Abstract
Objective
Male digit ratio (2D:4D) correlates positively with the national case fatality rate (CFR) for COVID-19. The severity of COVID-19 may be influenced by a counterbalance between the angiotensin-converting enzyme (ACE) and angiotensin-converting enzyme 2 (ACE2). SARS-CoV2 cleaves with ACE2 and enters cells leaving an unopposed effect of ACE in the lungs. Both 2D:4D and the ACE I/D polymorphism are covariates of oxygen metabolism. COVID-19 leads to lung damage and a reduction in oxygen saturation of the blood. Here, we examine the interrelationships between 2D:4D, ACE polymorphism, and COVID-19 CFR.
Methods
National frequencies/rates were obtained for 2D:4D from the BBC Internet study (n = 41), published values of ACE I/II (n = 39), and COVID-19 CFR from three World Health Organization situation reports (n = 41).
Results
2D:4D was negatively associated with national ACE I/II frequencies. However, there was a positive relationship between male 2D:4D and CFR (right and left 2D:4D, two, and three situation reports respectively). The relationships between ACE I/II and CFR were non-significant. Relationships between male 2D:4D and CFR's were independent of female 2D:4D and ACE I/II.
Conclusions
The ACE I/D polymorphism may influence 2D:4D such that ACE II individuals have lower 2D:4D than ACE DD individuals. Low 2D:4D and ACE II individuals show efficient oxygen metabolism. Therefore, low 2D:4D and ACE II together may protect against COVID-19 severity. The sex-dependent positive correlation between male 2D:4D and CFR is independent of ACE I/II, suggesting that the sex-dependent variation in the ACE2 gene may also influence the 2D:4D phenotype.
Keywords: Digit ratio, COVID-19, ACE, Polymorphism, Oxygen metabolism, Mortality
Highlights
-
•
Digit ratio (2D:4D) and the ACE I/D polymorphism are covariates of oxygen metabolism.
-
•
2D:4D was negatively associated with national ACE I/II frequencies.
-
•
Male 2D:4D correlates positive with the national case fatality rate for COVID-19.
-
•
ACE II individuals have lower 2D:4D than ACE DD individuals.
-
•
Low 2D:4D and ACE II together may protect against COVID-19 severity.
1. Introduction
The severity of COVID-19 is sex-dependent (males > females) [1,2]. Case fatality rates (CFR) vary across nations and correlate positively with national male (but not female) digit ratio (2D:4D) - a proxy for prenatal sex-steroids [[3], [4], [5]]. This relationship suggests that high prenatal testosterone to oestrogen ratios are protective against COVID-19 severity. Testosterone and oestrogen are important modulators of blood pressure through their regulatory effect on the renin-angiotensin system (RAS) [6]. In particular, angiotensin-converting enzyme (ACE) and its antagonist angiotensin-converting enzyme 2 (ACE2) play a key role in the RAS. Blood pressure is sex-dependent (males > females) and is determined by a balance of vasoconstriction (by ACE) and vasodilation (by ACE2) [6]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) enters cells by cleaving with membrane-bound ACE2, thus removing ACE2 from cell surfaces and leaving an unopposed effect of ACE on the lungs and other organs. The result is an increase of vasoconstriction, thrombosis, and inflammation of the lungs [7]. One important feature of the ACE gene is that it is highly polymorphic [8]. In the present study, we examine the relationship between 2D:4D, ACE polymorphism, and COVID-19 CFR across nations.
Digit ratio is a normally distributed sexually dimorphic trait (male 2D:4D < female 2D:4D) that is thought to be a negative correlate of prenatal testosterone and a positive correlate of prenatal oestrogen [9]. National values of male (but not female) 2D:4D correlate positively with national COVID-19 CFR's [3], i.e. nations with high COVID-19 mortality have male populations that have experienced low prenatal testosterone relative to oestrogen. The link between high male 2D:4D and COVID-19 severity may lie in the association between 2D:4D and oxygen metabolism. COVID-19 leads to lung damage and a reduction in oxygen saturation (SpO2) of the blood. Low presenting SpO2 in COVID-19 patients is predictive of high severity of outcomes such as pneumonia, acute respiratory distress syndrome, and admission to intensive care [10,11]. Low 2D:4D individuals may be resistant to oxygen depletion. Thus, high performances in endurance running and rowing are related to low 2D:4D, and this is particularly so for males [12,13]. Moreover, in high 2D:4D individuals (as compared to low 2D:4D individuals), low right-hand relative to left-hand 2D:4D is related to higher VO2max (maximum rate of oxygen consumption measured during the exercise of increasing intensity [14]), VO2peak (highest value of VO2 attained in an incremental exercise test [15]), and ventilatory threshold (the point at which ventilation increases at a faster rate than VO2 [16]).
