Abstract
As the SARS-CoV-2 pandemic unfolds across the globe, consistent themes are emerging with regard to aspects of SARS-CoV-2 infection and its associated disease entities in children. Overall, children appear to be less frequently infected by, and affected by, SARS-CoV-2 virus and the clinical disease COVID-19. Large epidemiological studies have revealed children represent less than 2% of the total confirmed COVID-19 cases, of whom the majority experience minimal or mild disease that do not require hospitalisation. Children do not appear to be major drivers of SARS-CoV-2 transmission, with minimal secondary virus transmission demonstrated within families, schools and community settings. There are several postulated theories regarding the relatively low SARS-CoV-2 morbidity and mortality seen in children, which largely relate to differences in immune responses compared to adults, as well as differences in angiotensin converting enzyme 2 distribution that potentially limits viral entry and subsequent inflammation, hypoxia and tissue injury. The recent emergence of a multisystem inflammatory syndrome bearing temporal and serological plausibility for an immune-mediated SARS-CoV-2-related disease entity is currently under investigation. This article summarises the current available data regarding SARS-CoV-2 and the paediatric population, including the spectrum of disease in children, the role of children in virus transmission, and host-virus factors that underpin the unique aspects of SARS-CoV-2 pathogenicity in children.
Key words: SARS-CoV-2, COVID-19, paediatrics, transmission, ACE2, multisystem inflammatory syndrome
Introduction
Since its identification in January 2020 to the time of writing, SARS-CoV-2 has caused coronavirus disease (COVID-19) in almost 10 million people worldwide and resulted in 500,000 deaths.1 However, COVID-19 morbidity and mortality exhibits significant variation across age groups. Early publications revealed SARS-CoV-2 could cause a wide spectrum of illness in adults, and children were seemingly under-represented with regard to both the total number of cases as well as the likelihood of experiencing severe disease.2 , 3 Comprehensive population-based epidemiological studies have confirmed this finding, revealing a lower prevalence of SARS-CoV-2 infection in children compared to adults.4, 5, 6 Furthermore, if children do acquire SARS-CoV-2, the vast majority experience mild disease not requiring hospitalisation,3 , 7, 8, 9 and as SARS-CoV-2 disseminates globally and chains of transmission are becoming increasingly recognised, children do not appear to be efficient transmitters of infection.10, 11, 12, 13
There are a number of hypotheses postulated to explain children's lower risk of being infected by, and affected by, SARS-CoV-2. These include differences in viral kinetics, a mucosal burden of competing pathogens, and alterations in their immune response including vascular susceptibility to SARS-CoV-2 infection. Most notably, a hyperinflammatory state, with features of acute respiratory distress syndrome (ARDS), appears to be a central feature of the pathogenesis of acute severe disease in adults. Children are seemingly acutely protected from this state for reasons that are as yet unclear, yet one of the leading hypotheses regarding their protection from acute illness and transmission relates to age-related differences in expression of angiotensin converting enzyme 2 (ACE2) in different tissue types. ACE2 is a key mediator of SARS-CoV-2 host cell entry and plays an integral role in the endothelial inflammatory response. However, with the recent recognition of potential late multisystem inflammatory sequelae from SARS-CoV-2 infection, differences in the immune-mediated response to SARS-CoV-2 is also of interest.
This article presents an overview of SARS-CoV-2 infection in children based on the available data to date, including the spectrum of disease, transmission and immunopathological underpinnings, as well the possible multi-system inflammatory sequelae that may ensue.
Spectrum of disease
Since the emergence of SARS-CoV-2 as a human pathogen in the Hubei Province of China, it has become evident that children are largely spared the acute severe features of COVID-19. China was the first country to experience intense community transmission, and a review of 72,314 cases by the Chinese Center for Disease Control (CDC) revealed that less than 1% of cases occurred in children aged less than 10 years, and the vast majority of these cases were well enough to be managed in the community.14 The United States (US) has subsequently emerged as the nation with the highest rates of infection and less than 2% of affected cases are in the paediatric age range,15 which is in keeping with data collated in Italy16 and Spain.17 In New South Wales, Australia, where community testing rates have been high, children contribute only 4% of the known COVID-19 cases in the community, despite comprising 23% of the population.18
Alongside a low prevalence of diagnosed disease, children also consistently exhibit mild (if any) illness following infection with SARS-CoV-2, including some traditionally ‘high risk’ children such as those with cancer,19 bone marrow transplant recipients,20 immunosuppressed children on biological agents,21 and those with cystic fibrosis.22
