Abstract
Background
Coronavirus disease 2019 (COVID-19) presents with myriad extra-pulmonary manifestation and a high mortality in patients with comorbidities. Its effect on patients with pre-existing acute pancreatitis is not known.
Methods
We hereby, present 3 cases with severe acute pancreatitis with persistent respiratory failure who acquired nosocomial COVID-19 during their hospital stay after recovery from respiratory failure. Their clinical course is highlighted which reflects on pathophysiology of organ dysfunction in these 2 disease states.
Results
None of the 3 patients with severe acute pancreatitis who developed nosocomial COVID-19 redeveloped respiratory failure due to COVID-19 despite having recently recovered from pancreatitis induced acute hypoxemic respiratory failure. Only one patient developed SARS-CoV2 induced moderate pneumonia.
Conclusion
These cases highlight that host responses and mechanisms of lung injury might be different in severe acute pancreatitis and COVID-19.
Keywords: COVID-19, Acute pancreatitis, Respiratory failure, Pneumonia
1. Introduction
Acute pancreatitis (AP) may lead to organ dysfunction most commonly respiratory failure with a high mortality [1]. During the COVID-19 pandemic, hospitalized patients are at risk of cross-infection with SARS-CoV-2 which has a tropism for angiotensin converting enzyme 2 (ACE2) receptors having high expression on the pulmonary alveolar epithelial cells and endothelial cells [2,3]. COVID-19 leads to a high mortality in patients with comorbidities [4]. What clinical course does COVID-19 run in patients with acute pancreatitis and does it differ from those with virus induced AP is unknown. We present a case-series of 5 patients of whom 3 with severe acute pancreatitis and respiratory failure acquired nosocomial COVID-19 while recovering from AP. Their clinical course highlights specific host responses leading to distinct pathophysiological mechanisms in different disease states affecting a common organ i.e. lung in the present context.
2. Methods
Our hospital has created separate facilities for non-COVID and COVID-19 patients. As a policy, all patients are tested before admission and re-tested during hospitalization on suspicion of SARS-CoV2 infection and managed in the appropriate facility. We collected the data of all hospitalized patients with AP who subsequently acquired nosocomial COVID-19 and patients who presented to dedicated COVID-19 facilities with features of AP from 1st April till June 30, 2020. The severity of acute pancreatitis was graded according to revised Atlanta classification [5].The clinical severity of COVID-19 was defined according to the Indian Ministry of Health and Family Welfare (MOHFW) criteria as follows-mild disease as patients with only upper respiratory tract symptoms without any signs of breathlessness and hypoxia; moderate severity as the presence of pneumonia with the respiratory rate (RR) between 24 and 30/minute and SpO2 between 90 and 94% on room air while the severe disease was defined by the presence of pneumonia with RR > 30/minute or SpO2<90% on room air or severe respiratory distress [6].
3. Results
Of a total of 22 patients with AP admitted to non-COVID inpatient ward, 3 patients with severe AP (Table) developed nosocomial COVID-19 infection. In addition, 2 patients presenting as AP had COVID-19 infection at diagnosis.
3.1. Case 1
A 31-year-old female patient with biliary pancreatitis presented to us on the day 12 of her illness after previous hospitalization elsewhere (Table 1 ). She had respiratory failure at admission with a PaO2/FiO2 of 250 and was started on non-invasive ventilation (NIV) which improved in next few days. She developed a large peripancreatic collection in the lesser sac and extending to left paracolic gutter and a percutaneous drain was placed in view of sepsis. Drain output showed intestinal contents and communication with bowel was suspected. In view of persistent systemic inflammatory response syndrome (SIRS) she underwent surgical diversion procedure. Necrosectomy of pancreatic bed was not attempted at that time in view of frozen abdomen. Endoscopic necrosectomy was done through the percutaneous sinus tract postoperatively as reported previously (Fig. 1a) [7]. She improved after necrosectomy and was running a stable course when she developed COVID-19 at the time of a mini localized outbreak of COVID-19 in the ward. She had mild illness with upper respiratory tract symptoms and did not develop any respiratory complication due to COVID-19. She improved and was discharged uneventfully.
Table 1.