In contrast to the continuous variation in 2D:4D, the genes of the RAS system have several discontinuous forms. We focus on one such, the insertion/deletion (I/D) polymorphism at intron 16 of the ACE gene. There have been >250 polymorphisms reported in the ACE gene, with the I/D polymorphism attracting the most attention [17]. This involves either the presence (I) or the absence (D) of a 287 bp fragment. The presence of the extra fragment is associated with lower circulating and tissue ACE activity, while its absence is associated with relatively higher ACE activity [18]. In common with low 2D:4D, the I allele is associated with endurance performance in sports such as distance running and rowing and fatigue resistance in skeletal muscle. However, these correlations are probably not as markedly sexually dimorphic as those related to 2D:4D [18,19].
Both 2D:4D and ACE I/D show geographic and ethnic variation. Mean 2D:4D is low in South-East Asian nations and the frequency of ACE I is high. In contrast, European nations have high mean 2D:4D and low frequencies of ACE I. Comparative analyses of mean national 2D:4D show that high national male 2D:4D is associated with high CFR from COVID-19. It is possible, therefore, that high national frequencies of ACE I are related to low CFR. However, Delanghe et al. [20] have reported a negative correlation between ACE DD frequency and national prevalence of COVID-19 (n/million). That is, countries with high frequencies of ACE II have a high prevalence of the virus. Prevalence is not the same as CFR, which is partly determined by the number of deaths (i.e. the severity of COVID-19 after infection). It is mortality that we focus on here.
We hypothesise that there are links between 2D:4D/ACE I, oxygen metabolism, and geographical variation that suggest that ACE polymorphism may influence both the 2D:4D phenotype and CFR from COVID-19. Thus, in the present study, we examined the relationships between (i) national values of male and female 2D:4D and ACE I/D, and (ii) national values of male and female 2D:4D, ACE I/D, and CFR's for COVID-19. Our prediction for (i) was that 2D:4D would be negatively related to the frequency of the ACE I allele and the ACE II genotype. Concerning (ii), we predicted the following relationships: a positive association between male 2D:4D and CFR, a null-relationship between female 2D:4D and CFR, and a negative relationship between ACE I or II and CFR. To address the issue of the temporal stability of our results we considered associations at three time points: WHO situation reports 113, 127 (which have been reported on earlier), and the more recent situation report 165.
2. Methods
National means for 2D:4D by sex and hand were obtained from a large online survey (the BBC Internet study). The data comprised means from 41 nations calculated from self-reported lengths of the 2nd (index finger) and 4th digits (ring finger) of 103,482 men and 83,366 women.
Mean national values of the allele ACE I and the genotype ACE II for the 41 BBC study nations were obtained principally from reviews of the worldwide genetic structure of the ACE gene [17,21]. Where nations in the BBC study were not represented in these reviews of ACE I/D we searched Pubmed (https://pubmed.ncbi.nlm.nih.gov), using keywords ‘angiotensin-converting enzyme’, ‘ACE’ ‘polymorphism’ and country names (e.g., ‘Belgium’). The search revealed publications for the following nations: Belgium [22,23], Bulgaria [24], Malaysia [25], Norway [26], Pakistan [27], Romania [28], Switzerland [29], United Arab Emirates [30].
National CFR's were calculated ([number of deaths / number of cases] ∗ 100) from three WHO situation reports 113 (May 12th 2020), 127 (May 26th 2020; see [4]), and 165 (July 3rd 2020). To ensure normality the CFR's were log-transformed.
3. Results
3.1. Descriptive statistics
The frequency of the ACE I allele was available for 38 nations (Table 1 ). Mean (SD) frequency of I was 0.474 (0.093) with a range of 0.268 to 0.692. For the ACE II genotype, there were values for 39 nations and the mean frequency was 0.228 (0.089) with a range of 0.05 to 0.475. The correlation between the ACE I and ACE II frequencies was high (r = 0.949, p < .0001).
Table 1.
The frequencies of the I allele and the II genotype (I/II) in the gene for angiotensinogen converting enzyme (ACE), mean male 2D:4D (right and left), mean female 2D:4D (right and left) and log case fatality rates (log CFR for WHO Situation Report 165, 3rd July 2020) for 41 nations.