The most common symptom reported in children is fever, with rhinorrhoea, cough, gastrointestinal symptoms, headache and myalgia also reported.15 , 23, 24, 25 However, a proportion of children with SARS-CoV-2 detected by real time polymerase chain reaction (RT-PCR) have no clinical or radiological features of disease.24 , 26 Mild bronchial thickening and ground-glass opacities are the main radiological findings on chest X-ray or computed tomography studies, and can be evident in asymptomatic children.24 , 27 While there are still limited data available regarding COVID-19 in infants, case reports regarding infants with SARS-CoV-2 infection (horizontally or otherwise acquired) have predominantly described asymptomatic or paucisymptomatic disease, with mild shortness of breath the most commonly reported symptom.28 Outcomes have been favourable in the vast majority of infants with SARS-CoV-2 infection, including those presenting with less common and potentially more severe signs, including encephalopathy.29 While vertical transmission has been postulated to occur,30 there is evidence suggesting perinatal acquisition is unlikely in the context of appropriate hygiene measures (adequate hand-hygiene and the use of a surgical mask in mothers positive for SARS-CoV-2) at delivery.31 Therefore, it is recommended that infants should continue to room-in with their mothers and breastfeeding should be encouraged, with these hygiene measures in place.32
Severe disease requiring admission to intensive care units (ICUs) in the acute phase of infection is rare in children. Of 1391 children assessed and tested for SARS-CoV-2 at Wuhan Children's Hospital, only 12.3% (n=171) were positive, of whom just three children (1.8%) required ICU admission.24 However, as has been evident in other reports (including a prospective surveillance study of paediatric oncology units in France),33 it is uncertain as to whether these children required ICU admission due to COVID-19 or their comorbid conditions. Whilst a review of 41 children with COVID-19 in Spain demonstrated a relatively high proportion requiring hospitalisation (25/41, 61%), only four required admission to ICU (4/41, 9.8%).17 In North America, the majority of paediatric cases have not required hospitalisation,8 , 34 and those requiring ICU admission frequently have a number of comorbidities and complex medical backgrounds that dictate their need for intensive care.35
Therefore, extrapolating conclusions from the observational data available on children who develop moderate to severe COVID-19 globally is difficult due to possible confounding by other factors. Published detailed clinical information is often lacking with regard to whether these children required high-level care due to COVID-19, or were simply RT-PCR SARS-CoV-2 positive and admitted due to an alternative diagnosis or active comorbidity at a time when community transmission of the virus in their geographic settings was high.27 Furthermore, a vast array of (not yet evidence-based) therapeutic options have been utilised in many of these descriptive studies, including antiviral agents, traditional medicine, antibiotics, and immunomodulatory agents, many of which have known adverse effects, further complicating the ability to ascertain whether complications in these cases were due to SARS-CoV-2 or other factors implicated in these presentations.36 , 37
The direct long-term outcomes for children from SARS-CoV-2 infection remains to be seen. Aside from the multisystem inflammatory sequelae potentially associated with SARS-CoV-2 (see section Multisystem inflammatory sequelae) there are no outcome data for children with SARS-CoV-2 infection beyond acute infection. There are preliminary adult data that raise concerns for the longer-term respiratory, cardiac and neurological sequelae. Observations from China in non-critical hospitalised SARS-CoV-2 adult survivors demonstrated persisting pulmonary function defects on discharge; the most common being diffusion capacity, followed by restrictive ventilatory defects, both of which correlated with the severity of acute infection.38 Myocardial involvement (as evidenced by elevated troponin levels) has been reported in up to 30% of adults hospitalised with SARS-CoV-2 infection, which may be related to myocarditis, myocardial ischaemia/infarction, cardiomyopathy or coronary vasculitis.39 The long-term prognosis for such patients is extrapolated from the long-term outcomes of other viral-associated myocardial injury (myocarditis, cardiomyopathy and vasculitis) and general myocardial insults (e.g., myocardial ischaemia/infarction). One concern that requires consideration is the long-term cardiac outcomes for children with SARS-CoV-2 who develop coronary artery abnormalities in the context of the suspected SARS-CoV-2 associated multisystem inflammatory conditions (see section Multisystem inflammatory sequelae). As may occur in other viral illnesses, there have been case reports of neurological complications in adults with SARS-CoV-2 including neuroinflammatory syndromes (such as encephalitis and acute demyelinating encephalomyelitis), stroke and peripheral nervous disorders (including Guillain–Barré syndrome, plexopathies, and cranial nerve palsies) that may lead to long-term morbidity (with prognoses extrapolated from other viral-associated and general neurological insults).40, 41, 42, 43, 44, 45, 46 However, as demonstrated in the differences regarding how children are affected by acute SARS-CoV-2 infection compared to adults, identifying and prognosticating the long-term outcomes for children must be independently evaluated to that of adults, and in the literature to date, there has not been a clear indication that these neurological complications predominate in children infected with SARS-CoV-2.