Demographic characteristics, investigations, and details of clinical course of the patients.
| Parameters | Case 1 | Case 2 | Case 3 | |||
|---|---|---|---|---|---|---|
| Age (years) | 31 | 40 | 42 | |||
| Sex | Female | Male | Male | |||
| Onset of pancreatitis | April 4, 2020 | May 2, 2020 | March 6, 2020 | |||
| Etiology of pancreatitis | Biliary | Alcohol | Biliary | |||
| Previous hospitalization | Yes | No | Yes | |||
| Date of admission | April 16, 2020 | May 5, 2020’ | May 20, 2020 | |||
| Severity of pancreatitis | Severe | Severe | Severe | |||
| Local complications | Infected WON | Infected WON | Infected WON | |||
| Systemic complications (worst grades) | Respiratory failure (grade 3) | Renal failure (grade 2) and respiratory failure (grade 3) | Respiratory failure (grade 3) | |||
| Onset of respiratory failure | April 16, 2020 | May 11, 2020 | 2nd week of March 2020a | |||
| Recovery from respiratory failure | April 20, 2020 | May 20, 2020 | 3rd week of March 2020a | |||
| Date of COVID -19 detection | June 4, 2020 | June 4, 2020 | June 4, 2020 | |||
| Severity of COVID-19 | Mild | Moderate | Mild | |||
| Date of discharge | June 12, 2020 | June 21, 2020 | June 20, 2020 | |||
| Investigations | 4/16/2020 | 3/6/2020 | 6/5/2020 | 6/18/2020 | 5/20//2020 | 6/13/2020 |
| Hb (g/dL) | 10.1 | 7.2 | 13.0 | 9.6 | 9.1 | 9 |
| TLC (x 103/mm3) | 23.5 | 11.2 | 11.9 | 4.9 | 14.3 | 8.6 |
| Platelet count (x 109/mm3) | 430 | 292 | 140 | 127 | 470 | 150 |
| Blood urea (mg/dL) | 78 | 35 | 39 | 23 | 21 | 24 |
| Creatinine (mg/dL) | 0.5 | 0.8 | 0.8 | 0.8 | 0.4 | 0.4 |
| Bilirubin (mg/dL) | 0.7 | 2.3 | 0.6 | 0.7 | 0.7 | 0.6 |
| AST (IU/L) | 37 | 47 | 46 | 134 | 32 | – |
| ALT (IU/L) | 32 | 47 | 103 | 96 | 21 | – |
| ALP (IU/L) | 222 | 389 | 210 | 116 | 265 | – |
| Total Protein (g/dL) | 5.2 | 6.7 | 5.7 | 7.0 | 6.5 | – |
| S. Albumin (g/dL) | 2.0 | 1.8 | 3.4 | 3.7 | 3.2 | – |
| INR | 1.5 | – | 1.1 | 1.2 | – | – |
| IL-6 level (pg/mL) | – | – | – | 48.70 | – | – |
| Calcium (mg/dL) | 8.2 | 7.9 | 8.1 | 8.4 | 8.5 | – |
| Procalcitonin (ng/mL) | 1.4 | – | 1.8 | – | – | – |
| Amylase at onset (IU/L) | 399 | – | 1054 | – | 900 | – |
Abbreviations- WON, walled off necrosis; Hb, hemoglobin; TLC, total leucocyte count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; INR, international normalised ratio; COVID-19, coronavirus disease 2019.
Exact date not known.
Fig. 1.
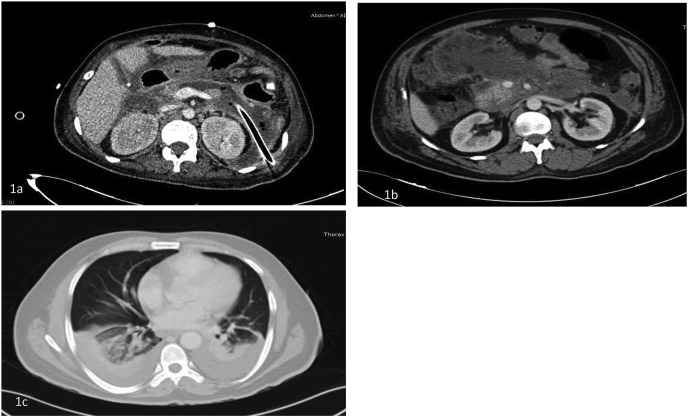
1a. Infected walled off necrosis in a patient with percutaneous catheter drain in situ who underwent percutaneous endoscopic necrosectomy, 1b. A contrast enhanced computed tomography (CT) scan of a patient showing features of acute necrotizing pancreatitis, 1c. CT scan of the chest of the same patient showing bilateral lung infiltrates after COVID-19.
3.2. Case 2
A 40-year-old male patient with chronic alcoholism presented on May 5, 2020 on the day 3 of illness. AP was diagnosed based on clinical features and laboratory investigations (Table 1). At presentation, he had acute kidney injury with a serum creatinine of 2.6 mg/dL and grade 2 respiratory failure with a PaO2/FiO2 ratio of 253. He tested negative for COVID-19 soon after the onset of respiratory failure. Renal and respiratory failure improved with conservative management. After a period of 2 weeks of gradual and continuous improvement, he developed new onset fever coinciding with the mini-outbreak of COVID-19 in the ward. A repeat reverse transcriptase-polymerase chain reaction (RT-PCR) was positive for SARS-CoV2 and he was shifted to the dedicated COVID-19 facility. He developed moderately severe COVID-19 as evidenced by lower respiratory tract involvement and presence of patchy ground glass opacities (GGOs) in bilateral lungs consistent with COVID-19 (Fig. 1b and c) requiring intermittent oxygen support but no respiratory failure. He improved with supportive therapy and was discharged.
