To the Editor
A novel coronavirus named COVID-19 has begun to spread in Wuhan, China from December 2019 and become a global health concern.1 Individuals with underlying diseases are at greater risk and more prone to be critically ill in case of infection so that mortality rate is 49% in subjects entering intensive care units.2 It has been reported that critically ill COVID-19 patients remarkably have elevated levels of pro-inflammatory cytokines including IL-6, G-CSF, IP10, MCP1, MIP1A, and TNFα, contributing to outbreak of cytokine storm.3 These turn of events lead to acute respiratory distress syndrome and ultimately maybe death2 and glucocorticoids were not efficient in reducing rate of mortality.4
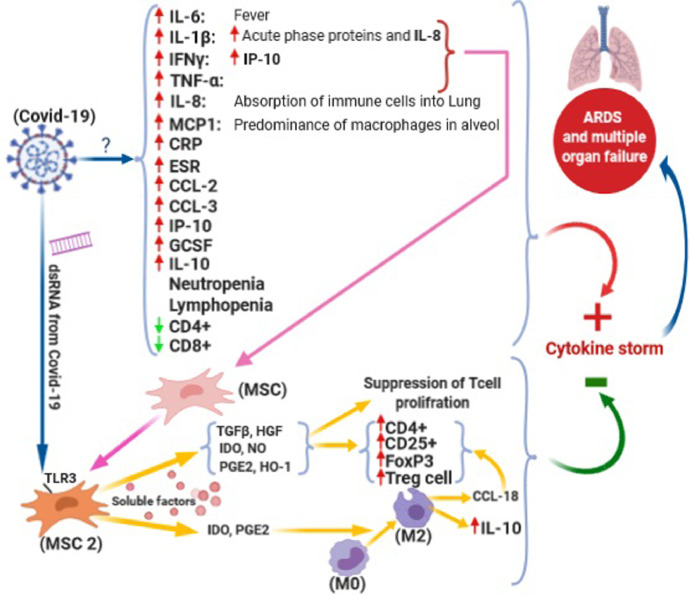
It has been suggested that mesenchymal stem cell (MSC) transplantation could be considered as a beneficial approach in treatment many disease. MSC exerts its positive impacts through immunomodulation and differentiation stimulation.5 It has proposed that pathogen molecules such as double-stranded RNA in virus is able to increase induction of toll-like receptors (TLRs) on MSC resulting in manifestation of immunomodulatory effects of MSC.6 Darwish et al. demonstrated that MSC is potentially able to treat H5N1 infection which has a cytokine profile similar to COVID-19.7 The mechanisms of action of MSC on COVID-19 treatment schematically are shown on Fig. 1 .
Fig. 1.
Mechanims of action MSC therapy on inflammatory process. COVID-19 cause a remarkable increase in pro-inflammatory and inflammatory biomarkers through unknown mechanisms which lead to a critical situation named “cytokine storm” and known as main cause of acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF). Mesenchymal stem cells (MSCs), as a new approach in COVID-19 treatment, convert to anti-inflammatory MSC (MSC2) due to the increase in levels of inflammatory factors such as interferon γ (IFN γ), TNF-α and Interleukin-1β (IL-1β). MSC2 suppress proliferation of T cells and give assistance to developing Treg cells by increase in secretion of soluble factors such as transforming growth factor beta (TGF-β), hepatocyte growth factor (HGF), Indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO), prostaglandin E2 (PGE2), and hemoxygenase (HO). The secreted IDO and PGE2 from MSC2 result in conversion of monocytes into macrophages M2 cells which produce anti-inflammatory cytokines such as Interleukin-10 (IL-10) and CC chemokine ligand 18 (CCL18). In addition to indirect impact of COVID-19 in production of MSC2 through inflammatory pathway, it also induces production of soluble factors from MSC2 through direct stimulation of toll-like receptor 3 (TLR3) in MSC2 membrane by its double stranded RNA (dsRNA). All of these processes lead to alleviation in the intensity of cytokine storm and therefore interruption in progress of ARDS and MOF. (Question mark (?): unknown mechanism; Upside arrow: Increase; Down side arrow: Decrease) (Figure is created at biorender.com)
Therefore, MSC therapy potentially could be considered as an efficacious and safe treatment approach in COVID-19-induced pneumonia. In this regard, Leng et al. recently demonstrated that with perfusion of 1 × 106 cells per kilogram of weight, almost all the clinical symptoms such as fever, breath shortness, and low oxygen saturation disappeared and the inflammation levels were relieved 2-4 days after MSC transplantation.8 Moreover, in a critically ill 65 years old woman, MSC transplantation with 5 × 107 cells three times resulted in significant decrease in CRP and increase in CD3+, CD4+ and CD8+ T cells to the normal ranges. Also, CT images implied to remarkable relieve in pneumonia.9 Furthermore, there are 30 registered studies investigating MSC therapy on COVID-19 to explore whether MSC transplantation could be able to shed the light in COVID-19 treatment. A summary of characteristics of registered studies are presented in Table 1 .