The role of children in transmission
By analogy to influenza, for which children are known to be major transmitters, there was initial concern regarding the potential role of children as ‘silent spreaders’ of SARS-CoV-2,47 , 48 and occasional early studies supported this perspective.49 As SARS-CoV-2 spreads globally, the transmission chains of SARS-CoV-2 are becoming apparent. Collectively, data suggest that children with SARS-CoV-2 acquire their infection from an adult contact, with minimal secondary transmission from children.50 Droplets are the main mechanism of transmission of SARS-CoV-2, and while some studies have revealed the presence of SARS-CoV-2 RNA detected in the stool of patients51 and on environmental surfaces,52 confirmation of viable virus of sufficient load to produce infection in other individuals has not yet been documented. To date, there are no published data confirming faecal-oral transmission of SARS-CoV-2, despite detectable virus genetic material documented in the stool of paediatric patients.53 , 54
Isolated case reports have suggested transmission from symptomatic children to household contacts,10 , 11 however these are rare. Simultaneously, other case reports support an absence of transmission even amongst symptomatic children, including one paediatric index case for whom not one of 172 contacts tested positive for SARS-CoV-2.55 One case series of household contacts revealed 3/31 (9.6%) clusters involved a child as a possible source case, compared to H5N1 avian influenza in which 54% of family clusters had a paediatric source case.12 Similarly, in school-based transmission studies in Australia involving 18 index COVID-19 cases, only two of 735 (0.3%) students became secondarily infected: one from an adolescent student, and another from an infected teacher.13 A systematic review on school closures for coronavirus control has found no conclusive evidence for a convincing impact on population-level viral transmission dynamics,56 and in Sweden (where schools and childcare centres have remained open throughout the pandemic) the prevalence of SARS-CoV-2 in children has remained very low.57
Concordant with these epidemiological findings, a recent modelling study demonstrated that susceptibility to SARS-CoV-2 infection in people under the age of 20 years is around half that of adults.58 Additionally, only 21% of infections are symptomatic in adolescents (aged 10–19 years), compared to 69% in older adults (>70 years). As a result, transmission-based interventions aimed at children, including school closures, are projected to have a relatively small impact of SARS-CoV-2 transmission dynamics.58
Indeed, there are now data from many geographic settings across Europe, Asia and America confirming that the proportion of children infected with SARS-CoV-2 in the community is low (varying from 1% in young children to 6% in older children),54 although further adequately powered seroprevalence studies are necessary to more clearly establish the transmission dynamics in children.
Despite the weight of evidence suggesting children are not major drivers of transmission of SARS-CoV-2 at a population level, children in many geographic areas have been subject to the same social isolation rules as adults, including widespread school closures. These policies have significant repercussions for education, development and wellbeing.59 Furthermore, reduced economic productivity at household as well as national levels, and negative impacts on the psychosocial wellbeing of parents and/or caregivers may be expected corollaries. Limiting transmission from adult source cases is essential through prioritisation of optimal hygiene and physical distancing measures; yet the need to enforce physical distancing in children with the same rigour (including universal school closures or restrictions) poses a risk of marked social and emotional morbidity which is not proportionate to the existing evidence for the role of children in the transmission of SARS-CoV-2.60
Diagnostic considerations
As with other human coronaviruses, the diagnostic gold standard for SARS-CoV-2 is via RT-PCR, commonly utilising genes encoding the internal RNA-dependent RNA polymerase and surface spike glycoprotein.61, 62, 63 Higher viral loads have been documented in the lower respiratory tract,64 consistent with what has been observed for SARS-CoV and Middle East respiratory syndrome (MERS).65 In some settings, where there is a strong clinical suspicion of SARS-CoV-2 yet a negative result on nasopharyngeal or throat swab, lower respiratory tract sampling is recommended;66 however, this should be reserved for the most unwell children in light of the risks associated with obtaining lower respiratory tract samples.
Buccal swabs have been explored as a more tolerable sampling method than the currently recommended nasopharyngeal or oropharyngeal specimens,67 yet studies investigating this method have included very small numbers of children and have revealed higher cycle thresholds when compared with nasal nasopharyngeal specimens, indicating a lower viral load assessed via this sampling method. Although saliva specimens have been investigated in studies involving small numbers of adults with SARS-CoV-2 infection,68 this has not been evaluated in the paediatric population. Most of the available data regarding the timing of viral detection via PCR is based on evidence obtained from the adult population, and indicates that viral detection via PCR tends to decline more quickly in upper respiratory tract samples than lower respiratory tract samples.63 SARS-CoV-2 RNA can be detected for longer periods in patients with more severe disease than in patients with mild illness.69
