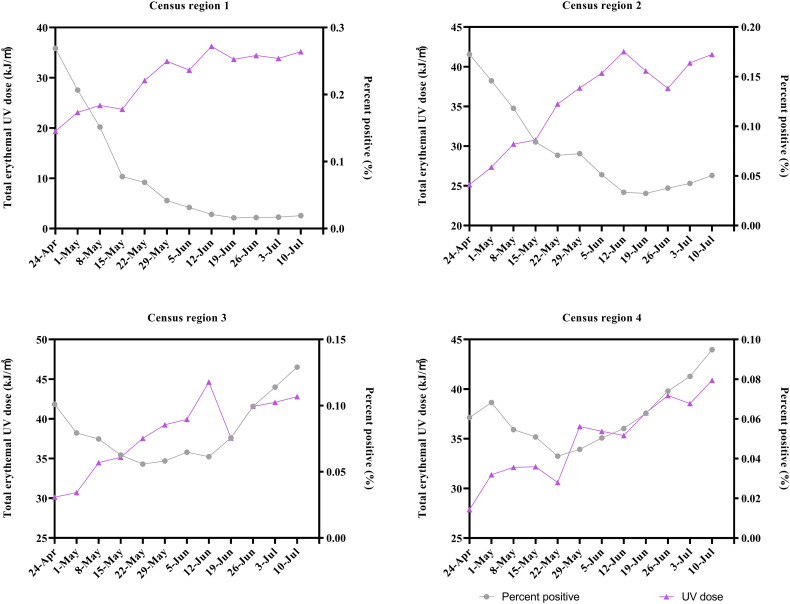
Since COVID-19 has spread further in the US, the number of total confirmed cases in the four census regions of the U.S. from April 17, 2020 to July 10, 2020 has shown different growth trends (Fig. 4). The association between the weekly average percent positive of SARS-CoV-2 in the four census regions and the corresponding weekly total sunlight UV radiation dose is shown in Fig. 5 and Table 2 . The results indicated that the weekly average percent positive of SARS-CoV-2 in census regions 1 and 2 (Northeast and Midwest regions) was significantly negatively correlated with the corresponding sunlight UV radiation dose, while that in census regions 3 and 4 (Southern and Western regions) appeared to be positively correlated. However, this association in census regions 3 and 4 was not statistically significant showing p-values 0.779 and 0.067, respectively, in the Spearman rank correlation test, and 0.784 and 0.1, respectively, in the Kendall rank correlation test.
Fig. 4.
The total number of COVID-19 confirmed cases from April 17, 2020 to July 10, 2020 in the four census regions of the U.S.
Fig. 5.
The weekly average percent positive of SARS-CoV-2 and the corresponding weekly total sunlight erythemal UV dose in the four census regions of the U.S.
Table 2.
The results of percent positive of SARS-Cov-2 and UV radiation dose in the U.S.
| UV dose variety | Spearman rank correlation |
Kendall rank correlation |
|||
|---|---|---|---|---|---|
| rs | p | τ | p | ||
| Census region 1 | Erythemal | −0.853⁎⁎ | 0 | −0.667⁎⁎ | 0.003 |
| VD | −0.853⁎⁎ | 0 | −0.667⁎⁎ | 0.003 | |
| DNA-damage | −0.776⁎⁎ | 0.003 | −0.606⁎⁎ | 0.006 | |
| Census region 2 | Erythemal | −0.853⁎⁎ | 0 | −0.727⁎⁎ | 0.001 |
| VD | −0.853⁎⁎ | 0 | −0.727⁎⁎ | 0.001 | |
| DNA-damage | −0.853⁎⁎ | 0 | −0.727⁎⁎ | 0.001 | |
| Census region 3 | Erythemal | 0.091 | 0.779 | 0.061 | 0.784 |
| VD | 0.049 | 0.88 | 0.03 | 0.891 | |
| DNA-damage | 0.049 | 0.88 | 0.03 | 0.891 | |
| Census region 4 | Erythemal | 0.545 | 0.067 | 0.364 | 0.1 |
| VD | 0.552 | 0.063 | 0.394 | 0.075 | |
| DNA-damage | 0.587⁎ | 0.045 | 0.424 | 0.055 | |
p significance level of the two-tailed test.
