Abstract
Objective:
Despite preliminary evidence of unique acute cognitive and psychopharmacological changes attributable to combined alcohol and cannabis use, few studies have investigated more chronic effects of same-day co-use, particularly during neurodevelopmentally sensitive periods. Therefore, relationships between past-month binge alcohol and cannabis co-use and cognitive functioning were examined in adolescents and young adults.
Method:
Data from the Imaging Data in Emerging Adults with Addiction (IDEAA) Consortium were used to assess cognitive functioning in emerging adults with a large range of substance use (n = 232; 15–26 years old) who were abstinent for at least 3 weeks. Multiple regressions assessed cognitive functioning by past-month binge episodes, cannabis use episodes, and same-day co-use, controlling for covariates (e.g., study site, sex, age).
Results:
After correcting for multiple comparisons, more past-month co-use episodes were related to decreased Ruff 2&7 selective attention accuracy (p = .036). Sex significantly covaried with California Verbal Learning Test–Second Edition initial learning.
Conclusions:
Although few significant relationships were found and effect sizes are modest, the persistence of an effect on attention despite a period of sustained abstinence highlights the need to carefully investigate patterns of substance use and potential independent and interactive effects on the developing brain.
Growing evidence suggests binge alcohol use and cannabis independently relate to deficits in cognitive functioning (Lisdahl et al., 2013), particularly during neurodevelopmentally sensitive periods (e.g., in adolescence and young adulthood). This is concerning given that cannabis use is positively correlated with alcohol use (Johnston et al., 2013) and 20% of high school seniors report simultaneously using both cannabis and alcohol in the past year, whereas 15% of young adults (ages 18–29 years old) report using both substances together in the past year (Subbaraman & Kerr, 2015; Terry-McElrath et al., 2013). The endocannabinoid system also undergoes neuromaturation during adolescence and emerging adulthood, making it more vulnerable to exogenous cannabinoids and their deleterious effects on the endocannabinoid system, morphological changes, and overall functioning (Schneider, 2008).
In humans, cannabinoid 1 (CB1) receptor activity is downregulated by chronic cannabis (Hirvonen et al., 2012) and alcohol use (Hirvonen et al., 2013). Other research suggests that manipulating the endocannabinoid system has been shown to alter cognitive performance in preclinical and human models (for review, see Mechoulam & Parker, 2013). Further, more robust psychopharmacological effects on cognition are suggested by some (e.g., Chait & Perry, 1994), although not all (e.g., Ballard & de Wit, 2011), studies of individuals who used the two substances together at the exact same/closely overlapping time point (also referred to as simultaneous use).
When examining the chronic effects of regular cannabis and alcohol use in adolescents, Winward and colleagues (2014) found that a more profound effect of combined use resulted in poorer working memory and mathematical abilities relative to controls and single-substance users. In contrast, Mahmood and colleagues (2010) found that individuals who used both cannabis and alcohol had better verbal memory in comparison with individuals who had more lifetime hangover or withdrawal symptoms and did not use cannabis.
Studies such as these (Mahmood et al., 2010; Winward et al., 2014) investigate “concurrent” substance use, wherein participants report using both substances but have no required overlap in use. Although these findings are important, they may be limited by considering group rather than dose-dependent impact of substance use and by not assessing for same-day co-use (wherein there is a set time frame of 1 day in which both substances need to be consumed). For instance, we recently reported that more past-month co-use episodes were related to lower white matter integrity (Wade et al., 2020), although cognitive correlates have not yet been assessed.
Given the potential for underlying mechanistic interactions that may relate to neuroanatomical and functional changes, additional research into the effects of co-occurring cannabis and alcohol use is needed. Despite preliminary evidence of unique acute cognitive and psychopharmacological changes due to combined alcohol and cannabis use, few studies have investigated more chronic effects of same-day co-use. Therefore, in the current study, relationships between past-month binge alcohol and cannabis co-use and cognitive functioning were examined. We predicted that past-month binge episodes, cannabis episodes, and same-day co-use would be adversely related to cognitive functioning, with co-use demonstrating a unique contribution.