SARS-CoV-2 infection can also be ascertained by the detection of antibodies to SARS-CoV-2, which may be helpful in aiding in the diagnosis of post-infectious inflammatory syndromes in children. The first detectable serological marker is total antibodies,70 although IgM and IgG enzyme-linked immunosorbent assay (ELISA) have been documented as positive as early as four days after symptom onset.63 Seroconversion typically occurs in the third to fourth week after illness and ELISA-based IgM and IgG antibody tests have revealed a high specificity for the diagnosis of SARS-CoV-2,63 although false positive results have been described71 and cross-reactivity between other coronaviruses may occur.72 While specific sensitivity quantification is difficult to delineate with regard to serology in children, it is known that paucisymptomatic patients (which describes the vast majority of paediatric cases) may have low antibody concentrations and in these circumstances, false negative results are more likely to occur.73
Mechanisms of disease
Immune response
Similar to SARS-CoV, which caused the 2003 SARS epidemic, several features of SARS-CoV-2 suggest the immune response is central to disease pathogenesis. For both viruses, the delay to onset of significant symptoms points to the priming of an immune response and high levels of inflammatory markers supports an inflammatory pathogenesis, while the worsening of disease in the presence of decreasing viral titres suggests that direct viral cytopathic effects may no longer be of relevance at that time.74
In adults, it has been established that infection with SARS-CoV-2 results in a robust antiviral CD4 and CD8 T cell response75 as well as generation of virus-specific antibodies, including neutralising antibodies.76 Using single cell ribonucleic acid sequencing (scRNA-seq) several studies have now established the immune profile of adult patients with COVID-19. One study found that in hospitalised adults with COVID-19, especially those with ARDS, there is depletion of natural killer (NK) cells, dendritic cells and CD16+ monocytes, but expansion of plasmablasts.77 Further analysis revealed that type I interferon induced signatures were strong (but not universal) in all patients, although cytokine expression was not elevated in peripheral blood immune cells, suggesting an alternative source of inflammatory cytokines in COVID-19.77 Indeed, scRNA-seq of bronchoalveolar lavage immune cells has revealed the presence of aberrant pro-inflammatory macrophages, especially in patients with severe COVID-19,78 suggesting that perhaps the immune cells of resident tissues are the source of inflammatory cytokines. Another study focused on the early recovery phase of COVID-19 and identified a prominent population of CD14IL1β monocytes in the peripheral blood of adults seven days after showing a negative PCR for SARS-CoV-2, which was associated with increased signalling by inflammatory cytokines (such as type 1 interferons and IL-6 in several immune cell subsets, including monocytes, T cells and B cells).79
Very little research currently exists regarding the immune response to SARS-CoV-2 in children. Data from New York city has shown that severe disease is associated with obesity in older children, and a pro-inflammatory profile demonstrated by a high C-reactive protein (CRP) and IL-6 levels at admission.80 Therefore, it seems that a pro-inflammatory state in predisposed children may be associated with severe adult-like COVID-19. Indeed, a key unanswered research area regarding SARS-CoV-2 is how the immune response in children differs from adults, and whether this difference may explain the spectrum of disease susceptibility and severity associated with COVID-19. Postulated reasons as to why most children appear to be protected from an acute hyperinflammatory response to SARS-CoV-2 include: (i) the capacity to mount a more controlled and efficient immune response; (ii) relatively fewer co-morbidities that predispose to a pre-existing pro-inflammatory state; and (iii) relatively lower viral loads that may not trigger as intense an inflammatory response.
Other host-virus factors
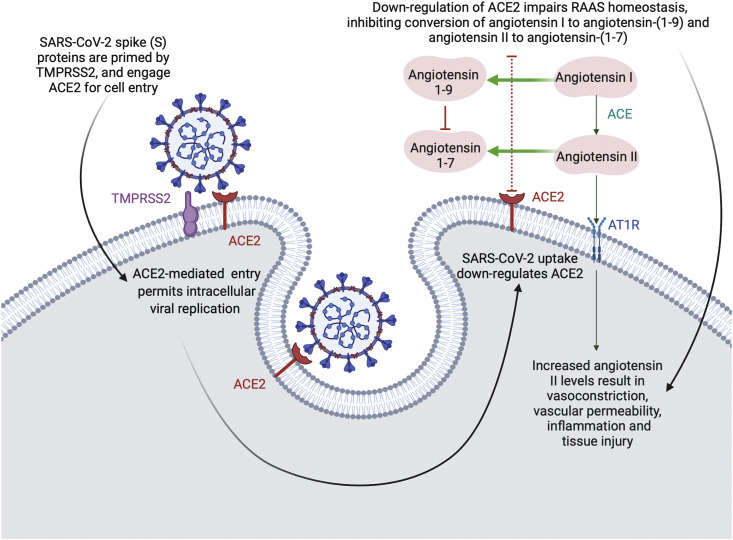
One of the most consistent and biologically plausible theories emerging in the literature regarding the mild (if any) disease SARS-CoV-2 causes in children surrounds age-related differences in ACE2. Cell entry of coronaviruses depends on the binding of the viral spike (S) protein to cellular receptors, as well as the cleavage or priming of this S protein by host cell proteases (Fig. 1 ).81 SARS-CoV-2 (and its predecessors SARS-CoV and the seasonal coronavirus, CoV-NL63) engage ACE2 for cell entry, and utilise the transmembrane protease serine 2 (TMPRSS2) for S protein priming.82 , 83 In the previous SARS-CoV epidemic, the efficiency of ACE2 usage in viral cell binding was found to be a key determinant of SARS-CoV transmissibility.84
Fig. 1.
Host cell interaction with SARS-CoV-2. ACE, angiotensin converting enzyme; AT1R, angiotensin 1 receptor; RAAS, renin-angiotensin-aldosterone system; TMPRSS2, transmembrane protease serine 2.
