Dear Editor,
The coronavirus disease 2019 (COVID-19) has spread globally since December 2019, affected several millions of infection, and had led to tens of thousands of death. Many countries implanted strict measures, including shutting down cities or community activities, banning international or domestic travel, confirmed case isolation and quarantine policy to contain this outbreak but also impact global economic and caused huge healthcare system burden. Since the studies of virological infectivity and dynamic transmissibility are ongoing, different policies had been applied in different countries. The interim guidance from Centers for Disease Control and Prevention (CDC), USA suggested that the test-based strategy, i.e., two consecutive negative results of oropharyngeal swabs by real-time-polymerase chain reaction (RT-PCR), could be the criterion for return to work or de-isolation. However, Taiwan CDC recommended three consecutive negative results as the de-isolation criterion for confirmed cases before Jun. 17, 2020. So far, the contagious period of symptomatic or asymptomatic cases of COVID-19 is not fully understood, and adoption of the stricter policy, three consecutive negative results, may avoid person-to-person transmission. However, prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in nasopharyngeal swabs or lower respiratory tract aspirates in the cases of COVID-19 after clinical resolution and seroconversion had been reported1 and prolonged isolation or hospitalization and the consumption of personal protective equipment were two major disadvantages of applying strict policy. This observation study is aimed to report the effect of different de-isolation criterion.
From Feb. 1 to Apr. 21, 2020, 12 patients of COVID-19 admitted to two hospitals at southern Taiwan were included for analysis. Their median age was 23.5 years with a range of 16–52 years, and 6 (50%) were males. Only one had underlying hypertension with regular medical control. Nine patients had fever and cough, but no patient experienced severe respiratory distress or needed ventilator support during hospitalization. Five patients reported anosmia and two of them only had anosmia without rhinorrhea or other discomfort. All patients except a naval crew were diagnosed within one week after symptom onset and their median interval between the symptom onset and diagnosis was 2 days, with a range of 1–6 days. Chest X-ray films showed pulmonary infiltrations or opacities in five patients, and four required supplemental oxygen during the initial week of hospitalization. Except four patients had prolonged anosmia during hospitalization, the median interval between diagnosis and symptom resolution in other eight patients was 3.5 days (range 0-7 days). Their symptoms improved before negative swab tests. Ten patients ever received oral hydroxycholoquine therapy. Oropharyngeal/nasopharyngeal swab specimens were collected every 2–4 days and were tested for viral RNA in one hospital, according to the laboratory method recommended by CDC, Taiwan2. Patients received at least four times and at most 26 times of oropharyngeal/nasopharyngeal swab sampling. De-isolation would be considered if patients experienced defervescence, symptom resolution, and had three consecutive negative SARS-CoV-2 RNA tests.
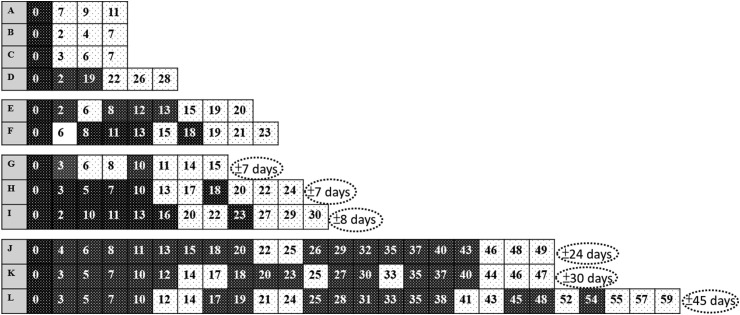
Total 146 clinical specimens were collected. The median hospital stay was 27 days with a range of 11–61 days. Fig. 1 depicted the sequential change between the days of confirmed date (D0) to each event of nasopharyngeal swab results during the hospitalization. Four patients (case A-D), including the naval crew, had three negative consecutive specimens following the first negative result of RT-PCR and the median time between confirmed diagnosis to the first negative result were 5 (range 2–22) days. The other eight patients had several times of intermittent negative results before three negative consecutive results. Five fulfilled the de-isolation criterion at the second time of negative results (case E, G, H, I and J), one the third time (case F), one the fourth time (case K), and one the fifth time (case L). Six patients had two negative consecutive results before three negative consecutive results (case G-L). If three consecutive negative results were applied as the de-isolation criterion, the median duration of extra-hospital stays will be 16 (range 7–45) days, as shown in Fig. 1.
Figure 1.
Sequential changes of SARS-CoV-2 PCR of oropharyngeal swab specimens in 12 patients with COVID-19. The alphabetic is the case No. and numerical values are the illness days of oropharyngeal swab sampling after the day of confirmed diagnosis of COVID-19 (D0). Black boxes indicate positive PCR results, and the boxes with dots negative results. The dotted circles show the gap intervals between two de-isolation criterion of two and three consecutive negative real-time polymerase–chain reaction results of oropharyngeal swabs.
Prolonged shedding of SARS-CoV-2 RNA in respiratory tract samples had been observed in convalescent children with enterovirus infection,3 and did not correlate with clinical infectivity. Our case series support that prolonged shedding of SARS-CoV-2 RNA and intermittent detection of viral RNA in COVID-19 patients is not uncommon. The stricter de-isolation criterion of three consecutive negative results prolonged the isolation duration for at least 1 week or at most more than 6 weeks, as compared with the criterion of two consecutive negative results. A recent study in Germany showed viral load was declining on day 10 after onset of symptoms and no live virus was isolated from specimens taken after day 8 in spite of ongoing high viral load.4 Another study also demonstrated that infectivity was reduced when RT-PCR cycle threshold (Ct) values were greater than 24.5 Moreover, a contact tracing and viral testing study from South Korea provided additional evidence to support that no infectivity in re-positive cases after their discharge from isolation.6 As the pandemic disease persists, shortage of personal protective equipment, and limited capacity of isolation rooms are real-world concerns. Earlier de-isolation and hospital discharge with home isolation after defervescence, symptom resolution with two consecutive negative results are acceptable. With the increasing clinical and virological data, Taiwan CDC revised two consecutive negative results, in addition to symptom resolution for 3 days and the elapse of 10 days after symptom onset for symptomatic cases or 10 days confirmed diagnosis for asymptomatic cases, as the de-isolation criterion for COVID-19 patients in Jun. 17, 2020.
Declaration of competing interest
All authors declare no conflicts of interest.
References
- 1.Liu W.D., Chang S.Y., Wang J.T., Tsai M.J., Hung C.C., Hsu C.L. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control, Taiwan Detection of 2019-nCoV viral nucleic acid. 2020. https://www.cdc.gov.tw/File/Get/BHL7va9Hwyc3kDX9aXn6-A version 2.2. [Chinese]
- 3.Richardson M., Elliman D., Maguire H., Simpson J., Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380–391. doi: 10.1097/00006454-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020:ciaa638. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.South Korea CDC. Findings from investigation and analysis of re-positive cases. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=367267&nPage=1#.