Method
Participants
The present study analyzed data collected by the Imaging Data in Emerging Adults with Addiction (IDEAA) Consortium (principal investigators: Krista M. Lisdahl, Staci A. Gruber, Francesca M. Filbey, and Susan F. Tapert), a post hoc data compilation of well-characterized emerging adult substance users at five different sites across the United States. Data from each site were included in a single combined data set drawn from the IDEAA Consortium principal investigators’ individual projects. Data from the University of Wisconsin–Milwaukee (UWM) and the University of California, San Diego (UCSD), were used in the present study because they had the greatest overlap of neurocognitive tasks and study protocol design, including an extended period of monitored abstinence.
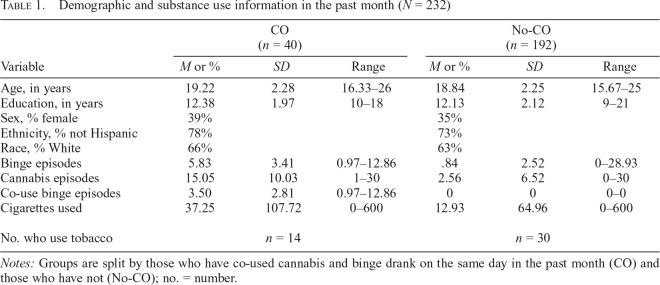
Data from 232 individuals, ages 15–26 years old, were used for the present study. The Institutional Review Boards at the UWM, Medical College of Wisconsin, and UCSD approved all aspects of this study, and all participants provided written informed consent (or parental informed consent and participant informed assent, if younger than 18 years old). Participants were recruited through flyers in the community and newspaper ads. Participants include individuals who had no substance use up to regular (at least weekly) substance users, as well as anyone in between those two extremes. To better understand those who co-use alcohol and cannabis, two groups were formed for descriptive purposes: a co-use group of those who have engaged in binge alcohol use and cannabis use on the same day in the past month (CO) and those who did not (No-CO; Table 1).
Table 1.
Demographic and substance use information in the past month (N = 232)
| Variable | CO (n = 40) |
No-CO (n = 192) |
||||
| M or % | SD | Range | M or % | SD | Range | |
| Age, in years | 19.22 | 2.28 | 16.33–26 | 18.84 | 2.25 | 15.67–25 |
| Education, in years | 12.38 | 1.97 | 10–18 | 12.13 | 2.12 | 9–21 |
| Sex, % female | 39% | 35% | ||||
| Ethnicity, % not Hispanic | 78% | 73% | ||||
| Race, % White | 66% | 63% | ||||
| Binge episodes | 5.83 | 3.41 | 0.97–12.86 | .84 | 2.52 | 0–28.93 |
| Cannabis episodes | 15.05 | 10.03 | 1–30 | 2.56 | 6.52 | 0–30 |
| Co-use binge episodes | 3.50 | 2.81 | 0.97–12.86 | 0 | 0 | 0–0 |
| Cigarettes used | 37.25 | 107.72 | 0–600 | 12.93 | 64.96 | 0–600 |
| No. who use tobacco | n = 14 | n = 30 | ||||
Notes: Groups are split by those who have co-used cannabis and binge drank on the same day in the past month (CO) and those who have not (No-CO); no. = number.
Inclusion criteria were completion of parent protocols from UCSD or UWM and at least 3 weeks of abstinence from alcohol, cannabis, or other drug use. Exclusion criteria were current use of psychotropic medication, being lefthanded, lifetime history of serious neurologic injuries or disorders, major medical illness, diagnosis of an independent Axis I psychiatric disorder in past year (except for substance abuse or dependence in any of the substance users), more than 50 lifetime use episodes of any other illicit substances, pregnancy, or magnetic resonance imaging contraindications (e.g., metal anywhere in or on the body, greater than 250 lbs., claustrophobia). Alcohol breath analysis and urine toxicology screens verified abstinence at study sessions.
