Highlights
-
•
Multicentre field trials with natural pathogen exposure complement challenge trials.
-
•
Beef calves housed with their dams were assessed for bovine respiratory disease (BRD).
-
•
Two commercial intranasal live vaccines for BRSV-bPI3V were evaluated.
-
•
New Vaccine A demonstrated non-inferiority compared to benchmarked Vaccine B.
-
•
Difference in BRD prevalence between Vaccines A and B was −0.4% (95% CI −1.6 to 0.8%).
Keywords: Bovine, Bovine respiratory syncytial virus, Bovine parainfluenza-3 virus, Calf, Intranasal vaccine, Vaccination
Abstract
Bovine respiratory syncytial virus (BRSV) and bovine parainfluenza-3 virus (bPI3V) are major causes of bovine respiratory disease (BRD) in newborn calves worldwide. Vaccination is widely used to prevent BRD, and intranasal vaccines for BRSV and bPI3V were developed to overcome interference from BRSV and bPI3V-specific maternally derived antibodies. Many experimental challenge trials have demonstrated that intranasal vaccines for BRSV and bPI3V are efficacious, but effectiveness under field conditions has been demonstrated less often, especially for newborn beef calves. The objective of this field trial was to compare the effectiveness of a newly available commercial BRSV-bPI3V intranasal vaccine with that of a benchmarked one in newborn beef calves reared in a cow-calf system. A total of 935 calves from 39 farms were randomized into two vaccine groups (Bovalto Respi Intranasal [Vaccine A], n = 468; Rispoval RS + PI3 Intranasal [Vaccine B], n = 467), and monitored during the in-house risk period up to three months after vaccination. Non-inferiority analysis was performed by calculating the difference in BRD prevalence between the two vaccine groups.
No significant differences were observed between vaccines regarding clinical outcomes of morbidity, mortality, duration between vaccination and BRD occurrence, or treatments required. Because the upper limit of the 2-sided 95% confidence interval of the difference in BRD prevalence between the two treatment groups (0.8%) was less than the margin of non-inferiority (δ = 5%), a non-inferiority of Vaccine A was concluded. In conclusion, Vaccine A is at least as effective as Vaccine B for the prevention of BRD in newborn beef cattle in a cow-calf system under field conditions.
Introduction
Bovine respiratory disease (BRD) is one of the main health issues encountered in non-weaned beef calves, and can lead to high economic losses (Assié et al., 2004a, Wang et al., 2018). Viral infections generally initiate BRD and predispose animals to secondary bacterial infections (Mosier, 2014). Bovine respiratory syncytial virus (BRSV), an Orthopneumovirus of the Pneumoviridae family, is a major virus involved in the BRD complex and is highly prevalent in both dairy and beef herds (Brodersen, 2010, Sacco et al., 2014, Valarcher and Taylor, 2007). Likewise, bovine parainfluenza-3 virus (bPI3V), a Respirovirus of the Paramyxoviridae family, is another virus involved in the BRD complex, widely prevalent in herds (Ellis, 2010). Vaccines against BRSV and bPI3V are widely used to control BRD, especially in beef calves. In a French study of 165 cow-calf herds in 2000, 116/186 (62%) batches of beef calves were vaccinated against BRSV (Assié et al., 2009).
The neonatal period is a significant risk period for BRD. The immune system of newborn calves differs from that of adults in several respects (Chase et al., 2008, Cortese, 2009). Although functional at birth, the immune system of a calf remains immature until six months of age (Hauser et al., 1986, Tizard, 2018), and the immune response during this time is weak, slow and more easily overcome by pathogenic microorganisms. In addition, maternally derived antibodies (MDA), which are transmitted through colostrum and remain present for up to six months, can interfere negatively with immunization of newborn calves after vaccination (Ellis et al., 2014, Kimman et al., 1989). To overcome interference between parenteral vaccines and MDA, intranasal vaccination strategies using modified live vaccines for respiratory diseases have been developed and used widely for many years (Windeyer and Gamsjäger, 2019). Intranasal vaccination is able to induce protective immunity in newborn calves despite the presence of MDA by priming mucosal immunization of the upper respiratory tract whereas protective immunity is inconsistent after parenteral vaccinations (Osman et al., 2018).
Veterinary vaccine efficacy is mainly evaluated in challenge trials under controlled conditions (Knight-Jones et al., 2014). The efficacy of BRSV intranasal vaccines has been proven in many challenge trials under controlled conditions even when vaccinations are performed in the presence of MDA (Ellis, 2017, Osman et al., 2018). However, these studies generally do not consider variations that occur under field conditions, such as exposure to other pathogens, or host and environmental factors. Field trials are therefore needed to reliably evaluate vaccine effectiveness (Knight-Jones et al., 2014). To our knowledge, only one study dedicated to BRSV intranasal vaccination effectiveness has been carried out under field conditions in newborn dairy calves. In that study, no decrease in BRD incidence or lung lesions associated with pneumonia was demonstrated, but an increase in average daily gain was observed (Ollivett et al., 2018). It should be noted, however, that the management of dairy calves is quite different from that of beef suckler calves. Indeed, in cow-calf systems, animals of different susceptibilities to respiratory diseases or with different immune statuses are mixed in collective barns, whereas dairy calves are classically housed in individual pens during the first eight weeks of life before being sorted and mixed into groups of similar age in collective barns.