ACE2 is an enzyme that functions to convert angiotensin II to its metabolite angiotensin-(1–7), particularly within the lung microenvironment, where ACE2 levels are intrinsically elevated.85 Angiotensin-(1–7) has a homeostatic role in the regulation of the renin-angiotensin-aldosterone system (RAAS), a finely tuned cascade of vasoactive peptides that orchestrates key processes of human physiology.86 , 87 The RAAS is an important regulatory pathway for the cardiovascular system and the counter-regulatory enzymes ACE and ACE2 have been identified as key elements of the inflammatory processes established in conditions such as cardiac hypertrophy, pulmonary hypertension, lung injury and sepsis.88 Diminished expression of ACE2 can result in chronic heart failure and pulmonary compromise, implying a protective mechanism of ACE2 and alluding to a mechanism whereby dysregulated ACE2 in COVID-19 may imbalance the angiotensin-II/angiotensin-(1–7) equilibrium to tend towards inflammation and hypoxia.85
After gaining entry to the host cell, SARS-CoV-2 appears to down-regulate ACE2 expression on cell surfaces, thereby minimising the ability of the enzyme to exhibit its protective effects on organs.87 , 89 , 90 While ACE2 expression has been found throughout the cardiorespiratory and gastrointestinal systems (with TMPRSS2 expressed even more broadly), nasal epithelial cells exhibit the highest expression of ACE2/TMPRSS2 within the entire respiratory tract, which may explain the prominence of the symptom of anosmia in patients with COVID-19.91, 92, 93
In the lower respiratory tract, where both SARS-CoV and SARS-CoV-2 appear to have their most acute pathogenic effects, ACE2/TMPRSS2 expression has been identified in both type I and type II pneumocytes.83 , 94 , 95 In children, a higher concentration of ACE2 in pneumocytes has been documented, which may explain the protection for this age group against the severe clinical manifestations of COVID-19.85 , 96 Age-dependent ACE2 gene expression has also been documented in the nasal epithelium, with the lowest gene expression evident in younger children and increasing expression evident with age.97 As this is the first point of contact for SARS-CoV-2 in the human body, lower ACE2 expression in the nasal epithelium of children may explain the infrequent infectivity in this age group.
The RAAS derangement that is evident in many underlying chronic cardiovascular conditions, which normally do not affect paediatric populations, may be implicated in the severity of COVID-19 seen in older populations. It is becoming evident that while age is the strongest predictor of COVID-19-related death, in keeping with findings revealed by prior coronavirus epidemics, the presence of coexisting conditions—particularly chronic cardiovascular disease and obesity—are proving key prognostic determinants for COVID-19.98, 99, 100, 101
It has been hypothesised that SARS-CoV-2 may directly attack vascular endothelial cells expressing high levels of ACE2, resulting in abnormal coagulation and a sepsis-like picture.102 Dysfunction of the vascular endothelium tends to result in organ ischaemia via a shift in the vascular equilibrium towards vasoconstriction, which further exacerbates tissue inflammation.103 , 104 SARS-CoV-2 may facilitate induction of endotheliitis, as autopsy evidence has revealed the presence of viral inclusion bodies within endothelial cells alongside an accumulation of inflammatory cells, impairing microcirculatory function and resulting in clinical ischaemia of multiple organs.103 Once again, this may explain a predisposition for patients with pre-existing endothelial dysfunction (as is associated with hypertension, diabetes, established cardiovascular disease and obesity) to have the highest risk of adverse outcomes in COVID-19.103
Finally, modified endovascular responses due to developmental differences in clotting factors and endothelial cells have also been proposed as an explanation for the attenuated response to SARS-CoV-2 in children.103 , 105 Indeed, there is clear evidence that haemostasis is a developmental continuum, with reduced vitamin K dependent coagulation factors and antithrombin evident in neonates, while lower protein C and S levels persist throughout childhood.106 This contrasts to the prothrombotic milieu evident in elderly populations, which may serve as yet another mechanism for driving severe disease in this population.107
Multisystem inflammatory sequelae
In April 2020, several severely unwell children with fever and shock were described in the United Kingdom (UK) in association with SARS-CoV-2 infection.108 No such cases had been reported prior to this from China, and a solitary case from the US of Kawasaki disease (KD) with concurrent detection of SARS-CoV-2 infection had been published.109 Further cases were later identified in the UK and the condition was labelled ‘paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2’ (PIMS-TS).108 Reports subsequently emerged from other high-burden settings regarding children with fever, shock, rash and abdominal pain, some of whom met diagnostic criteria for KD;110, 111, 112, 113 many of these children were seropositive for prior SARS-CoV-2 infection and a minority showed concurrent detection of SARS-CoV-2 via RT-PCR.108 , 110 , 111 The US Centers for Disease Control and Prevention (CDC) has named the syndrome ‘multisystem inflammatory syndrome in children associated with COVID-19’ (MIS-C).114
Although the link between SARS-CoV-2 and these multisystem inflammatory syndromes requires further elucidation, there is a growing consensus that this syndrome may represent an immune-mediated complication of SARS-CoV-2 infection in children and adolescents, in light of the presence of SARS-CoV-2 antibodies in most cases and 4-week delay in timing between peaks of community SARS-CoV-2 infections and case report clusters of children presenting with inflammatory syndromes in several locations.110 , 111