| Source/n of studies | Nation | Freq. ACE I/II | Male R2D:4D | Male L2D:4D | Female R2D:4D | Female L2D:4D | log CFR |
|---|---|---|---|---|---|---|---|
| Li et al., 2011/1 [21] | Argentina | 0.490/0.213 | 0.990 | 0.988 | 0.992 | 0.992 | 0.307 |
| Li et al., 2011/3 [21] | Australia | 0.460/0.209 | 0.981 | 0.982 | 0.990 | 0.988 | 0.114 |
| Li et al., 2011/4 [21] | Austria | 0.485/0.201 | 0.980 | 0.986 | 0.990 | 0.994 | 0.594 |
| Gu et al., 1994/1 [22] Li et al., 2007/1 [23] |
Belgium | –/0.238 | 0.981 | 0.984 | 0.989 | 0.989 | 1.199 |
| Li et al., 2011/3 [21] | Brazil | 0.373/0.136 | 0.979 | 0.980 | 0.994 | 0.991 | 0.622 |
| Stambolova et al., 2017/1 [24] | Bulgaria | 0.390/0.150 | 0.990 | 0.989 | 0.997 | 0.998 | 0.640 |
| Li et al., 2011/3 [21] | Canada | 0.422/0.194 | 0.981 | 0.981 | 0.994 | 0.992 | 0.916 |
| Li et al., 2011/88 [21] | China | 0.610/0.396 | 0.985 | 0.985 | 0.989 | 0.985 | 0.736 |
| Li et al., 2011/1 [21] | Croatia | 0.470/0.220 | 0.981 | 0.984 | 0.998 | 0.996 | 0.577 |
| Li et al., 2011/5 [21] | Czech | 0.467/0.224 | 0.984 | 0.986 | 1.000 | 0.999 | 0.460 |
| Li et al., 2011/2 [21] | Denmark | 0.495/0.241 | 0.982 | 0.988 | 0.987 | 0.990 | 0.675 |
| Li et al., 2011/3 [21] | Finland | 0.457/0.224 | 0.984 | 0.985 | 0.991 | 0.990 | 0.656 |
| Li et al., 2011/4 [21] | France | 0.430/0.201 | 0.983 | 0.987 | 0.990 | 0.986 | 1.275 |
| Li et al., 2011/5 [21] | Germany | 0.457/0.218 | 0.983 | 0.985 | 0.993 | 0.991 | 0.663 |
| Li et al., 2011/1 [21] | Greece | 0.380/0.173 | 0.986 | 0.987 | 0.997 | 0.998 | 0.744 |
| Li et al., 2011/1 [21] | Hungary | 0.513/0.254 | 0.986 | 0.988 | 0.999 | 0.995 | 1.149 |
| Iceland | 0.980 | 0.984 | 0.986 | 0.987 | −0.267 | ||
| Li et al., 2011/6 [21] | India | 0.540/0.299 | 0.986 | 0.986 | 0.997 | 0.992 | 0.464 |
| Ireland | 0.983 | 0.983 | 0.991 | 0.991 | 0.834 | ||
| Li et al., 2011/6 [21] | Israel | 0.397/0.153 | 0.987 | 0.987 | 1.001 | 0.996 | 0.084 |
| Li et al., 2011/10 [21] | Italy | 0.394/0.168 | 0.984 | 0.986 | 0.994 | 0.989 | 1.160 |
| Li et al., 2011/13 [21] | Japan | 0.651/0.422 | 0.984 | 0.982 | 0.985 | 0.982 | 0.709 |
| Ali et al., 2015/1 [25] | Malaysia | 0.689/0.452 | 0.976 | 0.976 | 0.992 | 0.991 | 0.146 |
| Li et al., 2011/7 [21] | Mexico | 0.599/0.318 | 0.976 | 0.977 | 0.989 | 0.984 | 1.090 |
| Li et al., 2011/1 [21] | N Zealand | 0.460/0.225 | 0.980 | 0.982 | 0.990 | 0.987 | 0.270 |
| Li et al., 2011/10 [21] | Netherlands | 0.482/0.226 | 0.981 | 0.985 | 0.990 | 0.992 | 1.084 |
| Goleva-Fjellet et al., 2020/1 [26] | Norway | 0.492/0.229 | 0.982 | 0.984 | 0.990 | 0.989 | 0.450 |
| Shaheen et al., 2019/1 [27] | Pakistan | 0.268/0.05 | 0.983 | 0.984 | 0.988 | 0.990 | 0.312 |
| Li et al., 2011/1 [21] | Philippines | 0.550/0.295 | 0.983 | 0.980 | 0.992 | 0.991 | 0.516 |
| Li et al., 2011/3 [21] | Poland | 0.570/0.256 | 0.984 | 0.989 | 0.999 | 0.997 | 0.628 |
| Li et al., 2011/1 [21] | Portugal | 0.440/0.170 | 0.983 | 0.983 | 0.988 | 0.986 | 0.569 |
| Marginean et al., 2015/1 [28] | Romania | 0.301/0.151 | 0.986 | 0.985 | 0.998 | 1.001 | 0.784 |
| Li et al., 2011/2 [21] | Russia | 0.480/0.233 | 0.976 | 0.986 | 0.996 | 1.002 | 0.169 |
| Li et al., 2011/4 [21] | Singapore | 0.692/0.475 | 0.977 | 0.974 | 0.989 | 0.986 | −1.229 |
| Li et al., 2011/9 [21] | Spain | 0.413/0.170 | 0.987 | 0.988 | 0.995 | 0.992 | 1.055 |
| Li et al., 2011/5 [21] | Sweden | 0.492/0.242 | 0.982 | 0.981 | 0.994 | 0.992 | 0.884 |
| Lovati et al., 2001/1 [29] |
Switzerland | 0.509/0.211 | 0.984 | 0.983 | 0.991 | 0.987 | 0.723 |
| Li et al., 2011/21 [21] | Turkey | 0.418/0.181 | 0.987 | 0.987 | 0.999 | 1.000 | 0.407 |
| Li et al., 2011/14 [21] | UK | 0.476/0.231 | 0.985 | 0.986 | 0.993 | 0.992 | 1.190 |
| Saeed et al., 2005/1 [30] | United Arab Emirates | –/0.06 | 0.981 | 0.983 | 1.001 | 0.993 | −0.193 |
| Li et al., 2011/5 [21] | USA | 0.440/0.196 | 0.984 | 0.984 | 0.996 | 0.993 | 0.680 |
Table 1 presents mean national 2D:4D values for 41 nations. Mean male right hand 2D:4D was 0.983 (0.003) with a range from 0.976 to 0.990, and mean left hand 2D:4D was 0.984 (0.003), ranging from 0.974 to 0.989. Right and left male 2D:4D correlated positively (r = 0.731, p < .0001, n = 41). Mean female right 2D:4D was 0.993 (0.004) with a range from 0.985 to 1.001, and mean left hand 2D:4D was 0.992 (0.005), ranging from 0.982 to 1.002. As for males, right and left female 2D:4D correlated positively (r = 0.809, p < .0001). Male national 2D:4D was lower than female national 2D:4D (right hand, Δ = −0.010, t = 14.88, p < .0001; left hand, Δ = −0.007, t = 11.22, p < .0001).