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
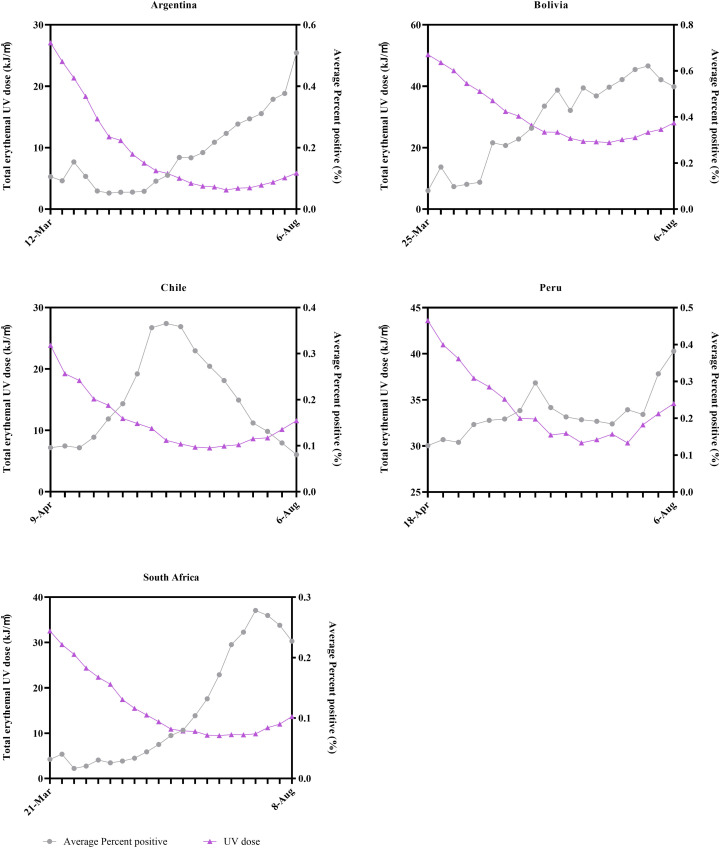
Furthermore, considering that the U.S. is located in the Northern Hemisphere where it is spring and summer from April to July, the current data did not include data of a full seasonal cycle yet. We calculated the weekly average percent positive of SARS-CoV-2 from March to August in five countries in the Southern Hemisphere with severe outbreaks (South Africa, Argentina, Chile, Bolivia, and Peru), and correlated them with the corresponding weekly total sunlight UV radiation dose, as supplementary. Due to the different seasonal patterns, it was autumn and winter in the Southern Hemisphere during this period, which may partly compliment the lack of seasonal data in the U.S. According to the results, the weekly percent positive of SARS-CoV-2 in four countries (South Africa, Argentina, Chile and Bolivia) showed a significant negative correlation with sunlight UV radiation dose while that in Peru showed negative correlation but without significant during that period (see Supplementary materials Fig. S3 and Table S4). The results indicated that a negative correlation may also exist between the weekly total UV radiation dose and the corresponding weekly average percent positive of SARS-CoV-2 during autumn and winter.
Fig. S3.
The weekly average percent positive of the SARS-CoV-2 and the corresponding weekly total sunlight erythemal UV dose in five countries in the Southern Hemisphere with severe outbreaks (South Africa, Argentina, Chile, Bolivia, and Peru).