One of the more comprehensive descriptive studies regarding this inflammatory syndrome depicted 58 children who presented with fever, with half having evidence of rash and gastrointestinal symptoms.115 Twenty-nine of these cases (50%) developed shock and required intensive care support. Twelve of these children (20%) met the diagnostic criteria for KD, while 24 children (40%) showed no features of KD. However, the patients described were generally older than KD cohorts and showed higher inflammatory markers than is common in KD. Eight (14%) cases developed coronary artery dilation or aneurysms, revealing a possible shared pathogenesis with KD. Almost all children recovered (1 death), with the majority receiving systemic immunomodulatory treatment. Other reports have described a high prevalence of macrophage activation syndrome in PIMS-TS, which is less common in endemic KD than in PIMS-TS.110
KD may provide a model for the pathogenesis of PIMS-TS, not least because KD has a strong association with seasonal infections, but also because it is rarely associated with intercurrent infection at the time of manifesting the vasculitic processes (as has been evident in the above described cohorts). A number of surveillance studies have been established to better monitor the emergence of this clinical entity.116 In Australia, surveillance undertaken by the Paediatric Active Enhanced Disease Surveillance network (www.paeds.org.au) has not shown an increased frequency of KD across the period of the COVID-19 epidemic, which may be anticipated in light of the low community prevalence of SARS-CoV-2 to date.
Specific, albeit provisional, recommendations for management of patients with possible PIMS-TS/MIS-C have been published by a group of specialist clinicians and endorsed by the Royal Australasian College of Physicians.117 SARS-CoV-2 serological testing during the acute presentation, and ideally prior to the administration of intravenous immunoglobulin, is a key priority.
Conclusion
Unlike many respiratory viruses, children have been relatively spared by severe illness in the global pandemic caused by SARS-CoV-2 and appear to be only minor contributors to virus transmission. These aspects of SARS-CoV-2 in children likely result from differences in their immune response to the virus, including a lower predisposition to pro-inflammatory states, fewer co-morbidities, and the differential expression of ACE2 in children, which may attenuate viral entry, as well as ongoing replication and subsequent inflammation, hypoxia and tissue injury.
At the time of writing, the multisystem inflammatory syndromes associated with SARS-CoV-2 (PIMS-TS/MIS-C), which present with KD-like clinical features, remain under investigation. Temporal and serological plausibility of a link with SARS-CoV-2 infection is supported by consistent data, but further studies are required to refine case definitions, establish SARS-CoV-2 as an inciting agent, understand the immune-mediated response to SARS-CoV-2 infection, and determine optimal management strategies.
There remain significant questions needing to be addressed regarding the intricate pathogenic mechanisms underlying the differences in SARS-CoV-2 infection between children and adults. Research to better define the immune response and assess the possibility of altered ACE2 tissue distribution and affinity for SARS-CoV-2 in the paediatric population should be a focus for future studies. Understanding the nature of host-virus interactions, and particularly which features of the paediatric immune system facilitate protection or (conversely) delayed multi-system inflammation, will shed light on SARS-CoV-2 pathogenesis and may lead to necessary improvements in therapeutic options. Furthermore, it is important that future epidemiological and clinical cohort data describe clinical comorbidities and coinfections in children with more precision, as well as delineating disease findings implicated in severe COVID-19 and potential SARS-CoV-2 multisystem inflammatory sequelae more clearly. This will enable clarification of risk factors for SARS-CoV-2-related disease and its associated disease entities in children.
Conflicts of interest and sources of funding
The authors state that there are no conflicts of interest to disclose.
References
- 1.World Health Organization Coronavirus disease (COVID-19): situation report–160. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200628-covid-19-sitrep-160.pdf?sfvrsn=2fe1c658_2 28 Jun 2020; cited 29 Jun 2020.
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. ; Mar 23: (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Gudbjartsson D.F., Helgason A., Jonsson H., et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stringhini S., Wisniak A., Piumatti G., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silveira M.F., Barros A.J.D., Horta B.L., et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26:1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 8.Pathak E.B., Salemi J.L., Sobers N., et al. COVID-19 in children in the United States: intensive care admissions, estimated total infected, and projected numbers of severe pediatric cases in 2020. J Public Health Manag Pract. 2020;26:325–333. doi: 10.1097/PHH.0000000000001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korean Society of Infectious Diseases Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, et al. Report on the Epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Q., Chen Y.C., Chen C.L., et al. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhen-Dong Y., Gao-Jun Z., Run-Ming J., et al. Clinical and transmission dynamics characteristics of 406 children with coronavirus disease 2019 in China: a review. J Infect. 2020;81:e11–e15. doi: 10.1016/j.jinf.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y., Bloxham C.J., Hulme K.D., et al. Children are unlikely to have been the primary source of household SARS-CoV-2 infections. medRxiv. 2020 ; 2020.03.26.20044826. [Google Scholar]
- 13.Macartney K., Quinn H.E., Pillsbury A.J., et al. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30251-0. ; Aug 3: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. ; Feb 24: (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 15.CDC COVID-19 Response Team Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. ; Mar 17: (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Tagarro A., Epalza C., Santos M., et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatrics. 2020;Apr 8 doi: 10.1001/jamapediatrics.2020.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NSW Health NSW COVID-19 case statistics. https://www.health.nsw.gov.au/Infectious/covid-19/Pages/stats-nsw.aspx Cited 24 May 2020.