One BRSV-bPI3V intranasal vaccine authorized for use in newborn calves to prevent BRD has been available for over 10 years in Europe (Vaccine B, Rispoval RS + PI3 Intranasal, Zoetis). The efficacy and the safety of Vaccine B have been demonstrated in several experimental studies (Vangeel et al., 2009, Vangeel et al., 2007). With this vaccine, nasal shedding of BRSV and bPI3V in vaccinated calves with or without MDA was reduced after challenges with BRSV and bPI3V respectively. Additionally, the severity of clinical disease was also reduced after BRSV in vaccinated calves with BRSV MDA. Moreover, this vaccine has been widely used in Europe and is now a benchmark for BRSV-bPI3V intranasal vaccines. Recently, several new BRSV intranasal vaccines have been launched in Europe. Our study aimed to compare the effectiveness of a new intranasal vaccine against BRSV and bPI3V (Vaccine A, Bovalto Respi Intranasal, Boehringer Ingelheim) with that of the benchmarked vaccine (Vaccine B) in terms of decreasing BRD morbidity in newborn beef calves reared in a cow-calf farming system. As these two vaccines were very similar in their composition (i.e. bivalent modified live vaccines against BRSV and bPI3V) and their indication for use (i.e. active immunization), a non-inferiority study was performed.
Materials and methods
The trial was carried out under the agreement of the Ethics Committee for Clinical and Epidemiological Veterinary Research of Oniris (CERVO, Nantes-Atlantic National College of Veterinary Medicine, Food Science and Engineering, France; Approval number, CERVO-2018-8-V; Approval date, 8 October, 2018).
Vaccines
Vaccines A and B contain BRSV and bPI3V strains administered as a single dose of 2 mL with an intranasal applicator. A dose of Vaccine A (evaluated vaccine) contains between 104.0 and 106.0 TCID50 of BRSV Bio 24/A strain and between 105.0 and 107.5 TCID50 of bPI3V strain Bio 23/A reconstituted with phosphate buffered saline. A dose of Vaccine B (benchmarked vaccine) contains between 105.0 and 107.2 50% tissue culture infective doses (TCID50) of BRSV 375 strain and between 105.0 and 108.6 TCID50 of temperature-sensitive mutant bPI3V strain RLB 103 reconstituted with saline.
Study design
Type of trial
A randomized non-inferiority multicentre trial was carried out to assess whether Vaccine A was at least as effective as Vaccine B, with a pre-stated margin of non-inferiority (δ) for the prevention of BRD in newborn beef cattle. The null hypothesis (H0) was that Vaccine A was inferior to Vaccine B in preventing BRD, whereas the alternative hypothesis (Ha) was that Vaccine A was not inferior by more than the predefined non-inferiority margin. The hypothesis statements may be summarized as follows:
H0: (PBRD[Vaccine A] – PBRD[Vaccine B]) ≥ δ
Ha: (PBRD[Vaccine A] – PBRD[Vaccine B]) < δ
where PBRD was the prevalence rate of calves treated for BRD during the study period and δ the non-inferiority margin.
Determination of the non-inferiority margin
Due to the lack of published field trials of the effectiveness of Vaccine B, it was not possible to determine the non-inferiority margin using a 2-step approach as described by Freise et al. (2013). However, efficacy and the safety of Vaccine B has been demonstrated in controlled challenge trials, and this vaccine has been until now been considered as the benchmarked BRSV-bPI3V IN vaccine. Thus, based on clinical judgment, because 5% was the largest loss of effectiveness of Vaccine A that would be considered clinically insignificant, the non-inferiority margin δ was defined as 5%.
Sample size determination
Sample size was determined using the package ‘TrialSize’ in R1 based on a non-inferiority trial with the prevalence of calves treated for BRD during the study period as the primary outcome. Four hundred and forty-six (446) calves per group were needed to demonstrate non-inferiority assuming α = 0.05, β = 0.20, δ = 0.05 and a prevalence of BRD in the active control vaccine group B of 10%. As it was a stratified multicentre individually randomized trial with two equal group sizes (A/B) and an equal number of calves in the two groups on each farm, the inflating factor was defined as 1 − ρ, with ρ the intraclass correlation coefficient (Vierron and Giraudeau, 2009, Vierron and Giraudeau, 2007). After assigning this inflation factor with ρ = 0.14 (Hendrick et al., 2013) and assuming 20% of loss to follow-up, it was decided to enroll at least 920 calves in the study.
Animals
Herd selection
Forty cow-calf farms with 110 ± 55 calvings (mean ± standard deviation, SD), located in four areas of France (Auvergne-Rhône-Alpes, Bretagne, Nouvelle-Aquitaine, Pays de La Loire), were selected for this study. Initial herd selection by veterinarians was based on the following criteria: (1) pure Charolais breed herds, (2) at least 30 calvings between December 2018 and April 2019, (3) calvings in stalls and a housing period as long as possible, ideally at least three months after vaccination, (4) ability to detect and treat sick animals and to record health events, and (5) no other BRD vaccination program during the study period (from birth to 3 months after enrolment). Herds were then enrolled in the study after farmers agreed to participate.