3.3. Case 3
A 42-year-old man was admitted with us after 10 weeks of onset of acute biliary pancreatitis with infected pancreatic necrosis. He had documented respiratory failure early during the course of his illness within first two weeks of onset for which he had required non-invasive ventilatory support in another hospital. He had not responded to antibiotics and percutaneous catheter drainage (Table 1). He underwent two sessions of endoscopic lavage of the infected necrotic collection via percutaneous route at our center and improved. While improving from sepsis, he acquired COVID-19. He also did not develop any respiratory symptoms related to COVID-19 and had a mild disease which improved over 5 days.
3.4. Acute pancreatitis due to SARS-CoV2 infection
Two patients were admitted in the dedicated COVID-19 facility with upper abdominal pain in June 2020. On evaluation, they had mild cough and infiltrates on chest X-ray on day 3 of illness but no fever. Serum amylase levels were elevated (>3 times of upper limit of normal) and an abdominal ultrasound examination showed bulky pancreas with no evidence of gallstones. There was no history of intake of alcohol, trauma, or drug predisposing to AP. Both had normal serum triglycerides and calcium levels. One patient also had concomitant elevation in liver enzymes and renal injury which improved over next 5 days. Both the patients were diagnosed as moderate COVID-19 illness and mild acute pancreatitis. They improved with conservative management and discharged home over the next 10–14 days.
4. Discussion
The most important observation was that despite all 3 patients having had respiratory failure due to acute pancreatitis per se, none developed severe respiratory complication due to COVID-19. The possible explanations could be: (i) These patients had marked systemic inflammation during the initial phase of AP due to a dysregulated immune response leading to organ failure and subsequently developed compensatory anti-inflammatory response syndrome, a state of immune exhaustion [8]. Hence, they could not mount a strong inflammatory response to SARS-CoV-2; (ii) The host immune response to a viral infection is fundamentally different from that due to damage-associated molecular patterns (DAMPs) in AP and its severity may be determined by host genetic predisposition [9]; (iii) Another possibility could be that AP primed immune response which might have modulated the inflammatory response to SARS-CoV2; (iv) The pathophysiology of organ failure in COVID-19 might be unrelated to the cytokine storm and could be due to endothelialitis and vascular thrombosis seen in <10% of patients [3]; and (v) COVID-19 is mild in ∼80% of patients particularly in younger age group. The risk of developing acute respiratory distress syndrome (ARDS) is age dependent and is 5.4% for patients less than 60 years as compared to 16.9% in those over 60 years [10]. A larger prospective study evaluating the relationship between age and severity is required to support that patients with acute pancreatitis are at no greater risk than general population for developing ARDS from COVID-19 infection.
Thus, differential pathophysiological mechanisms depending on specific host responses to the inciting injury i.e. DAMPs in acute pancreatitis or virus in COVID-19 may determine the extent of lung involvement regardless of the tropism of the latter.
Despite the fact that COVID-19 is associated with poor outcomes in patients with comorbid diseases like diabetes mellitus, hypertension, obesity, coronary artery disease and COPD, the same may not hold true for patients with AP since it is an acute illness and the same may be applicable for other acute illnesses as well. The outcomes of COVID-19 may be independent of the outcome of acute pancreatitis. This is an important observation because more cases with AP might develop COVID-19 during the ongoing pandemic. This should be reassuring to both patients and treating physicians.
Declaration of competing interest
The authors do not have any conflict of interest or financial disclosure.
References
- 1.Garg P.K. Annals for hospitalists inpatient notes - clinical pearls—acute pancreatitis. Ann Intern Med. 2019 Feb 19;170(4) doi: 10.7326/M19-0008. HO2–3. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z., et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020 Jun 1;69(6):1010–1018. [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020 July 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020 May 26;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013 Jan;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Management Protocol for COVID19.pdf [Internet]. [cited 2020 Jun 14]. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf.
- 7.Jain S., Padhan R., Bopanna S., et al. Percutaneous endoscopic step-up therapy is an effective minimally invasive approach for infected necrotizing pancreatitis. Dig Dis Sci. 2020;65:615–622. doi: 10.1007/s10620-019-05696-2. [DOI] [PubMed] [Google Scholar]
- 8.Garg P.K., Singh V.P. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156(7):2008–2023. doi: 10.1053/j.gastro.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., et al. Genomewide association study of severe COVID-19 with respiratory failure. N Engl J Med [Internet] 2020 Jun 17 doi: 10.1056/NEJMoa2020283. https://www.nejm.org/doi/10.1056/NEJMoa2020283 [cited 2020 Jun 30]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L., et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin Infect Dis. 2020;28(71):740–747. doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]