Procedure
Eligible participants completed each respective parent study protocol; youth were asked to remain abstinent from all substance use other than cigarettes for a minimum of 3 or 4 weeks before session start for UWM and UCSD, respectively. For the present analyses, data from UWM and UCSD were used because they were the most similarly harmonized for the particular study questions regarding cognitive functioning after sustained abstinence. All sites completed breath alcohol analysis and toxicology tests to confirm abstinence through weekly visits and pregnancy tests for female subjects. Positive results on either the pregnancy, toxicology, or breath alcohol analysis tests rendered participants ineligible. Participants were asked not to smoke cigarettes within an hour of their study sessions, and they were not allowed to smoke during the study session. Participants were given questionnaires to assess mood and psychological variables and then completed the neuroimaging and neurocognitive testing protocols, with neurocognitive data presented here. All participants were compensated for their time.
Measures
Recent drug use.
Drug use history was collected using the Timeline Followback (TLFB; Sobell & Sobell, 1992). Using a calendar to cue special dates and holidays, participants were asked to recount when they used alcohol at binge levels (episodes of >4 standard drinks for females and >5 standard drinks for males), cannabis (episodes), and co-occurring alcohol and cannabis (days of co-use). Data for past month of use were used for this study (30 days), as sites collected varying lengths of time (up to 1 year), with past-month substance use calculated from the first day of study participation and immediately before the initiation of the abstinence requirement. TLFB was used to calculate past-month cannabis use, alcohol use, co-use days, binge alcohol episode, and co-use-binge days to allow for dose-dependent analyses; all substance use data were calculated orthogonally due to multicollinearity concerns in some analyses. Data measurement for both sites was coded by trained resident assistants for the IDEAA Consortium to ensure consistency.
Cognitive tasks.
Participants at both UCSD and UWM completed a harmonized neuropsychological battery, including the following: (1) Ruff 2&7 (Ruff & Allen, 1996), a measure of selective attention subdivided into accuracy and speed (total standard score for each; n = 101); (2) Delis–Kaplan Executive Function System (D-KEFS; Delis et al., 2001) trail making tests (Condition 4: switching/inhibition; Condition 5: motor speed; n = 232), verbal fluency (using letters F-A-S; n = 232), and Color–Word Interference Test (inhibition/switching condition; n = 151); (3) California Verbal Learning Test–Second Edition (CVLT-II; Delis et al., 2000) initial and total learning (Trial 1 and total Trials 1–5, respectively; n = 231) and memory (delayed free recall; n = 230); (4) Weschler Adult Intelligence Scale–Third Edition (WAIS-III; Weschler, 1997) Letter-Number Sequencing (n = 231); and (5) Rey Complex Figure Task (RCFT; Meyers & Meyers 1995) copy and delayed recall (n = 224).
Normed scores were used in all analyses, with the exception of the RCFT and CVLT-II Trials 1–5, as sites differed in calculation of normed performance and therefore raw performance scores were used. Differences in sample sizes for administered tasks are largely due to the release of findings that suggested certain tasks (i.e., Ruff 2&7 and D-KEFS Color–Word Interference Test) are highly relevant for substance-using adolescents after protocol onset; thus, protocols were amended to include these tasks. Given differences in sample size for the Ruff 2&7 and Color–Word Interference Test, differences for those who completed these tasks and those who did not were assessed. For both tasks, there were no substance use differences between task completers and noncompleters. However, in both instances, groups did differ by age, Ruff 2&7: F(232) = 68.57, p < .001; Color-Word Interference Test: F(232) = 37.126, p < .001. Because both tasks are age-normed, age was not included as a covariate.
Data analysis
Multiple regression analyses assessed cognitive functioning by past-month binge episodes, cannabis use episodes, and same-day binge co-use, controlling for covariates (i.e., study site, sex, age, as applicable). Study site was included as a covariate for any neurocognitive measures that differed in performance by study site (Ruff 2&7 Accuracy and Speed, RCFT Copy and Delay, D-KEFS Trail 4; ps < .05). Regressions for dependent variables (DVs) that included normed age did not covary for age. Twelve regression analyses were completed which assessed for the influence of outliers; if outliers were discovered, findings were confirmed without the outlier; in no instance did this change the overall findings. All primary regression analyses were corrected for multiple comparisons using the false discovery rate (FDR) method (Benjamini & Hochberg, 1995). Covariates included study site (when sites differed by performance on a particular task), age (when tasks did not include age-normed scores), and sex. Past-month cigarette use was also considered as a potential covariate; results remained unchanged whether or not it was included.