The CFR's reported in WHO 113 correlated positively with those of WHO 127 (r = 0.996, p < .0001), and WHO 127 CFR's correlated positively with those of WHO 165 (r = 0.985, p < .0001).
3.2. 2D:4D, ACE I, and ACE II
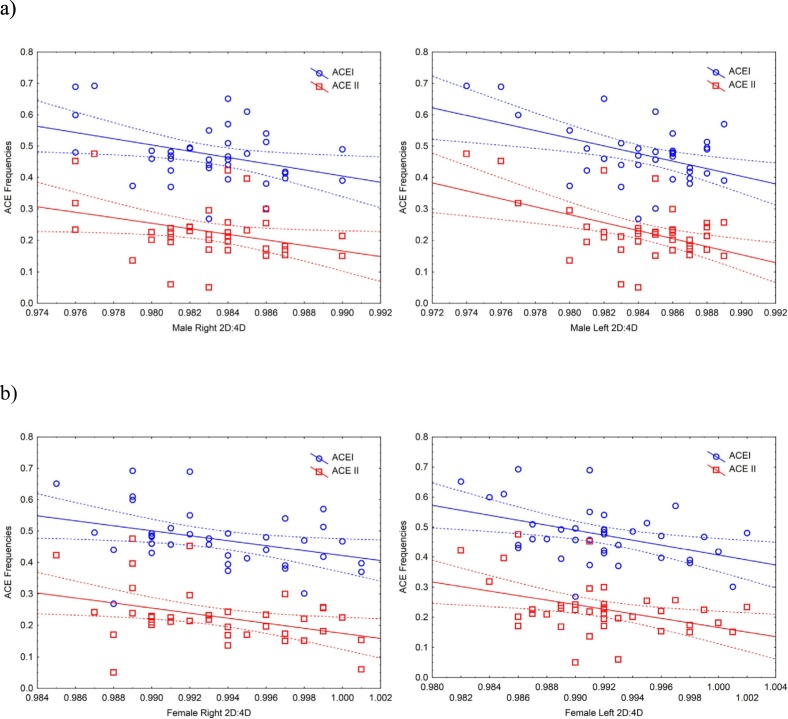
There were significant negative associations between male and female national 2D:4D and the national frequency of the I allele of ACE (n = 38, males: right 2D:4D, r = −0.367, p = .02, left 2D:4D, r = −0.458, p = .004; females: right 2D:4D, r = −0.362, p = .03, left 2D:4D, r = −0.429, p = .007). A similar pattern of associations was also found between male and female national 2D:4D and the national frequency of the II genotype of ACE (n = 39, males: right 2D:4D, r = −0.335, p = .04, left 2D:4D, r = −0.492, p = .002; females: right 2D:4D, r = −0.381, p = .02, left 2D:4D, r = −0.404, p = .01) (Fig. 1 ).
Fig. 1.
The relationship between the national frequencies of the ACE I and ACE II alleles and national mean male (a) and female (b) right and left 2D:4D in 38 nations. ACE I: males, right hand r2 = 0.13, left hand r2 = 0.21; females right hand r2 = 0.13, left hand r2 = 0.18. ACE II: males, right hand r2 = 0.11, left hand r2 = 0.24; females right hand r2 = 0.15, left hand r2 = 0.16.
3.3. 2D:4D, ACE I, ACE II and log CFR
The correlations between male national 2D:4D and log CFR for WHO 113, 127, 165 were all positive and varied from right 2D:4D = 0.293 (WHO 165) to left 2D:4D = 0.387 (WHO 165). Five were significant with p values ranging from 0.01 to 0.03 and one was non-significant with p = .06. National female 2D:4D did not correlate with log CFR, with values of r ranging from 0.006 (p = .97, WHO 165) to 0.054 (p = .74, WHO 113) (Table 2 ).
Table 2.