Calf selection
On each farm, 1 to 3 blocks of 10 calves were enrolled (3, 6 and 32 farms had respectively 1, 2 or 3 blocks). As soon as there were more than 10 calves over 10 days of age, a clinical examination of each calf was performed by a veterinarian. Only calves in good health (no intercurrent illness detected during the clinical examination) at the time of enrolment were included. If a calf had been ill and treated for BRD or other diseases before the day of inclusion, enrolment was possible only after full clinical recovery of the animal.
Randomization and vaccination protocols
Randomization was performed with a 1:1 allocation ratio. In each block of 10 enrolled calves from each farm, calves were sorted by decreasing age. A predefined randomized spreadsheet was prepared for each block: a random drawing was performed using the Rand function of MS Excel to assign the vaccine to the oldest calf, then the following calves were vaccinated alternately with one of the two vaccines.
Vaccines were administered by one veterinary investigator with calves restrained by the farmer, while another veterinary investigator reconstituted vaccines and recorded data. The vaccines were administered according to the manufacturer's recommendations: for Vaccine A, 1 mL in each nostril with the specific intranasal applicator (cannula and disk) provided by the manufacturer; for Vaccine B, 2 mL in one nostril with the specific intranasal applicator (cannula) provided by the manufacturer.
Intranasal applicators were changed between each calf. Due to the different administration modalities for the two vaccines, it was not possible to carry out a blinded vaccination. However, data were collected and registered by another veterinary investigator who kept randomization sheets and was not involved in the monitoring of calves after vaccination. To avoid potential bias in later husbandry, farmers were asked to not read the tag numbers of calves during vaccination. After vaccination, cow-calf couples were raised in the same pen according to the usual rearing conditions on each farm.
Outcomes and data collection
The protocol started with vaccination and ended three months later. The primary outcome for analysis was BRD events, defined by at least one respiratory sign (such as cough, dyspnea, and/or nasal discharge) associated with at least one general clinical sign (hyperthermia, depression, and/or anorexia). Secondary outcomes included the time between vaccine administration and the occurrence of BRD, calf mortality, and the number of calves treated with antibiotics or anti-inflammatory drugs during the study.
For each calf, demographic data (tag number, date of birth, sex, and parity of the dam) were extracted from the official identification databases, and medical history was gathered from animal health records of the farm. For three months following vaccination, the farmers, under supervision of the veterinary investigators, had to monitor and record all observations and treatments carried out: date, clinical signs, diagnosis (respiratory disorders and others) and drug administration (antibiotic and/or anti-inflammatory preparations).
In the event of mortalities during the study, a veterinary investigator performed required necropsy examinations to identify the cause of death. In the case of bronchopneumonia, lung samples (approximately 5 cm3) were frozen at −20 °C for PCR analysis. Multiplex real-time PCR assays (Pack Respiratory 8 Bio-T kit, Biosellal) designed for the detection of eight BRD agents (BRSV, bPI3V, bovine Coronavirus, Influenza D, Mannheimia haemolytica, Pasteurella multocida, Mycoplasma bovis, Histophilus somni) were performed in Agrivalys 71.
Post-admission exclusion
Calves with incomplete data, vaccinated before 10 days or after 60 days of age, or treated for BRD without at least two clinical signs being recorded, or as part of a metaphylaxis protocol, or vaccinated after the beginning of the grazing period were excluded.
Statistical analysis
The characteristics of calves assigned to the two vaccine groups were compared to assess homogeneity: Student's t-test was used for continuous variables (i.e., age at vaccination, risk period) after checking normality, and chi-squared or Fisher tests were used for categorical variables (i.e., parity of dam, sex, and disease occurrence before vaccination).
The statistical analysis of the primary outcome was performed using a mixed logistic regression model (with ‘calf’ as the statistical unit). The primary explanatory variable of interest was vaccine. Other variables were also tested (sex, parity of dam, age at vaccination, risk period) and kept in the model if P < 0.2 in the univariable analysis. A backward stepwise elimination of variables was then performed until all explanatory variables with P < 0.05 were included in the final model, taking into account potential confounders. Herd (categorical variable) was included as a random effect. The variable risk period was kept in the model to adjust BRD occurrence to the variation of the duration of exposition to pathogens between calves. The variables vaccine and risk period were forced in the final model, written as:
where BRD ij is the occurrence of a BRD case diagnosed during the study Risk Period for the calf i of the herd j with a probability of occurrence p ij, is the intercept, vaccine ij is either Vaccine A or B, risk period ij is the duration of the in-house risk period, X ij is sex, parity of dam and age at vaccination variables and v j is the random effect for herd j. Herd random effect followed a normal distribution with mean 0 and variance .
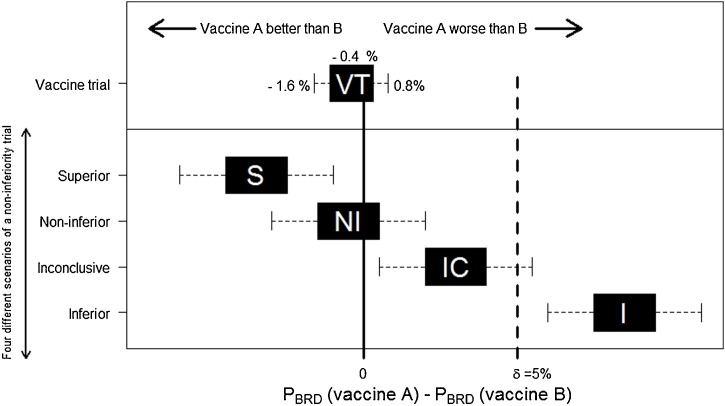
The difference in BRD prevalence between vaccine groups and its 95% confidence interval (CI) was calculated from the model. Non-inferiority of Vaccine A compared with Vaccine B was concluded if the upper bound of the 2-sided 95% CI of the difference of BRD prevalence between the two vaccines was smaller than the non-inferiority margin δ (Fig. 1 ).