Results
More past-month co-use episodes of cannabis and binge alcohol use related to poorer Ruff 2&7 accuracy (β = -.343; t = -2.920, p = .004; FDR p = .036; ΔR = .084). Although there were several cannabis-only and alcohol-only relationships demonstrated, they did not survive correction for multiple comparisons (see Supplemental Material). (Supplemental Material appears as an online-only addendum to this article on the journal’s website.)
Sex was significantly associated with CVLT-II Trial 1 recall (β = -.241; t = -3.697, p < .001; FDR p = .001; ΔR = .057), with males demonstrating better performance. As sex significantly covaried with some findings, additional post hoc analyses were conducted to assess whether there may be an interaction between sex and substance use patterns in cognitive performance associated with sex (i.e., CVLT-II Trial 1). Sex did not significantly moderate the aforementioned main findings.
Detailed results for regression models and post hoc analyses are reported in the Supplemental Material.
Discussion
The present results suggest a differential influence of patterns of substance use on cognition after a period of at least 3 weeks of abstinence in adolescents and young adults. Specifically, same-day binge alcohol and cannabis co-use related to poorer selective attention accuracy after controlling for cannabis-only and alcohol-only drinking days.
Of particular interest, the present results revealed that co-use was associated with poorer attention. Such results are consistent with prior evidence of a more negative relationship between co-use and working memory (Winward et al., 2014), which relies heavily on attention capacity. In addition, this maps onto prior structural findings of more past-month co-use episodes being related to poorer white matter integrity in the cingulate gyrus in an overlapping sample of participants (Wade et al., 2020). The cingulum is key in many aspects of higher order cognitive functioning, including attention (Catani & Thiebaut de Schotten, 2012). Co-use may, therefore, influence structural pathways necessary to maintain attention, representing important brain–behavior relationship changes. However, because the present data are cross-sectional, the exact directionality of these findings should be confirmed in future longitudinal studies.
In contrast to our hypotheses, no other cognitive domains showed a significant relationship with either co-use or other substance use patterns after 3 weeks of abstinence. Although cannabis use was linked with poorer verbal memory, this did not survive correction for multiple comparisons. A growing body of evidence suggests that, following 1 week to a month of abstinence, cognitive functioning in substance users (and particularly in cannabis users) is no different than in healthy controls (Hanson et al., 2010; Roten et al., 2015; Schuster et al., 2018; Wallace et al., 2020). Although the domains of recovery have differed (e.g., some show recovery in verbal memory, whereas others show recovery in attention) and there are differences in the age group sampled, results are hopeful for teens and young adults who seek to abstain or reduce substance use for extended periods. This further fits with the suggested underlying mechanism of endocannabinoid system downregulation relating to cognitive functioning, as the binding within the endocannabinoid system recovers following abstinence from cannabis (Hirvonen et al., 2012). Many emerging adults are placed in high-pressure situations that depend on attention, memory, and executive functioning for classroom or standardized testing situations (e.g., SAT and ACT exams). Therefore, interventions that encourage reduction of or abstinence from substance use for a minimum of several days may be beneficial for achieving optimal cognitive functioning and improving outcomes at key lifetime points (e.g., during finals week).
Few significant relationships were found, and effect sizes are modest, consistent with a recent meta-analysis of cognitive relationships with cannabis use following extended abstinence beyond 3 days (Scott et al., 2018). Regardless, it is concerning that any significant relationships were found, given that participants were abstinent for at least 21 days, and co-occurring use of alcohol and cannabis has been linked to decreased white matter integrity (Wade et al., 2020), poorer treatment outcomes, higher rates of depression, higher positive expectancies of use, and—in turn—increased use of any substance (Aharonovich et al., 2005; Lopez-Quintero et al., 2011). On balance, it may be that individuals with lower cognitive performance (or other outcomes) may be more drawn to use substances in the first place. Because the impact of even slight changes in cognition and brain-behavior relationships may have great implications for life outcomes, large, prospective longitudinal studies are necessary. The Adolescent Brain Cognitive Development Study (www.abcd-study.org) has superior statistical power and a prospective longitudinal design that will be better able to delineate characteristics that may be most related to cognitive functioning.