Relationships between national frequencies of the ACE I allele, ACE II genotype and 2D:4D (male right and left; female right and left) and log CFR's from WHO Situation Reports 113, 127 and 165.
| Log CFR's situation report 113 |
Log CFR's situation report 127 |
Log CFR's situation report 165 |
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| ACE I allele n = 38 |
−0.240 | 0.15 | −0.240 | 0.15 | −0.225 | 0.18 |
| ACE II genotype n = 39 |
−0.242 | 0.14 | −0.233 | 0.15 | −0.211 | 0.20 |
| Male R2D:4D n = 41 |
0.358 | 0.02 | 0.349 | 0.03 | 0.293 | 0.06 |
| Male L2D:4D n = 41 |
0.373 | 0.02 | 0.376 | 0.02 | 0.387 | 0.01 |
| Female R2D:4D n = 41 |
0.054 | 0.74 | 0.045 | 0.78 | 0.014 | 0.93 |
| Female L2D:4D n = 41 |
0.010 | 0.95 | 0.016 | 0.92 | 0.006 | 0.97 |
The correlations of ACE I and ACE II with log CFR were both negative. They ranged from r = −0.211 (p = .20, ACE II, WHO 165) to r = −0.242 (p = .14, ACE II, WHO 113). None were significant with p values ranging from 0.14 to 0.18 (Table 2).
We performed four multiple regressions with independent variables male 2D:4D (right or left), female 2D:4D (right or left), and ACE (I or II) for each of the dependent variables log CFR 113, 127 and 165 (i.e. 12 associations in all). Male national 2D:4D was positively associated with log CFR independent of female 2D:4D and ACE in nine relationships across the three WHO Situation reports (three for right 2D:4D and six for left 2D:4D). There were no significant associations between female 2D:4D and log CFR or ACE (I or II) and log CFR (Table 3 ).
Table 3.
Multiple regressions with independent variables (i) male right 2D:4D, female right 2D:4D, ACE I, (ii) male left 2D:4D, female left 2D:4D, ACEI, (iii) male right 2D:4D, female right 2D:4D, ACE II, (iv) male left 2D:4D, female left 2D:4D, ACE II. Dependent variables log CFR 113, log CFR 127 and log CFR 165.
| Log CFR situation report 113 |
Log CFR situation report 127 |
Log CFR situation report 165 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| r | t | p | r | t | p | r | t | p | |
| Male R2D:4D |
0.363 | 2.104 | 0.04 | 0.353 | 2.037 | 0.0495 | 0.294 | 1.663 | 0.11 |
| Female R2D:4D |
−0.176 | 1.019 | 0.32 | −0.182 | 1.051 | 0.30 | −0.193 | 1.096 | 0.28 |
| ACE I allele | −0.170 | 0.976 | 0.34 | −0.176 | 1.010 | 0.32 | −0.187 | 1.048 | 0.30 |
| Male L2D:4D | 0.50 | 2.780 | 0.009 | 0.499 | 2.768 | 0.009 | 0.526 | 2.939 | 0.006 |
| Female L2D:4D | −0.333 | 1.882 | 0.07 | −0.323 | 1.820 | 0.08 | −0.333 | 1.891 | 0.07 |
| ACE I allele | −0.153 | 0.884 | 0.38 | −0.150 | 0.862 | 0.40 | −0.126 | 0.732 | 0.47 |
| Male R2D:4D |
0.344 | 2.039 | 0.049 | 0.337 | 1.991 | 0.054 | 0.282 | 1.634 | 0.11 |
| Female R2D:4D |
−0.228 | 1.326 | 0.19 | −0.230 | 1.330 | 0.19 | −0.238 | 1.350 | 0.19 |
| ACE II genotype | −0.214 | 1.249 | 0.22 | −0.208 | 1.210 | 0.23 | −0.207 | 1.181 | 0.25 |
| Male L2D:4D | 0.495 | 2.724 | 0.01 | 0.499 | 2.739 | 0.01 | 0.531 | 2.934 | 0.006 |
| Female L2D:4D | −0.338 | 1.958 | 0.06 | −0.326 | 1.878 | 0.07 | −0.334 | 1.942 | 0.06 |
| ACE II genotype | −0.135 | 0.782 | 0.44 | −0.119 | 0.687 | 0.50 | −0.085 | 0.491 | 0.63 |
4. Discussion
We have found that national means for 2D:4D are negatively related to national values of the frequency of the ACE I alleles and the ACE II genotype. Nations with high frequencies of I also have populations with low right- and left-hand 2D:4D in both males and females. The frequency of the I allele explained 13% to 21% of the variance in 2D:4D and the frequency of the II genotype 11% to 24% of 2D:4D. We acknowledge the important limitation that this relationship is that between populations (i.e. nations) and not individuals. However, it does suggest that at the individual level the genotype of ACE (II, ID, DD) influences 2D:4D such that II genotypes have lower 2D:4D than ID or DD genotypes. This relationship is significant for both males and females.