Fig. 1.
Four possible scenarios of a non-inferiority trial comparing Vaccine A to Vaccine B for preventing BRD. The margin of non-inferiority (δ) is drawn by a vertical dashed line. PBRD is the prevalence of BRD cases diagnosed during the study risk period of housing after vaccination. Error bars indicate 2-sided 95% confidence interval (CI) of the difference in BRD incidence (Piaggio et al., 2012). S: if the CI lies wholly to the left of zero, Vaccine A is superior. NI: if the CI lies to the left of δ and includes zero, Vaccine A is non-inferior. IC: if the CI includes δ and zero, the difference is non-significant but the result regarding non-inferiority is inconclusive. I: if the CI is wholly above δ, Vaccine A is inferior. VT is the representation of the main outcome of this non-inferiority trial. The black block indicates the difference in BRD incidence between Vaccine A group and Vaccine B group. Non-inferiority of Vaccine A compared to Vaccine B at a margin of 5% is demonstrated because the 95% CI lies to the left of δ (=5%) and includes zero.
Secondary outcomes were compared between the two vaccine groups using the chi-squared test, Fisher test or Student's t-test. All analyses were carried out using R software1 and a statistical significance at P ≤ 0.05 was used.
Results
Descriptive results
A total of 1120 calves from 40 farms were enrolled in the study. Data from 185 calves were excluded: 40 because of incomplete data, 85 for date of vaccination before 10 days or after 60 days of age, 30 for being treated for BRD without at least two clinical signs or in a metaphylaxis protocol, and 30 for being vaccinated after the beginning of the grazing period (no housing period). Thus, a total of 935 calves from 39 herds were used in the study analysis: 24 ± 7 calves (mean ± SD) per herd. The two experimental groups were homogeneous in regard to age at vaccination, duration of in-house risk period, parity of dams, sex ratio, and occurrence of diseases before vaccination (Table 1 ).
Table 1.
Calf characteristics in vaccine groups A and B.
| Variable | Vaccine group (n = 935) |
P | |
|---|---|---|---|
| A (n = 468) | B (n = 467) | ||
| Age at vaccination in days (mean ± SD) | 25.97 ± 11.62 | 25.85 ± 11.54 | 0.88 |
| Duration of in-house risk period in days (mean ± SD) | 56.52 ± 28.25 | 57.00 ± 27.93 | 0.80 |
| Parity | |||
| 1 (n = 279) | 143 | 136 | 0.51 |
| 2 (n = 157) | 72 | 85 | |
| 3 and more (n = 499) | 253 | 246 | |
| Sex | |||
| Male (n = 447) | 235 | 212 | 0.14 |
| Female (n = 488) | 233 | 255 | |
| Occurrence of diseases before vaccination | |||
| No disease (n = 873) | 437 | 436 | 1 |
| Respiratory (n = 6) | 3 | 3 | |
| Other than respiratory a (n = 56) | 28 | 28 | |
SD, Standard deviation.
All neonatal diseases were diagnosed and treated (i.e., septicaemia, diarrhea, umbilical infection, others).
Primary outcome
The occurrence of BRD during the in-house risk period between the two vaccine groups was similar (Table 2 ). Using least squares means of model outcome (BRD events), the difference PBRD(Vaccine A) − PBRD(Vaccine B) was estimated at −0.4% with a 95% CI between −1.6% and 0.8%. Non-inferiority of Vaccine A compared to Vaccine B was concluded since the upper limit of the 2-sided 95% CI (0.8%) of the difference in prevalence of calves diagnosed with BRD between the two vaccines was smaller than δ (Fig. 1). BRD incidence in our study was 0.74 cases per 1000 calf-days at risk (Table 3 ).
Table 2.
Multivariable results of mixed logistic regression model of bovine respiratory disease (BRD) prevalence after intranasal vaccination of non-weaned beef calves.
| Variable | Category | Number of calves evaluated | Odds ratio of BRD occurrence | 95% Confidence interval | Pa |
|---|---|---|---|---|---|
| Vaccine | Vaccine B | 467 | Reference | ||
| Vaccine A | 468 | 0.61 | 0.30–1.25 | 0.17 | |
| Duration of in-house risk period in days | (0–45) | 313 | Reference | ||
| (45–67.5) | 243 | 8.88 | 1.07–73.66 | 0.04 | |
| (67.5–90) | 379 | 6.61 | 0.86–50.99 | 0.07 |
For each variable, refers to level of significance between the category under consideration and the reference category.
Table 3.
Incidence of bovine respiratory disease cases in the two vaccine groups.
| Vaccine group | Number of |
Incidence ratea | |
|---|---|---|---|
| Calf-days at risk | Cases | ||
| Vaccine A | 25,538 | 15 | 0.59 |
| Vaccine B | 26,031 | 23 | 0.88 |
| Total | 51,569 | 38 | 0.74 |
Per 1000 calf-days at risk.