Results suggest that males in this sample performed better on a verbal learning task. Other research suggests that males and females are more similar than different, but modest cognitive differences do exist (Hyde, 2016). Although verbal memory is typically associated with better performance in females, recent research suggests that it may be an age by sex quadratic effect because in females, verbal memory abilities increase until middle adulthood then taper off, whereas in males, verbal memory peaks in young adulthood (Graves et al., 2017), perhaps consistent with the present results. Sex, then, warrants greater consideration in any assessment of cognitive functioning, including in future research of co-occurring substance use.
Limitations to the present results include a lack of common measurement of IQ, preventing inclusion of IQ as a potential covariate. Results are cross-sectional, and therefore no causal relationships can be established. The length of abstinence, although novel in its ability to potentially elucidate long-term associations, does not clarify immediate or short-term relationships between patterns of substance co-use and cognitive functioning. Finally, co-use is a nuanced issue that requires complex measurement. Data here are presented orthogonally, but other methods with larger samples may be better suited to more carefully delineate the unique contribution of each pattern of substance use to related cognitive performance.
Taken together, results highlight the need to carefully investigate patterns of co-substance use and potential independent and interactive effects. This is particularly important in emerging adults, as the impact on the developing brain may be greater than use later in life. Further, given the changing landscape of public policy around cannabis use and understanding its nuanced effects, including co-use with alcohol, are important in preventing further public health issues that may arise.
Footnotes
Data drawn from the Imaging Data in Emerging Adults with Addiction (IDEAA) Consortium, a National Institute on Drug Abuse (NIDA)-funded project providing combined neuroimaging and neurocognitive data from well-characterized cannabis users. The IDEAA Consortium was supported by NIDA Grants R01 DA032646 (principal investigator: Staci A. Gruber) and R01 DA030354 (principal investigator: Krista M. Lisdahl). Staci A. Gruber’s work was supported by Grants DA032646,DA016695,andDA021241.Susan F.Tapert’s work was supported by Grants R01AA03419, P20 DA024194, and R01 DA021182. Francesca M. Filbey’s work was supported by Grants R01 DA042490, R01 DA030344, R01 AA023658, and the Bert Moore Chair in BrainHealth. Manuscript preparation was supported by Grants T32AA013525 (principal investigator: Edward Riley/Susan F. Tapert) and U01 DA041025 (principal investigator: Krista M. Lisdahl).
References
- Aharonovich E., Liu X., Samet S., Nunes E., Waxman R., Hasin D. Postdischarge cannabis use and its relationship to cocaine, alcohol, and heroin use: A prospective study. American Journal of Psychiatry. 2005;162:1507–1514. doi: 10.1176/appi.ajp.162.8.1507. doi:10.1176/appi.ajp.162.8.1507. [DOI] [PubMed] [Google Scholar]
- Ballard M. E., de Wit H. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers. Pharmacology, Biochemistry, and Behavior. 2011;97:627–631. doi: 10.1016/j.pbb.2010.11.013. doi:10.1016/j.pbb.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B. Methodological. 1995;57:289–300. doi:10.1111/j.2517-6161.1995.tb02031.x. [Google Scholar]
- Catani M., Thiebaut de Schotten M. Atlas of human brain connections. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Chait L. D., Perry J. L. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994;115:340–349. doi: 10.1007/BF02245075. doi:10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., Kramer J. H. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. California Verbal Learning Test–Second Edition. Adult Version. Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Graves L. V., Moreno C. C., Seewald M., Holden H. M., Van Etten E. J., Uttarwar V., Gilbert P. E. Effects of age and gender on recall and recognition discriminability. Archives of Clinical Neuropsychology. 2017;32:972–979. doi: 10.1093/arclin/acx024. doi:10.1093/arclin/acx024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. L., Winward J. L., Schweinsburg A. D., Medina K. L., Brown S. A., Tapert S. F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. doi:10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J., Goodwin R. S., Li C. T., Terry G. E., Zoghbi S. S., Morse C., Innis R. B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. doi:10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J., Zanotti-Fregonara P., Umhau J. C., George D. T., Rallis-Frutos D., Lyoo C. H., Heilig M. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Molecular Psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012.100. doi:10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J. S. Sex and cognition: Gender and cognitive functions. Current Opinion in Neurobiology. 2016;38:53–56. doi: 10.1016/j.conb.2016.02.007. doi:10.1016/j.conb.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Johnston L. D., O’Malley P. M., Bachman J. G., Schulenberg J. E. Monitoring the Future national survey results on drug use, 1975-2012: Volume 1, Secondary school students. Ann Arbor, MI: Institute for Social Research; 2013. [Google Scholar]
- Lisdahl K. M., Gilbart E. R., Wright N. E., Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. doi:10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C., Hasin D. S., de Los Cobos J. P., Pines A., Wang S., Grant B. F., Blanco C. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction. 2011;106:657–669. doi: 10.1111/j.1360-0443.2010.03194.x. doi:10.1111/j.1360-0443.2010.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood O. M., Jacobus J., Bava S., Scarlett A., Tapert S. F. Learning and memory performances in adolescent users of alcohol and marijuana: Interactive effects. Journal of Studies on Alcohol and Drugs. 2010;71:885–894. doi: 10.15288/jsad.2010.71.885. doi:10.15288/jsad.2010.71.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R., Parker L. A. The endocannabinoid system and the brain. Annual Review of Psychology. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. doi:10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Meyers J. E., Meyers K. R. Rey complex figure test and recognition trial: Professional manual. Odessa, Florida: Psychological Assessment Resources; 1995. [Google Scholar]
- Roten A., Baker N. L., Gray K. M. Cognitive performance in a placebo-controlled pharmacotherapy trial for youth with marijuana dependence. Addictive Behaviors. 2015;45:119–123. doi: 10.1016/j.addbeh.2015.01.013. doi:10.1016/j.addbeh.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R., Allen C. Ruff 2&7 Selective Attention Test. Manual. Stockholm: Psykologiförlaget AB; 1996. [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addiction Biology. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. doi:10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schuster R. M., Gilman J., Schoenfeld D., Evenden J., Hareli M., Ulysse C., Evins A. E. One month of cannabis abstinence in adolescents and young adults is associated with improved memory. Journal of Clinical Psychiatry. 2018;79:17m11977. doi: 10.4088/JCP.17m11977. doi:10.4088/JCP.17m11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. C., Slomiak S. T., Jones J. D., Rosen A. F. G., Moore T. M., Gur R. C. Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585–595. doi: 10.1001/jamapsychiatry.2018.0335. doi:10.1001/jamapsychiatry.2018.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J., Litten R. Z., editors. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Subbaraman M. S., Kerr W. C. Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcoholism: Clinical and Experimental Research. 2015;39:872–879. doi: 10.1111/acer.12698. doi:10.1111/acer.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath Y. M., O’Malley P. M., Johnston L. D. Simultaneous alcohol and marijuana use among U.S. high school seniors from 1976 to 2011: Trends, reasons, and situations. Drug and Alcohol Dependence. 2013;133:71–79. doi: 10.1016/j.drugalcdep.2013.05.031. doi:10.1016/j.drugalcdep.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade N. E., Thomas A. M., Gruber S. A., Tapert S. F., Filbey F. M., Lisdahl K. M. Binge and cannabis co-use episodes in relation to white matter integrity in emerging adults. Cannabis and Cannabinoid Research. 2020;5:62–72. doi: 10.1089/can.2018.0062. doi:10.1089/can.2018.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A. L., Wade N. E., Lisdahl K. M. Journal of the International Neuropsychological Society. 1–9. Advance online publication; 2020. Impact of 2 weeks of monitored abstinence on cognition in adolescent and young adult cannabis users. doi:10.1017/S1355617720000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III Administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Winward J. L., Hanson K. L., Tapert S. F., Brown S. A. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. Journal of the International Neuropsychological Society. 2014;20:784–795. doi: 10.1017/S1355617714000666. doi:10.1017/S1355617714000666. [DOI] [PMC free article] [PubMed] [Google Scholar]