Table 1.
Characteristics of registered studies investigating the effect of stem cell therapy on COVID-19 outcomes.
| Applicant and registry number | Study design | Country | Disease | Sample size | Age (year) | Duration | Intervention | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cao Yang ChiCTR2000029580 |
RCT | China | COVID-19 with severe pneumonia | 70 | 18-75 | NR | G1: Ruxolitinib + MSC G2: SCT |
Safety, efficacy, improvement rates at 7-days and 1-month, pulmonary function, long-term disability rates and quality of life |
| 2 | Huang Guoxin ChiCTR2000029569 |
RCT | China | Severe and critical type of COVID-19 | 30 | ≥18 | NR | G1: SCT G2: MSC + SCT |
PSI, arterial blood gas analysis, mortality, hospitalization day |
| 3 | Charlie Xiang ChiCTR2000029606 |
RCT | China | COVID-19 pneumonia | 63 | 1-99 | NR (IV infusion) | G1: MSC + SCT G2: SCT G3: SCT + Artificial liver therapy G4: SCT + MSC + Artificial liver therapy G5: SCT |
Mortality, improvement rate, incidence of shock and multiple organ failure, days in hospital and ICU, ventilation modes and parameters |
| 4 | Fu-Sheng Wang NCT04252118 |
Non-RCT | China | COVID-19 pneumonia | 40 | 18-70 | 180 days (IV infusion at Day 0, Day 3, Day 6) |
G1: MSC + SCT G2: SCT |
Size of chest lesion by CT, side effects, 28 day mortality rate, CD4+ and CD8+ T cell, CRP, ALT, Creatine kinase |
| 5 | Ouyang Qi ChiCTR2000030866 |
Non-RCT | China | COVID-19 (without severe type) | 30 | ≥18 | 28 days (IV infusion at Day 0, Day 3, Day 6) | G1: MSC + SCT | Oxygenation index, mortality, total T cells, CD4 + T cells, CD8 + T cells, B cells, NK cells, IL-1β, IL-2, IL-6, IL-10, TNF-α, APACHE II score, D-dimer, CRP, procalcitonin |
| 6 | ZhiYong Peng NCT04269525 |
Non-RCT | China | COVID-19 pneumonia | 10 | 18-75 | 28 days (IV infusion at Day 1, Day 3, Day 5, Day 7) | G1: Umbilical Cord-Derived MSC | Oxygenation index, 28 day mortality, hospital stay, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, γ-IFN |
| 7 | Yongxiang Yi ChiCTR2000030300 |
Non-RCT | China | COVID-19 pneumonia | 9 | 18-75 | NR | G1: MSC | Time and rate of coronavirus become negative |
| 8 | Tianhe Stem Cell Biotech, Inc. NCT04299152 |
RCT | USA | COVID-19 pneumonia | 20 | 18-60 | 4 weeks | G1: MSC G2: SCT |
Feasibility, activated T cells, Th17, chest CT scan |
| 9 | Fu-Sheng Wang NCT04288102 |
RCT | China | COVID-19 pneumonia | 60 | 18-70 | 28 days (IV infusion at Day 0, Day 3, Day 6) |
G1: SCT + MSC G2: SCT + placebo |
Improvement in CTI, side effects of the MSC, all-cause mortality, duration of oxygen therapy and hospitalization |
| 10 | Ruijin Hospital NCT04276987 |
A Pilot Clinical Trial | China | COVID-19 with severe pneumonia | 30 | 18-75 | 28 days (aerosol inhalation at Day 1, Day 2, Day 3, Day 4, Day 5) | G1: aerosol inhalation of MSCs-derived exosomes | Adverse reaction, TTIC, duration of mechanical ventilation and ICU monitoring, rate of mortality, CRP, pro-BNP, IL-1β, IL-6, IL-8, IL-2R |
| 11 | Robert Chunhua Zhao ChiCTR2000029990 |
RCT | China | COVID-19 with Pneumonia(moderate to severe) | 120 | 18-95 | NR | G1: MSC G2: placebo (saline) |