Secondary outcome
The two experimental groups were similar in regard to time between vaccination and occurrence of BRD, treatments and mortality (Table 4 ). For the six calves which died during the study period, BRSV was not detected from the samples collected during the necropsy procedure (Table 5 ).
Table 4.
Comparisons of secondary outcomes between the two vaccine groups.
| Outcome | Vaccine group (n = 935) |
P | |
|---|---|---|---|
| A (n = 468) | B (n = 467) | ||
| Calves treated for BRD with | |||
| Antibiotics (%) | 15 (3.2) | 23 (4.9) | 0.23 |
| Non-steroidal anti-inflammatories (%) | 12 (2.6) | 17 (3.6) | 0.34 |
| Steroidal anti-inflammatories (%) | 1 (0.2) | 2 (0.4) | 0.50 |
| Mortalities (%) | 1 (0.2) | 5 (1.1) | 0.11 |
| Time between vaccination and occurrence of BRD in days (mean ± SD) | 33 ± 20 | 28 ± 22 | 0.45 |
BRD, Bovine respiratory disease; SD, standard deviation.
Table 5.
Results of multiplex real-time PCR on lung samples for detection of eight respiratory pathogens from necropsies of dead calves.
| Calf number | Vaccine group | Herd number | Pathogen detected in multiplex real-time PCRa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRSV | bPI3V | Mh | Pm | Mb | Hs | bCo | ID | |||
| 14 | B | 1 | No sample | |||||||
| 244 | B | 13 | No sample | |||||||
| 913 | B | 37 | − | + | + | + | − | + | − | − |
| 941 | A | 38 | − | − | − | + | − | + | + | − |
| 955 | B | 38 | − | − | − | + | − | − | + | − |
| 967 | B | 38 | − | + | − | + | − | − | − | − |
BRSV, Bovine respiratory syncytial virus; bPI3V, bovine Parainfluenza Virus type 3; Mh, Mannheimia haemolytica; Pm, Pasteurella multocida; Mb, Mycoplasma bovis; Hs, Histophilus somni; bCo, bovine Coronavirus; ID, Influenza D virus.
Pack Respiratory 8 Bio-T kit, Biosellal.
Discussion
The objective of this study was to compare the effectiveness of the newly available BRSV-bPI3V intranasal vaccine to the benchmarked vaccine under field conditions in newborn beef calves reared in a cow-calf system. Based on our results, non-inferiority of Vaccine A compared to Vaccine B was concluded. Due to the lack of studies dedicated to the effectiveness of Vaccine B compared to a placebo, the non-inferiority margin δ was defined as 5% only, based on a clinical judgment. This margin is narrow compared to the one used in most vaccine trials, with δ usually fixed at 10% as reviewed by Donken et al. (2015). Choosing a more conservative non-inferiority margin required an increased sample size and improved the clinical significance of the trial. Indeed, based on the experiences of the authors, an increase of more than 5% of BRD events (i.e., the primary outcome) in the Vaccine A group compared to the Vaccine B group was considered unfavorable.
In this study, calves from the two vaccine groups were mixed together in order to homogenize as much as possible environmental conditions and exposure to pathogens. This design is often chosen in field studies dealing with vaccine effectiveness (Schunicht et al., 2003, Stilwell et al., 2008, Wildman et al., 2008). Moreover, in a cow-calf system, this design enables the absence of separation of paired calves of the two vaccine groups after randomization, and improves blind assessment of calf health in a single group. However, a bias in vaccine effectiveness evaluation could be introduced with this method. The reduction of virus shedding after vaccination of the calves of one vaccine group contributes to the protection of the calves of the other vaccine group reared in the same environment (Smith, 2019, Smith, 2014, Stokka, 2010). Indeed, apparent effectiveness of the test vaccine could be improved if the comparison involved a reference vaccine with a better shedding reduction efficacy. Moreover, Vaccines A and B are both modified live vaccines. Cross-immunization thus could occur between the two vaccine groups. In previous studies using commercial vaccines including the reference vaccine, nasal shedding of vaccine-origin viruses was detected by PCR in nasal swabs during 14 days in most of the vaccinated calves after vaccination and was detected up to 28 days post vaccination in a few calves (Timsit et al., 2009, Walz et al., 2017).
The study was designed to determine the effectiveness of two commercial vaccines under conditions as close as possible to those encountered by calves reared in beef herds. Although the minimum age at vaccination recommended by the manufacturers is 9 and 10 days of age for Vaccines B and A, respectively, under our conditions the mean age (±SD) at vaccination was 26 ± 12 days. This delay in administrating vaccines was due to the distribution of births on each farm and the packaging of the vaccines in 5-dose bottles. Since both vaccines are available in a 5-dose bottle, 10 calves had to be over 10 days of age before being vaccinated in order to randomize them into two equal groups of five calves. However, this difference between the recommended and actual age at vaccination was the same in both groups and is common in French cow-calf systems.
Most of the efficacy studies for BRSV and bPI3V intranasal vaccination include a controlled challenge, but challenges may not reproduce natural exposure under variable host and environmental factors (Knight-Jones et al., 2014). It has been observed that many BRSV infection models failed to reproduce the severe clinical signs of the disease, complicating the evaluation of vaccine efficacy (Belknap et al., 1995, Blodörn et al., 2015, Taylor, 2013). In these studies, efficacy was demonstrated in newborn calves both in the absence of BRSV-specific MDAs (Ellis et al., 2007, Vangeel et al., 2007) and in their presence (Ellis et al., 2013, Hill et al., 2012). However, the absence of maternal antibody interference is not always observed (Ellis et al., 2010). In our study, colostral immunity in calves was not systematically controlled for practical reasons, and the BRSV and bPI3V serological status of calves at the time of vaccination was unknown. Due to the high prevalence of BRSV in France, most of the vaccinations were likely to have been performed in the presence of BRSV-specific MDAs.