- 19.Boulad F., Kamboj M., Bouvier N., et al. COVID-19 in children with cancer in New York City. JAMA Oncol. 2020; May 13 doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balduzzi A., Brivio E., Rovelli A., et al. Lessons after the early management of the COVID-19 outbreak in a pediatric transplant and hemato-oncology center embedded within a COVID-19 dedicated hospital in Lombardia, Italy. Estote parati. Bone Marrow Transplant. 2020 doi: 10.1038/s41409-020-0895-4. ; Apr 20: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner D., Huang Y., Martín-de-Carpi J., et al. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2020;70:727–733. doi: 10.1097/MPG.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poli P., Timpano S., Goffredo M., et al. Asymptomatic case of Covid-19 in an infant with cystic fibrosis. J Cyst Fibros. 2020;19:e18. doi: 10.1016/j.jcf.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu H., Wu J., Hong L., et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NSW Health COVID-19 weekly surveillance in NSW: epidemiological week 21, ending 23 May 2020. https://www.health.nsw.gov.au/Infectious/covid-19/Documents/COVID-19-weekly-surveillance-report-20200527.pdf Cited 27 May 2020.
- 26.Kam K.Q., Yung C.F., Cui L., et al. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis. 2020;71:847–849. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castagnoli R., Votto M., Licari A., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1467. ; Apr 22: (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Zeng L., Xia S., Yuan W., et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz N., Treptow A., Schmidt S., et al. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J. 2020;39:e212. doi: 10.1097/INF.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 30.Vivanti A.J., Vauloup-Fellous C., Prevot S., et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvatore C.M., Han J.Y., Acker K.P., et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30235-2. ; Jul 29: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization Breastfeeding and COVID-19. https://www.who.int/publications/i/item/10665332639 Cited 4 Aug 2020.
- 33.Andre N., Rouger-Gaudichon J., Brethon B., et al. COVID-19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatrics. 2020 doi: 10.1001/jamapediatrics.2020.1948. ; May 11: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun D., Li H., Lu X.X., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray P.E., Belessis Y. The use of traditional Chinese medicines to treat SARS-CoV-2 may cause more harm than good. Pharmacol Res. 2020;156:104776. doi: 10.1016/j.phrs.2020.104776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.026. ; Jun 26: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020 doi: 10.1093/brain/awaa240. ; Jul 8: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriguchi T., Harii N., Goto J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyrouti R., Adams M.E., Benjamin L., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poyiadji N., Shahin G., Noujaim D., et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toscano G., Palmerini F., Ravaglia S., et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanin L., Saraceno G., Panciani P.P., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162:1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelvin A.A., Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis. 2020;20:633–634. doi: 10.1016/S1473-3099(20)30236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passanisi S., Lombardo F., Salzano G., et al. Are children most of the submerged part of SARS-CoV-2 iceberg? Front Pediatr. 2020;8:213. doi: 10.3389/fped.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bi Q., Wu Y., Mei S., et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei M., Yuan J., Liu Y., et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dona D., Minotti C., Costenaro P., et al. Fecal-oral transmission of SARS-CoV-2 in children: is it time to change our approach? Pediatr Infect Dis J. 2020;39:e133–e134. doi: 10.1097/INF.0000000000002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Y., Li X., Zhu B., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X., Xu W., Dozier M., et al. The role of children in transmission of SARS-CoV-2: a rapid review. J Glob Health. 2020;10 doi: 10.7189/jogh.10.011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danis K., Epaulard O., Bénet T., et al. Cluster of coronavirus disease 2019 (Covid-19) in the French Alps, 2020. Clin Infect Dis. 2020;71:825–832. doi: 10.1093/cid/ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viner R.M., Russell S.J., Croker H., et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4:397–404. doi: 10.1016/S2352-4642(20)30095-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hildenwall H., Luthander J., Rhedin S., et al. Paediatric COVID-19 admissions in a region with open schools during the two first months of the pandemic. Acta Paediatr. 2020 doi: 10.1111/apa.15432. ; Jun 21: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davies N.G., Klepac P., Liu Y., et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 59.Roberton T., Carter E.D., Chou V.B., et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e901–e908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munro A.P.S., Faust S.N. Children are not COVID-19 super spreaders: time to go back to school. Arch Dis Child. 2020;105:618–619. doi: 10.1136/archdischild-2020-319474. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.8259. ; May 6: (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 64.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Memish Z.A., Al-Tawfiq J.A., Makhdoom H.Q., et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Unites States National Institutes of Health COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/critical-care/laboratory-diagnosis/ Cited 4 Aug 2020. [PubMed]