blood oxygen saturation, recovery time |
| 12 | CAR-T Biotechnology Co., Ltd. NCT04302519 |
A Pilot Clinical Trial | China | COVID-19 pneumonia | 24 | 18-75 | 14 days (IV infusion at Day 1, Day 3, Day 7) | G1: MSC | Disappear time of ground-glass shadow in the lungs, changes of blood oxygen |
| 13 | Yang Jin NCT04273646 |
RCT | China | COVID-19 with severe pneumonia | 48 | 18-65 | 12 weeks (IV infusion at Day 1, Day 3, Day 5, Day 7) | G1: SCT + MSC G2: SCT + placebo |
Pneumonia severity index, oxygenation index, side effects, 28 day mortality rate, CRP, procalcitonin, CD3+, CD4+ and CD8+ T cell, lymphocyte count |
| 14 | Jianjun Li ChiCTR2000030484 |
RCT | China | COVID-19 pneumonia | 90 | 18-70 | NR (IV infusion at Day 1, Day 7) |
G1: MSC G2: MSC + exosomes G3: placebo |
PaO2 / FiO2 or respiratory rate, number of chest lesion by CT, time for cough and dyspnea to become mild or absent, CRP, PCT, SAA |
| 15 | Li Weilin ChiCTR2000030173 |
RCT | China | COVID-19 pneumonia | 60 | 18-60 | NR | G1: MSC G2: SCT |
pulmonary function, pulmonary CT, chest radiography, nucleic acid test |
| 16 | Jian Bo ChiCTR2000030138 |
RCT | China | COVID-19 pneumonia | 60 | 16-75 | NR (IV infusion) | G1: MSC G2: SCT + placebo |
Clinical index |
| 17 | Liu Yu ChiCTR2000030116 |
RCT | China | COVID-19 with severe pneumonia | 16 | 18-75 | 28 days | G1: MSC G2: MSC in different dose |
28 day mortality rate, incidence of long-term complications, serum inflammatory cytokines |
| 18 | Ningkun Zhang ChiCTR2000030088 |
RCT | China | COVID-19 with severe pneumonia | 40 | 18-80 | NR (IV infusion) | G1: MSC G2: normal saline |
nucleic acid of COVID-19, CT scan of ground glass shadow disappeared |
| 19 | Liu Sha ChiCTR2000030020 |
Non- RCT | China | COVID-19 pneumonia | 20 | 18-70 | NR (4 times IV infusion) | G1: MSC | FEV1, lymphocyte subpopulation changes, symptoms improved, inflammation |
| 20 | Stem Cells Arabia NCT04313322 |
Non- RCT | Jordan | COVID-19 pneumonia | 5 | ≥18 | 8 weeks (IV infusion) | G1: MSC | Improvement of clinical symptoms, Side effects, Real-Time Polymerase Chain Reaction |
| 21 | Ye Qingsong NCT04336254 |
RCT | China | COVID-19 with severe pneumonia | 20 | 18-65 | 28 days (IV infusion at Day 1, Day 4, Day 7) | G1: SCT + MSC G2: SCT + normal saline |
TTCI, Lung lesion, IL-1β, IL- 2, TNF-a, ITN-γ, IL- 4, IL- 6, IL- 10, Immunoglobulins, Lymphocyte counts, CRP |
| 22 | Azidus Brasil NCT04315987 |
Non- RCT | Brazil | COVID-19 with severe pneumonia | 66 | ≥18 | 28 days (IV infusion at Day 1, Day 3, Day 7) | G1: SCT + MSC |
Disappearance of ground-glass shadow in lung, 28 day mortality rate, CD4+ and CD8+, blood oxygen |
| 23 | Qi Zhou NCT04331613 |
Non- RCT | China | COVID-19 with severe pneumonia | 9 | ≥18 | 28 days (IV infusion) | G1: 3 G2: 5 G3: 10 million cell/kg |
Adverse reactions, Time to SARS-CoV-2, Improvement of clinical symptoms, Lymphocyte count, CRP, ALT, IL-1beta, IL-2, IL-6, IL-8 |
| 24 | Assistance Publique NCT04333368 |
RCT | France | COVID-19 with severe pneumonia | 60 | ≥18 | 28 days (IV infusion at Day 1, Day 3, Day 5) | G1: MSC G2: placebo | PaO2/FiO2 ratio, oxygenation index, mortality, IL1, IL6, IL8, TNF-alpha, IL10, TGF-beta, sRAGE, Ang2 |