As observed in a previous study, BRSV and bPI3V infections were found in 71% and 80% of cow-calf farms respectively (Assié et al., 2004b). Furthermore, challenge trials do not reproduce the variability of host and environmental conditions that may be encountered in the field, such as variable passive immune transfer (Raboisson et al., 2016), variable calf housing (Assié et al., 2009, Dubrovsky et al., 2019a, Maier et al., 2019) and variable seasonal or weather conditions (Buczinski et al., 2018, Dubrovsky et al., 2019b). For these reasons, a multicentre study was chosen in order to reproduce this variability of environmental factors. Both efficacy assessment in experimental challenges and effectiveness assessment in field trials have limits but are complementary.
Contrary to challenge trials, the exposure of calves to pathogens, in particular to BRSV and bPI3V, is rarely controlled in a field study (Ellis, 2017, Ollivett et al., 2018). The authors acknowledge that monitoring BRSV and bPI3V exposure (by means such as PCR or virus isolation on deep nasal swabs or fluid of transtracheal aspiration or bronchoalveolar lavage, or serology on sentinels) would have allowed us to assess specifically the effectiveness of vaccines against BRSV and bPI3V, and not only the prevention of BRD. As previously reported, monitoring exposure to pathogens in a vaccinated population is very difficult for both practical and economic reasons (Ellis, 2017). Indeed, the short viremia would require repeated samplings of a large population. In a recent field trial evaluating BRSV and bPI3V intranasal vaccination in dairy calves, Ollivett et al. (2018) similarly did not assess the exposure of calves to BRSV and bPI3V, and monitored BRD morbidity alone.
However, the exposure of calves to respiratory pathogens in our study can be attested by the measurement of BRD incidence, which was 0.74 cases per 1000 calf-days at risk. Although this BRD incidence was low, it remains consistent with the incidence observed in another French study in a comparable breeding system in which respiratory vaccination was inconsistent: 1.89 cases per 1000 calf-days at risk in 137 farms (Assié et al., 2004a). Circulation of respiratory pathogens in the study farms can also be attested by the viruses (bPI3V, bovine Coronavirus) and bacteria (Mannheimia haemolytica, Pasteurella multocida, Histophilus somni) identified at necropsy in dead animals. To overcome the variability in the exposure to pathogens under field conditions, our study would need to be repeated.
Conclusions
The effectiveness of a newly available commercial BRSV-bPI3V intranasal vaccine to control BRD has been demonstrated under field conditions. To the authors’ knowledge, this is the first study under field conditions assessing BRSV and bPI3V intranasal vaccination effectiveness in newborn beef calves in a cow-calf system. Data from challenge studies or from dairy calf field studies cannot be extrapolated to beef calves. Beef cattle from different age groups with different immune statuses against respiratory pathogens are mixed together in a specific in-house environment, in contrast to dairy calves which are typically housed in individual pens or in collective pens with animals of the same age.
Conflict of interest statement
This study was funded by Boehringer Ingelheim, which supports the European College of Bovine Health Management (ECBHM) residency program of the first author. Boehringer Ingelheim played no role in the study design, in data collection, analysis or interpretation, or in the writing of the report and the decision to submit the manuscript for publication. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of the paper.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors acknowledge the assistance of the veterinarians of the ‘Réseau Cristal’ and the farmers participating in this study. The financial support from Boehringer Ingelheim France is gratefully acknowledged.
Footnotes
See: R Foundation for Statistical Computing, Vienna, Austria, http://w***ww.R-project.org (Accessed 16 August 2020).