3.3. Correlation between the sunlight UV radiation and the early transmission of COVID-19
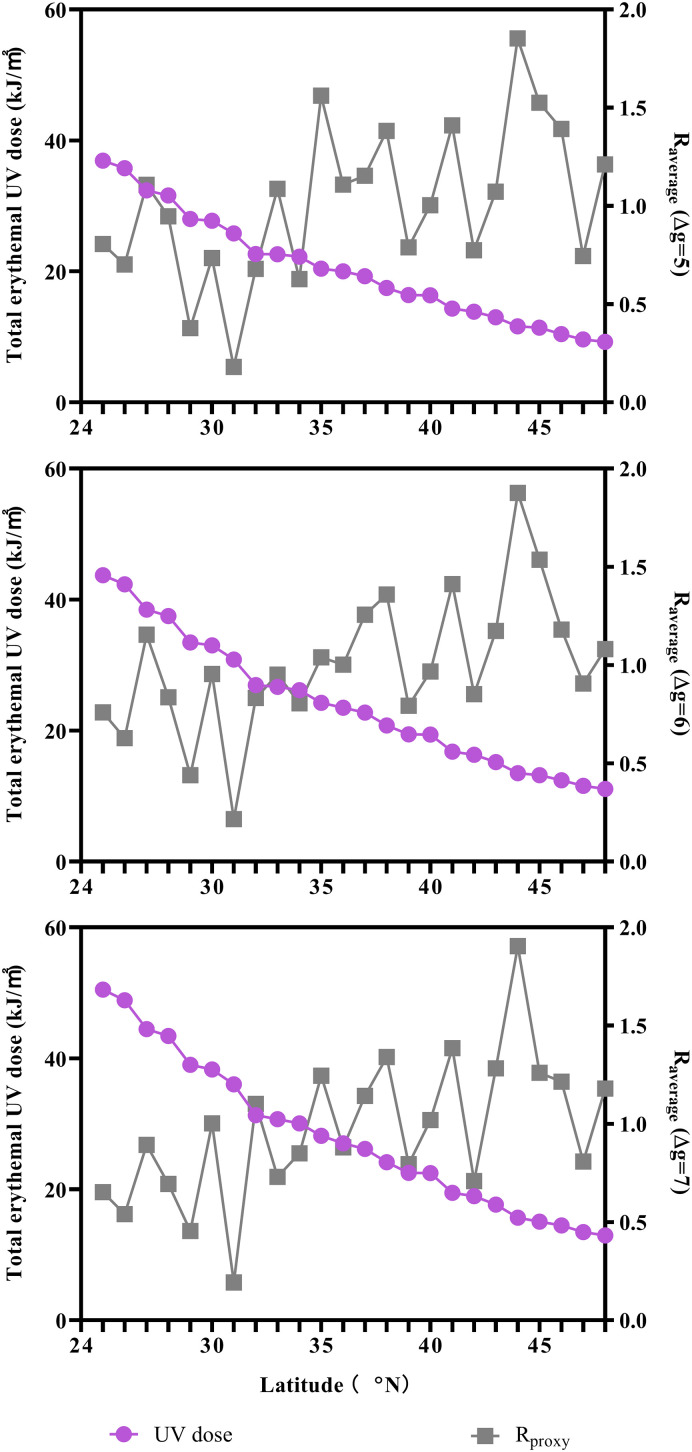
The infectivity of COVID-19 from April 17, 2020 to April 30, 2020 in the U.S. was investigated to study the relationship between the sunlight UV radiation dose and COVID-19 early transmission. The 11-, 13-, and 15-day total sunlight erythemal UV doses and the corresponding Raverage values at the different generation intervals are shown in Fig. 6 . Spearman and Kendall rank correlation tests indicated significant negative association between sunlight UV radiation and COVID-19 infectivity during the early transmission period outside the State of New York, as shown in Table 3 . Despite the sunlight UV radiation dose variations can be caused by latitude or seasonal reason, similar negative correlation between sunlight UV radiation and the transmission of COVID-19 was observed. This further suggested that COVID-19 and other human coronavirus diseases might show similar seasonality.
Fig. 6.
The Raverage values at the different generation intervals (Δg selected as 5, 6, and 7 days) and the corresponding total sunlight erythemal UV doses (11-, 13-, and 15-day durations, respectively).
Table 3.
The correlations between sunlight UV radiation dose and infectiousness of COVID-19 during the early transmission.
| Generation interval | Spearman rank correlation |
Kendall rank correlation |
|||
|---|---|---|---|---|---|
| rs | p | τ | p | ||
| US | Δg = 5 | −0.506⁎ | 0.012 | −0.333⁎ | 0.022 |
| Δg = 6 | −0.6⁎⁎ | 0.002 | −0.42⁎⁎ | 0.004 | |
| Δg = 7 | −0.612⁎⁎ | 0.001 | −0.42⁎⁎ | 0.004 | |
p significance level of the two-tailed test.