Despite the negative association between ACE I or II and 2D:4D, the former did not correlate with CFR but the latter did. CFR's are sex-dependent (males > females) as are 2D:4D's (males < females). Thus, the variation in 2D:4D that is not dependent on ACE I/D may be influenced by a sex-dependent trait. This possibility is supported by a positive correlation between national 2D:4D and CFR, independent of ACE I/D and female national 2D:4D.
Our findings may help in part in understanding the between-individual pattern of COVID-19 severity. In this regard, we focus on the links between ACE II, 2D:4D, and oxygen metabolism. ACE II and low 2D:4D are associated with high performance in endurance sports such as distance running and rowing [12,13,18,19]. For example, 2D:4D correlated positively with finishing times in a half marathon for both men and women, i.e. those with low 2D:4D ran faster than those with high 2D:4D. However, the relationship was significantly stronger for men (right hand: r = 0.45, p < .001; left hand: r = 0.42, p < .001) than for women (right hand: r = 0.26, p < .01; left hand: r = 0.23, p = .02) [13]. The explanation may lie in higher prenatal androgenisation in the former compared to the latter. Between-individual differences in oxygen metabolism may account for much of the variance in endurance running times. Low cardiorespiratory fitness (quantifiable as VO2max) is associated with a high risk of cardiovascular disease [31]. Thus, between-individual variation in cardio-vascular fitness may contribute to between-individual variation in COVID-19 severity. At first sight, this may seem unlikely because COVID-19 mortality is higher in males than females and VO2max is higher in males than females [32]. However, we suggest that males who have experienced low prenatal testosterone and high prenatal oestrogen (high 2D:4D) have low cardiovascular fitness and high COVID-19 mortality. This group is driving high sex-dependent CFR's. Cardiovascular disease is associated with high 2D:4D [33] and the ACE DD genotype [8], and the severity of COVID-19 increases in individuals with hypertension and other cardiovascular problems [1,2].
Polymorphism at the ACE locus may influence the 2D:4D phenotype but variation in the former is discontinuous and in the latter it is continuous. Therefore, other loci may be involved. These could include genes in the classical RAS axis (ACE→angiotensin II → AT1/AT2 receptor) in which ACE is a key component of the vasoconstrictor angiotensin II production. The ACE2 → angiotensin1 – 7 → Mas receptor axis accomplishes the conversion of angiotensin II to the vasodilator angiotensin1 – 7 [7]. COVID-19 severity is sex-dependent and 2D:4D is sexually dimorphic and established in utero. Therefore, it is of interest if the genes in these two axes are polymorphic, X-linked, and involved in embryogenesis, steroidogenesis, or gametogenesis [[34], [35], [36]]. Three such genes are AT2 receptor, ACE2, and the Mas receptor. The AT2 gene is X-linked and the receptors are highly expressed in foetal tissues, although their expression dramatically decreases after birth, being restricted to a few organs, including the cardiovascular system. This suggests the gene might play a role in foetal cardiovascular development [34]. The ACE2 gene is also X-linked and has at least 32 variants. It is expressed in tissues other than the lungs and is abundant in the testes including the Leydig and Sertoli cells, suggesting its involvement in steroidogenesis in the former and spermatogenesis in the latter [35]. The Mas receptor is also expressed in the testes and deletion mutations in Mas perturb spermatogenesis reducing ejaculate size [36]. Thus, variation in the phenotype of 2D:4D may be dependent on polymorphisms in AT2, ACE2, and Mas receptor genes. If so this may explain the links between 2D:4D, prenatal androgen:oestrogen ratios, spermatogenesis, and performance in endurance sports.
We have thus far focussed on a hypothesized relationship between 2D:4D and a reduction in cardio-respiratory reserve when ACE2 is removed from the cell surfaces of the lungs. However, the eventual outcomes of COVID-19 depend in large part on complement and coagulation dysfunction (e.g., increases in fibrinogen and D-dimers) with hyper-inflammation leading to increases in severity and fatality rates [[37], [38], [39]]. Such associations depend on sex (males > females) and age, which may explain the positive relationship between male (but not female) 2D:4D and CFR's. There is accumulating evidence that fibrinogen independently predicts diseases associated with coagulation dysfunction such as myocardial infarction (MI). Fibrinogen levels are higher in males than females, increase with age and correlate positively with 2D:4D in men (i.e. men who experienced low prenatal testosterone and high oestrogen have high fibrinogen), and research suggest that high 2D:4D in men is associated with MI (for review see [33]). Cardiovascular problems are one of the comorbidities of high COVID-19 severity. High 2D:4D in men together with coagulation dysfunction may characterize several comorbidities associated with COVID-19 severity. Thus, prenatal sex steroids (as measured by 2D:4D) may relate to a complex pattern of COVID-19 severity through relationships with ACE and ACE2 function, leading to age- and sex-dependent hyper-inflammation.