References
- Assié S., Bareille N., Beaudeau F., Seegers H. Management- and housing-related risk factors of respiratory disorders in non-weaned French Charolais calves. Prev. Vet. Med. 2009;91:218–225. doi: 10.1016/j.prevetmed.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Assié S., Seegers H., Beaudeau F. Incidence of respiratory disorders during housing in non-weaned Charolais calves in cow-calf farms of Pays de la Loire (Western France) Prev. Vet. Med. 2004;63:271–282. doi: 10.1016/j.prevetmed.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Assié S., Seegers H., Ogier de Baulny M., Beaudeau F. Pathogens and incidence of respiratory disorders on non-weaned calves in Charolais cow-calf farms of the Pays de la Loire (France) 11ème Journées Rencontres Recherches Ruminants. Presented at the 11ème Journées Rencontres Recherches Ruminants, Paris (France) 2004:329–332. [Google Scholar]
- Belknap E.B., Ciszewski D.K., Baker J.C. Experimental respiratory syncytial virus infection in calves and lambs. J. Vet. Diagn. Investig. 1995;7:285–298. doi: 10.1177/104063879500700226. [DOI] [PubMed] [Google Scholar]
- Blodörn K., Hägglund S., Gavier-Widen D., Eléouët J.-F., Riffault S., Pringle J., Taylor G., Valarcher J.F. A bovine respiratory syncytial virus model with high clinical expression in calves with specific passive immunity. BMC Vet. Res. 2015;11:76. doi: 10.1186/s12917-015-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen B.W. Bovine respiratory syncytial virus. Vet. Clin. N. Am.: Food Anim. Pract. 2010;26:323–333. doi: 10.1016/j.cvfa.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Buczinski S., Borris M.E., Dubuc J. Herd-level prevalence of the ultrasonographic lung lesions associated with bovine respiratory disease and related environmental risk factors. J. Dairy Sci. 2018;101:2423–2432. doi: 10.3168/jds.2017-13459. [DOI] [PubMed] [Google Scholar]
- Chase C.C.L., Hurley D.J., Reber A.J. Neonatal immune development in the calf and its impact on vaccine response. Veterinary Clinics of North America: food animal practice. Dairy Heifer Manag. 2008;24:87–104. doi: 10.1016/j.cvfa.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese V.S. Neonatal immunology. Veterinary Clinics of North America: food animal practice. Bovine Neonatol. 2009;25:221–227. doi: 10.1016/j.cvfa.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donken R., de Melker H.E., Rots N.Y., Berbers G., Knol M.J. Comparing vaccines: a systematic review of the use of the non-inferiority margin in vaccine trials. Vaccine. 2015;33:1426–1432. doi: 10.1016/j.vaccine.2015.01.072. [DOI] [PubMed] [Google Scholar]
- Dubrovsky S.A., Van Eenennaam A.L., Karle B.M., Rossitto P.V., Lehenbauer T.W., Aly S.S. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10 K study. J. Dairy Sci. 2019;102:7306–7319. doi: 10.3168/jds.2018-14774. [DOI] [PubMed] [Google Scholar]
- Dubrovsky S.A., Van Eenennaam A.L., Karle B.M., Rossitto P.V., Lehenbauer T.W., Aly S.S. Bovine respiratory disease (BRD) cause-specific and overall mortality in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019;102:7320–7328. doi: 10.3168/jds.2018-15463. [DOI] [PubMed] [Google Scholar]
- Ellis J., Gow S., Bolton M., Burdett W., Nordstrom S. Inhibition of priming for bovine respiratory syncytial virus-specific protective immune responses following parenteral vaccination of passively immune calves. Can. Vet. J. 2014;55:1180–1185. [PMC free article] [PubMed] [Google Scholar]
- Ellis J., Gow S., West K., Waldner C., Rhodes C., Mutwiri G., Rosenberg H. Response of calves to challenge exposure with virulent bovine respiratory syncytial virus following intranasal administration of vaccines formulated for parenteral administration. J. Am. Vet. Med. Assoc. 2007;230:233–243. doi: 10.2460/javma.230.2.233. [DOI] [PubMed] [Google Scholar]
- Ellis J.A. How efficacious are vaccines against bovine respiratory syncytial virus in cattle? Vet. Microbiol. 2017;206:59–68. doi: 10.1016/j.vetmic.2016.11.030. [DOI] [PubMed] [Google Scholar]
- Ellis J.A. Bovine parainfluenza-3 virus. Vet. Clin. N. Am.: Food Anim. Pract. 2010;26:575–593. doi: 10.1016/j.cvfa.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Ellis J.A., Gow S.P., Goji N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J. Am. Vet. Med. Assoc. 2010;236:991–999. doi: 10.2460/javma.236.9.991. [DOI] [PubMed] [Google Scholar]
- Ellis J.A., Gow S.P., Mahan S., Leyh R. Duration of immunity to experimental infection with bovine respiratory syncytial virus following intranasal vaccination of young passively immune calves. J. Am. Vet. Med. Assoc. 2013;243:1602–1608. doi: 10.2460/javma.243.11.1602. [DOI] [PubMed] [Google Scholar]
- Freise K.J., Lin T.-L., Fan T.M., Recta V., Clark T.P. Evidence-based medicine: the design and interpretation of noninferiority clinical trials in veterinary medicine. J. Vet. Intern. Med. 2013;27:1305–1317. doi: 10.1111/jvim.12211. [DOI] [PubMed] [Google Scholar]
- Hauser M.A., Koob M.D., Roth J.A. Variation of neutrophil function with age in calves. Am. J. Vet. Res. 1986;47:152–153. [PubMed] [Google Scholar]
- Hendrick S.H., Bateman K.G., Rosengren L.B. The effect of antimicrobial treatment and preventive strategies on bovine respiratory disease and genetic relatedness and antimicrobial resistance of Mycoplasma bovis isolates in a western Canadian feedlot. Can. Vet. J. 2013;54:1146–1156. [PMC free article] [PubMed] [Google Scholar]