| 25 | Qingsong Ye ChiCTR2000031319 |
RCT | China | COVID-19 pneumonia | 20 | 18-65 | NR (IV infusion) | G1: SCT + MSC G2: SCT + placebo |
TTCI, Immune biomarkers, Improvement of clinical symptoms, |
| 26 | Lei Shi ChiCTR2000031430 |
Non- RCT | China | COVID-19 pneumonia | 200 | 18-80 | NR (IV infusion at Day 0, Day 3, Day 6) | G1: SCT + MSC G2: SCT |
Electrocardiogram, chest CT, Blood gas analysis, blood routine, Liver and kidney function, cytokine analysis, immunoglobulins, CRP, D-Dimer |
| 27 | Leng Nannan ChiCTR2000031494 |
Non- RCT | China | COVID-19 with severe pneumonia | 36 | 18-90 | NR (IV infusion) | G1: SCT + MSC G2: SCT |
Chest imaging, lung function |
| 28 | Nader Tavakoli IRCT20140528017891N8 |
RCT | Iran | COVID-19 pneumonia | 10 | 18-95 | 28 days (IV infusion at Day 1, Day 3, Day 6) | G1: SCT + MSC G2: SCT + placebo |
Death, pneumonia severity index, oxygen index, CRP, procalcitonin, lymphocyte count, CD3 +, CD4 + and CD8 +, chest CT |
| 29 | Hassan Abolghasemi IRCT20200325046860N2 |
Non- RCT | Iran | COVID-19 pneumonia | 10 | 18-70 | 28 days (IV infusion at Day 1, Day 3, Day 6) | G1: SCT + MSC | mortality rate; duration of hospital stay, chest CT |
| 30 | Abbas Pardakhty IRCT20140911019125N6 |
Non- RCT | Iran | COVID-19 pneumonia | 10 | 18-95 | 28 days (IV infusion at Day 1) | G1: SCT + MSC | Pulmonary condition, Lymphocytes count, clinical signs |
All the registered studies are conducted on both genders
MSC: mesenchymal stem cells; SCT: supportive and conventional treatment; NR: not reported; RCT: randomized controlled trial; IV: intravenous; CT: computed tomography; G: group; CTI: critical treatment index; TTCI: time to clinical improvement; pro-BNP: pro-type B natriuretic peptide; ALT: alanine aminotransferase.
Author contribution
Masoud Khorshidi: Conceptualiation, Investigation, Methodology, Software, Visualization; Meysam Zarezadeh: Data curation, Investigation, Validation, Visualization, Writing - original draft, Writing - review and editing; Mohammadreza Emami: Data curation, Visualization; Beheshteh Olang: Data curation, Visualization, Validation; Omid Moradi Moghaddam: Conceptualization, Project administration, Supervision, Visualization.
Declaration of Competing Interest
Authors declare that there is no conflict of interest.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;17 doi: 10.1016/j.ijantimicag.2020.105924. 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arabi Y.M., Mandourah Y., Al-Hameed F. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 5.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 6.Li W., Ren G., Huang Y. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darwish I., Mubareka S., Liles W.C. Immunomodulatory therapy for severe influenza. Expert Rev Anti-Infect Ther. 2011;9(7):807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- 8.Leng Z., Zhu R., Hou W. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang B., Chen J., Li T. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine. 2020;31(99):31. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]