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
4. Discussion
Our study mainly aimed to provide evidence on the correlation between the sunlight UV radiation dose and human coronaviruses. According to our analyses based on the data from the U.S. during 2018–2020, the sunlight UV radiation dose showed significant negative correlation with four common human coronaviruses (CoVHKU1, CoVNL63, CoVOC43, and CoV229E). The seasonal variation in sunlight UV radiation dose could partly explain the seasonality of four common human coronaviruses which were reported in previous studies (Friedman et al., 2018; Killerby et al., 2018).
The other coronavirus, SARS-CoV-2, also showed significant negative correlation with the sunlight UV radiation dose in some regions (census regions 1 and 2) of the U.S. However, in census regions 3 and 4 of the U.S., the association between the weekly percent positive and the corresponding sunlight UV radiation dose was not statistically significant, since the p-values obtained in the Spearman and Kendall rank correlation tests did not meet the standard level of significance (p < 0.05). This uncertain correlation in census regions 3 and 4 might be related to the different public health policies in different regions.
Recent studies have suggested that the severity of the COVID-19 epidemic may be closely related to climatic factors, including the UV radiation dose (Babu et al., 2020; Sfîcă et al., 2020; Suhaimi et al., 2020). And the role of particulate matter (PM) and aerosol in COVID-19 transmission has been increasingly recognized (Hsiao et al., 2020; Mutuku et al., 2020; Razzini et al., 2020). Severe COVID-19 outbreaks in some areas may be associated with lower UV radiation dose in those areas (Sfîcă et al., 2020). A study of the provincial-level regions in China from December 2019 to April 2020 showed a negative correlation between the number of infections and the latitude (Sun et al., 2020). The results of these existing studies supported our finding that the UV radiation dose might play an important role in COVID-19 transmission. Additionally, PM and aerosol were reported to be important vectors for COVID-19 transmission, and the chemical properties of aerosols might affect SARS-CoV-2 survival and transmission (Razzini et al., 2020; Tung et al., 2020; Wathore et al., 2020). According to a previous study in Italy, the average concentration of PM on the ground in Milan was strongly associated with the number of COVID-19 confirmed cases (Zoran et al., 2020). Inactivation of pathogens in aerosols and PM by sunlight UV radiation might be the underlying mechanism for this correlation.
However, our study still had some limitations. First, the sunlight UV radiation dose is the cumulative total of the effective UV irradiance received from sunlight over a period. For areas with different sunshine intensities, higher UV radiation dose did not necessarily correspond to higher UV radiation intensity. Therefore, the relationship between the UV radiation intensity and human coronaviruses remains to be studied further. Second, the study on the role of sunlight UV radiation in the early transmission of COVID-19 is limited to the source of the data, which was not discussed in depth in this study. Third, although the percent positive of SARS-CoV-2 in the five countries in the Southern Hemisphere, where it was autumn and winter, showed a similar negative correlation with the sunlight UV dose, the data of the U.S. has not reached a fully seasonal cycle yet. Hence, a clearer relationship between the percent positive of SARS-CoV-2 and the sunlight UV radiation dose in the U.S. still requires more data and further confirmation. In addition, COVID-19 is an emerging infectious disease and more research must be performed on the COVID-19 transmission processes. Thus, the role of the sunlight UV radiation dose in the COVID-19 epidemic, especially for the effects of the sunlight UV radiation on the seasonality and early transmission of COVID-19, may be very complicated. At least, our research indicated that the sunlight UV radiation probably showed a negative correlation with COVID-19 infectivity during its early transmission period, and this may contribute to future research.
5. Conclusion
This study provided some evidence on the possible negative correlations between the sunlight UV radiation dose and the percent positive of five human coronaviruses (SARS-CoV-2, CoVHKU1, CoVNL63, CoVOC43, and CoV229E). Our study indicated significant negative correlation between the percent positive of four common human coronaviruses (CoVHKU1, CoVNL63, CoVOC43, and CoV229E) and the sunlight UV radiation dose in the U.S. Higher sunlight UV radiation dose might be related to lower percent positive of human coronaviruses. However, although SARS-CoV-2 might show a similar negative correlation with the sunlight UV radiation dose, further research and verification based on the update data in the future are needed to account for the variations in the seasonal cycles. We will continue to focus on this study area in future studies, since they may be beneficial for providing more insights into COVID-19 prevention and treatment.