In conclusion, national means for 2D:4D correlate negatively with the national frequencies of ACE I and ACE II. The relationships were significant for mean 2D:4D of men and women. The association between low 2D:4D and high ACE I/II is expected because both are linked to high performance in endurance sports and high capacity in oxygen metabolism. ACE acts as a vasoconstrictor and its action is counterbalanced by the vasodilatory effect of ACE2. COVID-19 severity is thought to initially arise because SARS-CoV2 removes ACE2 from cell surfaces thus nullifying its counterbalancing effect on ACE. There is a positive correlation between male (but not female) national 2D:4D and CFR for COVID-19 and no correlation between ACE I/II and CFR. We hypothesise that the polymorphisms in the genes of the ACE→angiotensin II → AT1/AT2 receptor and the ACE2 → angiotensin1 – 7 → Mas receptor axes may explain variation in the 2D:4D phenotype and in CFR for COVID-19. Further work should clarify the relationship of sex-and age-dependent relationships of 2D:4D and the severity of COVID-19 at the individual level.
CRediT authorship contribution statement
John Manning: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing. Bernhard Fink: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
None.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y., Liang W., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to coronavirus infection severe acute respiratory syndrome. J. Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning J.T., Fink B. Understanding COVID-19: digit ratio (2D:4D) and sex differences in national case fatality rates. Early Hum. Dev. 2020;146 doi: 10.1016/j.earlhumdev.2020.105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning J.T., Fink B. Evidence for (mis-)understanding or obfuscation in the COVID-19 and digit ratio relationship? A reply to Jones et al. (2020) Early Hum. Dev. 2020:105100. doi: 10.1016/j.earlhumdev.2020.105100. (In press) [DOI] [PubMed] [Google Scholar]
- 5.Manning J.T., Fink B. Sex differences in the relationship between digit ratio (2D:4D) and national case fatality rates for COVID-19: a reply to Sahin (2020) Early Hum. Dev. 2020;148:105120. doi: 10.1016/j.earlhumdev.2020.105120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White M.C., Fleeman R., Arnold A.C. Sex differences in the metabolic effects of the renin-angiotensin system. Biol. Sex Differ. 2019;10:31. doi: 10.1186/s13293-019-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdecchiaa P., Cavallinia C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Int. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gard P.R. Implications of the angiotensin converting enzyme gene insertion/deletion polymorphism in health and disease: a snapshot review. Int. J. Mol. Epidemiol. Genet. 2010;1:145–157. [PMC free article] [PubMed] [Google Scholar]
- 9.Manning J.T., Scutt D., Wilson J., Lewis-Jones D.I. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- 10.Rubin S.J.S., Falkson S.R., Degner N., Blish C. 2020. Clinical Characteristics Associated With COVID-19 Severity in California. medRxiv. 03.27.2004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute of Clinical Excellence (NICE) Guidelines for the use of pulse oximetry. 23rd April, 2020. https://www.nice.org.uk/guidance/ng165/chapter/3-Diagnosis-and-assessment
- 12.Manning J.T., Morris L., Caswell N. Endurance running and digit ratio (2D:4D): implications for fetal testosterone effects on running speed and vascular health. Am. J. Hum. Biol. 2007;19:416–421. doi: 10.1002/ajhb.20603. [DOI] [PubMed] [Google Scholar]
- 13.Longman D., Wells J.C., Stock J.T. Can persistence hunting signal male quality? A test considering digit ratio in endurance athletes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill R., Simpson B., Manning J.T., Kilduff L. Right-left digit ratio (2D:4D) and maximal oxygen uptake. J. Sport Sci. 2012;30:129–134. doi: 10.1080/02640414.2011.637947. [DOI] [PubMed] [Google Scholar]
- 15.Holzapfel S.D., Chomentowski P.J., III, Summers L.A.M., Sabin M.J. The relationship between digit ratio (2D:4D), VO2max, ventilatory threshold and running performance. Int. J. Sports Sci. Fit. 2016;6:1–30. [Google Scholar]
- 16.M.P. Lombardo, S. Otieno, The associations between digit ratio, aerobic fitness, physical skills, and overall physical fitness of elite youth distance runners. Am. J. Hum. Biol. doi: 10.1002/ajhb.23448. (In press). [DOI] [PubMed]
- 17.Saab Y.B., Gard P.R., Overall D.J. The geographic distribution of the ACE II genotype: a novel finding. Genet. Res. 2007;89(89):259–267. doi: 10.1017/S0016672307009019. [DOI] [PubMed] [Google Scholar]
- 18.Woods D.R., Humphries S.E., Montgomery H.E. The ACE I/D polymorphism and human physical performance. Trends Endocrinol. Metab. 2000;11:416–420. doi: 10.1016/s1043-2760(00)00310-6. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Ooi F.K., Zilfalil B.A., Yusoff S. The influence of angiotensin-converting enzyme gene ID polymorphism on human physical fitness performance in European and other populations. Sport Sci. Health. 2017;13:495–506. doi: 10.1007/s11332-016-0340-7. [DOI] [Google Scholar]