- 67.Kam K.Q., Yung C.F., Maiwald M., et al. Clinical utility of buccal swabs for severe acute respiratory syndrome coronavirus 2 detection in coronavirus disease 2019-infected children. J Pediatr Infect Dis Soc. 2020;9:370–372. doi: 10.1093/jpids/piaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azzi L., Carcano G., Gianfagna F., et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81:e45–e50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou B., Li T., Zheng S., et al. Serology characteristics of SARS-CoV-2 infection since the exposure and post symptoms onset. medRxiv. 2020 doi: 10.1183/13993003.00763-2020. ; 2020.03.23.20041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan S., Nakajima R., Jain A., et al. Analysis of serologic cross-reactivity between common human coronaviruses and SARS-CoV-2 using coronavirus antigen microarray. bioRxiv. 2020 ; 2020.03.24.006544. [Google Scholar]
- 72.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peeling R.W., Wedderburn C.J., Garcia P.J., et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30517-X. ; Jul 17: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grifoni A., Weiskopf D., Ramirez S.I., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suthar M.S., Zimmerman M., Kauffman R., et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. medRxiv. 2020 doi: 10.1016/j.xcrm.2020.100040. ; 2020.05.03.20084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilk A.J., Rustagi A., Zhao N.Q., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao M., Liu Y., Yuan J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 79.Wen W., Su W., Tang H., et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zachariah P., Johnson C.L., Halabi K.C., et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a Children's Hospital in New York City, New York. JAMA Pediatr. 2020;Jun 3 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li W., Moore M.J., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsuyama S., Nagata N., Shirato K., et al. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W., Zhang C., Sui J., et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cristiani L., Mancino E., Matera L., et al. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. 2020;55:2000749. doi: 10.1183/13993003.00749-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tikellis C., Bernardi S., Burns W.C. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens. 2011;20:62–68. doi: 10.1097/MNH.0b013e328341164a. [DOI] [PubMed] [Google Scholar]
- 87.Vaduganathan M., Vardeny O., Michel T., et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaddam R.R., Chambers S., Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets. 2014;13:224–234. doi: 10.2174/1871528113666140713164506. [DOI] [PubMed] [Google Scholar]
- 89.Glowacka I., Bertram S., Herzog P., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sungnak W., Huang N., Becavin C., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., et al. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30293-0. ; Apr 15: (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamming I., Timens W., Bulthuis M.L., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donaldson S.H., Hirsh A., Li D.C., et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- 96.Lee P.I., Hu Y.L., Chen P.Y., et al. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Al-Baadani A.M., Elzein F.E., Alhemyadi S.A., et al. Characteristics and outcome of viral pneumonia caused by influenza and Middle East respiratory syndrome-coronavirus infections: a 4-year experience from a tertiary care center. Ann Thorac Med. 2019;14:179–185. doi: 10.4103/atm.ATM_179_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alraddadi B.M., Watson J.T., Almarashi A., et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H., Liu L., Zhang D., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 105.Shi Y., Wang Y., Shao C., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Favaloro E.J., Lippi G. Translational aspects of developmental hemostasis: infants and children are not miniature adults and even adults may be different. Ann Transl Med. 2017;5:212. doi: 10.21037/atm.2017.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Riphagen S., Gomez X., Gonzalez-Martinez C., et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones V.G., Mills M., Suarez D., et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 110.Verdoni L., Mazza A., Gervasoni A., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toubiana J., Poirault C., Corsia A., et al. Outbreak of Kawasaki disease in children during COVID-19 pandemic: a prospective observational study in Paris, France. medRxiv. 2020 doi: 10.1136/bmj.m2094. ; 2020.05.10.20097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.New York City Health Department Health Alert #13: pediatric multi-system inflammatory syndrome potentially associated with COVID-19. https://www.yu.edu/sites/default/files/inline-files/20200504_HAN13_COVID19_FINAL_0.pdf Cited 11 Jun 2020.
- 113.European Centre for Disease Prevention and Control Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children – 15 May 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf Cited 11 Jun 2020.
- 114.United States Centers for Disease Control and Prevention Emergency preparedness and response: multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). https://emergency.cdc.gov/han/2020/han00432.asp Cited 11 Jun 2020.
- 115.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020; Jun 8 doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paediatric Active Enhanced Disease Surveillance (PAEDS) Network and Royal Australasian College of Physicians. Advice for clinicians: paediatric inflammatory multisystem syndrome temporally associated with SARS-COV-2 (PIMS-TS): a statement from the Acute Inflammatory Vasculitis Working Group and the Paediatric Active Enhanced Disease Surveillance (PAEDS) Network. National Centre for Immunisation Research and Surveillance,; Sydney: 2020. http://ncirs.org.au/sites/default/files/2020-06/PIMS-TS/statement_Final_June/2020.docx.pdf [Google Scholar]