The following are the supplementary data related to this article.
The geographical locations and total confirmed cases up to 30 April,2020 of 24 selected counties in the U.S.
The results of Spearman and Kendall rank correlation tests between the monthly average percent positive of four common human (CoVHKU1, CoVNL63, CoVOC43, CoV229E) and the corresponding monthly total sunlight vitamin D UV dose.
The results of Spearman and Kendall rank correlation tests between the monthly average percent positive of four common human (CoVHKU1, CoVNL63, CoVOC43, CoV229E) and the corresponding monthly total sunlight DNA-damage UV dose.
The results of Spearman and Kendall rank correlation tests between the weekly average percent positive of SARS-CoV-2 and the corresponding weekly total sunlight erythemal UV dose among five Southern Hemisphere Countries.
CRediT authorship contribution statement
Research concept and design, Liwei Tang and Min Liu; data collection and statistical analysis, by Min Liu and Bingyu Ren; drafting of the manuscript, Liwei Tang, Min Liu, Bingyu Ren, Zongting Wu, Xunci Yu, Chen Peng, and Jing Tian.
All authors read and approved the final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgements
We would like to thank Dr. Jari Hovila from Finnish Meteorological Institute for his useful suggestions on collecting UV radiation dose data. We would also like to thank the three anonymous reviewers and the editors for their helpful and constructive comments.
This study was financially supported by grants from the Science and Technology Innovation Committee of Shenzhen Municipality (JCYJ20190808152613121), Shenzhen-HongKong Institute of Brain Science-Shenzhen Fundamental Research Institutions (2019SHIBS0003) and a special fund for fighting the COVID-19 epidemic from Shenzhen University.
Editor: Jay Gan
References
- Babu S.R., Rao N.N., Kumar S.V., Paul S., Pani S.K. Plausible role of environmental factors on COVID-19 transmission in the Megacity Delhi, India. Aerosol Air Qual. Res. 2020;20 [Google Scholar]
- Bonett D.G., Wright T.A. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika. 2000;65:23–28. [Google Scholar]
- Dalmay F., Preux P.M., Druet-Cabanac M., Vergnenegre A. What is a non-parametric test? Rev. Mal. Respir. 2003;20:955–958. [PubMed] [Google Scholar]
- D’Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int. J. Mol. Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003;16:246–255. [PubMed] [Google Scholar]
- Fioletov V.E., McArthur L.J.B., Mathews T.W., Marrett L. On the relationship between erythemal and vitamin D action spectrum weighted ultraviolet radiation. Journal of Photochemistry and Photobiology B-Biology. 2009;95:9–16. doi: 10.1016/j.jphotobiol.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin. Microbiol. Infect. 2012;18:946–954. doi: 10.1111/j.1469-0691.2012.03968.x. [DOI] [PubMed] [Google Scholar]
- Friedman N., Alter H., Hindiyeh M., Mendelson E., Shemer Avni Y., Mandelboim M. Human coronavirus infections in Israel: epidemiology, clinical symptoms and summer seasonality of HCoV-HKU1. Viruses. 2018;10 doi: 10.3390/v10100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt E.R., Hardie A., Claas E.C., Simmonds P., Templeton K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J. Clin. Microbiol. 2010;48:2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020:12. [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessling M., Hones K., Vatter P., Lingenfelder C. Ultraviolet irradiation doses for coronavirus inactivation - review and analysis of coronavirus photoinactivation studies. GMS Hyg Infect Control. 2020;15:Doc08. doi: 10.3205/dgkh000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F., Chen T.C., Lu Z., Sauter E. Vitamin D and skin physiology: a D-lightful story. J. Bone Miner. Res. 2007;22:V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- Hsiao T.-C., Chuang H.-C., Griffith S.M., Chen S.-J., Young L.-H. COVID-19: an aerosol’s point of view from expiration to transmission to viral-mechanism. Aerosol Air Qual. Res. 2020:905–910. [Google Scholar]
- Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman C., Bozdag C.E., Ruan D., Ozok A.F. Fuzzy sets approaches to statistical parametric and nonparametric tests. Int. J. Intell. Syst. 2004;19:1069–1087. [Google Scholar]
- Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I., et al. Human coronavirus circulation in the United States 2014-2017. J. Clin. Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. Vitamin D physiology. Progress in Biophysics & Molecular Biology. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Mansur J.L. Letter: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020;52:411–412. doi: 10.1111/apt.15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menebo M.M. Temperature and precipitation associate with Covid-19 new daily cases: a correlation study between weather and Covid-19 pandemic in Oslo, Norway. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.139659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku J.K., Hou W.-C., Chen W.-H. An overview of experiments and numerical simulations on airflow and aerosols deposition in human airways and the role of bioaerosol motion in COVID-19 transmission. Aerosol Air Qual. Res. 2020;20:1172–1196. [Google Scholar]
- Pani S.K., Lin N.H., RavindraBabu S. Association of COVID-19 pandemic with meteorological parameters over Singapore. Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N.V., et al. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J.M., Subramanian S., Laird E., Kenny R.A. Letter: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Authors’ reply. Aliment. Pharmacol. Ther. 2020;52:412–413. doi: 10.1111/apt.15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagripanti J.L., Lytle C.D. Estimated inactivation of coronaviruses by solar radiation with special reference to COVID-19. Photochem. Photobiol. 2020;96(4):731–737. doi: 10.1111/php.13293. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfîcă L., Bulai M., Amihăesei V.-A., Ion C., Ștefan M. Weather conditions (with focus on UV radiation) associated with COVID-19 outbreak and worldwide climate-based prediction for future prevention. Aerosol Air Qual. Res. 2020;20 [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhaimi N.F., Jalaludin J., Latif M.T. Demystifying a possible relationship between COVID-19, air quality and meteorological factors: evidence from Kuala Lumpur, Malaysia. Aerosol Air Qual. Res. 2020;20:1520–1529. [Google Scholar]
- Sun Z., Zhang H., Yang Y., Wan H., Wang Y. Impacts of geographic factors and population density on the COVID-19 spreading under the lockdown policies of China. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N.T., Cheng P.-C., Chi K.-H., Hsiao T.-C., Jones T., BéruBé K., et al. Particulate matter and SARS-CoV-2: a possible model of COVID-19 transmission. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.141532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinga J., Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proceedings of the Royal Society B-Biological Sciences. 2007;274:599–604. doi: 10.1098/rspb.2006.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathore R., Gupta A., Bherwani H., Labhasetwar N. Understanding air and water borne transmission and survival of coronavirus: insights and way forward for SARS-CoV-2. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.141486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A.R., Slaper H., Koepke P., Schmalwieser A.W. Know your standard: clarifying the CIE erythema action spectrum. Photochem. Photobiol. 2011;87:483–486. doi: 10.1111/j.1751-1097.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- Welch D., Buonanno M., Grilj V., Shuryak I., Crickmore C., Bigelow A.W., et al. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018;8:2752. doi: 10.1038/s41598-018-21058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Zhang L., Shen F.M., Chen F., Lin Z. Origin and evolution of the 2019 novel coronavirus. Clin. Infect. Dis. 2020;71(15):882–883. doi: 10.1093/cid/ciaa112. (Jul 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The geographical locations and total confirmed cases up to 30 April,2020 of 24 selected counties in the U.S.
The results of Spearman and Kendall rank correlation tests between the monthly average percent positive of four common human (CoVHKU1, CoVNL63, CoVOC43, CoV229E) and the corresponding monthly total sunlight vitamin D UV dose.
The results of Spearman and Kendall rank correlation tests between the monthly average percent positive of four common human (CoVHKU1, CoVNL63, CoVOC43, CoV229E) and the corresponding monthly total sunlight DNA-damage UV dose.
The results of Spearman and Kendall rank correlation tests between the weekly average percent positive of SARS-CoV-2 and the corresponding weekly total sunlight erythemal UV dose among five Southern Hemisphere Countries.