- 20.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin. Chim. Acta. 2020;505:192–193. doi: 10.1016/j.cca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Sun X., Jin L., Xue F. World-wide spatial genetic structure of angiotensin converting enzyme gene: a new evolutionary ecological evidence for the thrifty genotype hypothesis. Eur. J. Hum. Genet. 2011;19:1002–1008. doi: 10.1038/ejhg.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu X., Spaepen M., Guo C., Fagard R., Amery A., Lijnen P., Cassiman J.J. Lack of association between the I/D polymorphism of the angiotensin converting enzyme gene and essential hypertension in a Belgian population. J. Hum. Hypertens. 1994;8:683–685. [PubMed] [Google Scholar]
- 23.Li Y., Zagato L., Kuznetsova T., Tripodi G., Zerbini G., T.R., Thijs L., Manunta P., Wang Ji-G., Bianchi G., Staessen J.A. Angiotensin-converting enzyme I/D and α-adducin Gly460Trp polymorphisms from angiotensin-converting enzyme activity to cardiovascular outcome. Hypertension. 2007;49:1291–1297. doi: 10.1161/hypertensionhaa.106.085498. [DOI] [PubMed] [Google Scholar]
- 24.Kostadinova E.S., Miteva L.Y.D., Stanilova S.A. ACE serum level and I/D gene polymorphism in children with obstructive uropathies and other congenital anomalies of the kidney and urinary tract. Nephrology. 2017;22:609–616. doi: 10.1111/nep.12824. [DOI] [PubMed] [Google Scholar]
- 25.Ali A., Vasudevan R., Ismail P., Seong C.L.T., Chakravarthi S. Analysis of insertion/deletion polymorphisms of the angiotensin converting enzyme gene in Malaysian end-stage renal disease patients. J. Renin-Angiotensin-Aldosterone Syst. 2015;16:1337–1343. doi: 10.1177/1470320310392096. [DOI] [PubMed] [Google Scholar]
- 26.Goleva-Fjellet S., Bjurholt A.M., Kurel E.H., Larsen I.K., Støren Ø., Sæbø M. Distribution of allele frequencies for genes associated with physical activity and/or physical capacity in a homogenous Norwegian cohort- a cross-sectional study. BMC Genet. 2020;21:8. doi: 10.1186/s12863-020-0813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaheen G., Sajid S., Mazhar S.B., Afsar T., Almajwal A., Alam I., Jahan S. Role of ACE I/D polymorphism in pathological assessment of preeclampsia in Pakistan. Mol. Genet. Genomic Med. 2019;7:e799. doi: 10.1002/mgg3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marginean C.O., Banescu C., Duicu C., Voidazan S., Marginea C. Angiotensin-converting enzyme gene insertion/deletion polymorphism in nutritional disorders in children. Eur. J. Nutr. 2015;54:1245–1254. doi: 10.1007/s00394-014-0802-0. [DOI] [PubMed] [Google Scholar]
- 29.Lovati E., Richard A., Frey B.M., Frey F.J., Ferrari P. Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int. 2001;60(2001):46–54. doi: 10.1046/j.1523-1755.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 30.Saeed M., Saleheen D., Siddiqui S., Khan A., Butt Z.A., Frossard P.M. Association of angiotensin converting enzyme gene polymorphisms with left ventricular hypertrophy. Hypertens. Res. 2005;28:345–349. doi: 10.1291/hypres.28.345. [DOI] [PubMed] [Google Scholar]
- 31.Ross R., Blair S.N., Arena R., Church T.S., Despres J.-P., Franklin B.A., Haskell W.L., Kaminski L.A., Levine B.D. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 32.Eisenmann J.C., Laurson K.R., Welk G.J. Aerobic fitness percentiles for U.S. adolescents. Am. J. Prev. Med. 2011;41(2011):S106–S110. doi: 10.1016/j.amepre.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Manning J.T., Bundred P.E., Kasielska-Trojan A., Smith-Straney T., Mason L. Digit ratio (2D:4D), myocardial infarction and fibrinogen in men. Early Hum. Dev. 2019;133:18–22. doi: 10.1016/j.earlhumdev.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Li X.-hui, Yuan H. Angiotensin II type-2 receptor-specific effects on the cardiovascular system. Cardiovasc. Diagn. Ther. 2012;2:56–62. doi: 10.3978/j.issn.2223-3652.2012.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leal M.C., Pinheiro S.V.B., Ferreira A.J., Santos R.A.S., Bordoni L.S., Alenina N., Bader M., França L.R. The role of angiotensin-(1–7) receptor Mas in spermatogenesis in mice and rats. J. Anat. 2009;214:736–743. doi: 10.1111/j.1469-7580.2009.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramlall V., Thangaraj P.M., Meydan C., Foox J., Butler D., Kim J., May B., De Freitas J.K., Glicksberg B.S., Mason C.E., Tatonetti N.P., Shapira S.D. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020:3. doi: 10.1038/s41591-020-1021-2. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matacic C. Blood vessel injury may spur disease’s fatal second phase. Science. 2020;368:1039–1040. doi: 10.1126/science.368.6495.1039. [DOI] [PubMed] [Google Scholar]
- 39.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]