- Hill K.L., Hunsaker B.D., Townsend H.G., van Drunen Littel-van den Hurk S., Griebel P.J. Mucosal immune response in newborn Holstein calves that had maternally derived antibodies and were vaccinated with an intranasal multivalent modified-live virus vaccine. J. Am. Vet. Med. Assoc. 2012;240:1231–1240. doi: 10.2460/javma.240.10.1231. [DOI] [PubMed] [Google Scholar]
- Kimman T.G., Westenbrink F., Straver P.J. Priming for local and systemic antibody memory responses to bovine respiratory syncytial virus: effect of amount of virus, virus replication, route of administration and maternal antibodies. Vet. Immunol. Immunopathol. 1989;22:145–160. doi: 10.1016/0165-2427(89)90057-3. [DOI] [PubMed] [Google Scholar]
- Knight-Jones T.J.D., Edmond K., Gubbins S., Paton D.J. Veterinary and human vaccine evaluation methods. Proc. R. Soc. B: Biol. Sci. 2014;281 doi: 10.1098/rspb.2013.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier G.U., Love W.J., Karle B.M., Dubrovsky S.A., Williams D.R., Champagne J.D., Anderson R.J., Rowe J.D., Lehenbauer T.W., Van Eenennaam A.L., Aly S.S. Management factors associated with bovine respiratory disease in preweaned calves on California dairies: the BRD 100 study. J. Dairy Sci. 2019;102:7288–7305. doi: 10.3168/jds.2018-14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. Review of BRD pathogenesis: the old and the new. Anim. Health Res. Rev. 2014;15:166–168. doi: 10.1017/S1466252314000176. [DOI] [PubMed] [Google Scholar]
- Ollivett T.L., Leslie K.E., Duffield T.F., Nydam D.V., Hewson J., Caswell J., Dunn P., Kelton D.F. Field trial to evaluate the effect of an intranasal respiratory vaccine protocol on calf health, ultrasonographic lung consolidation, and growth in Holstein dairy calves. J. Dairy Sci. 2018;101:8159–8168. doi: 10.3168/jds.2017-14271. [DOI] [PubMed] [Google Scholar]
- Osman R., Malmuthuge N., Gonzalez-Cano P., Griebel P. Development and function of the mucosal immune system in the upper respiratory tract of neonatal calves. Annu. Rev. Anim. Biosci. 2018;6:141–155. doi: 10.1146/annurev-animal-030117-014611. [DOI] [PubMed] [Google Scholar]
- Piaggio G., Elbourne D.R., Pocock S.J., Evans S.J.W., Altman D.G., Group for the CONSORT Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. J. Am. Med. Assoc. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- Raboisson D., Trillat P., Cahuzac C. Failure of passive immune transfer in calves: a meta-analysis on the consequences and assessment of the economic impact. PLOS ONE. 2016;11:e0150452. doi: 10.1371/journal.pone.0150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R.E., McGill J.L., Pillatzki A.E., Palmer M.V., Ackermann M.R. Respiratory syncytial virus infection in cattle. Vet. Pathol. 2014;51:427–436. doi: 10.1177/0300985813501341. [DOI] [PubMed] [Google Scholar]
- Schunicht O.C., Booker C.W., Jim G.K., Guichon P.T., Wildman B.K., Hill B.W. Comparison of a multivalent viral vaccine program versus a univalent viral vaccine program on animal health, feedlot performance, and carcass characteristics of feedlot calves. Can. Vet. J. 2003;44:43–50. [PMC free article] [PubMed] [Google Scholar]
- Smith D.R. Herd immunity. Vet. Clin. N. Am.: Food Anim. Pract. 2019;35:593–604. doi: 10.1016/j.cvfa.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Smith D.R. Field epidemiology to manage BRD risk in beef cattle production systems. Anim. Health Res. Rev. 2014;15:180–183. doi: 10.1017/S1466252314000243. [DOI] [PubMed] [Google Scholar]
- Stilwell G., Matos M., Carolino N., Lima M.S. Effect of a quadrivalent vaccine against respiratory virus on the incidence of respiratory disease in weaned beef calves. Prev. Vet. Med. 2008;85:151–157. doi: 10.1016/j.prevetmed.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokka G.L. Prevention of respiratory disease in cow/calf operations. The Veterinary Clinics of North America: food animal practice. Bovine Respir. Dis. 2010;26:229–241. doi: 10.1016/j.cvfa.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. Challenges and Opportunities for Respiratory Syncytial Virus Vaccines, Current Topics in Microbiology and Immunology. Springer; Berlin, Heidelberg: 2013. Bovine model of respiratory syncytial virus infection; pp. 327–345. [DOI] [PubMed] [Google Scholar]
- Timsit E., Le Dréan E., Maingourd C., Belloc C., Guattéo R., Bareille N., Seegers H., Douart A., Sellal E., Assié S. Detection by real-time RT-PCR of a bovine respiratory syncytial virus vaccine in calves vaccinated intranasally. Vet. Record. 2009;165:230–233. doi: 10.1136/vr.165.8.230. [DOI] [PubMed] [Google Scholar]
- Tizard I. Veterinary Immunology. Elsevier; Saint Louis, Missouri: 2018. Immunity in the fetus and newborn; pp. 247–260. [Google Scholar]
- Valarcher J.-F., Taylor G. Bovine respiratory syncytial virus infection. Vet. Res. 2007;38:153–180. doi: 10.1051/vetres:2006053. [DOI] [PubMed] [Google Scholar]
- Vangeel I., Antonis A.F.G., Fluess M., Riegler L., Peters A.R., Harmeyer S.S. Efficacy of a modified live intranasal bovine respiratory syncytial virus vaccine in 3-week-old calves experimentally challenged with BRSV. Vet. J. 2007;174:627–635. doi: 10.1016/j.tvjl.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Vangeel I., Ioannou F., Riegler L., Salt J.S., Harmeyer S.S. Efficacy of an intranasal modified live bovine respiratory syncytial virus and temperature-sensitive parainfluenza type 3 virus vaccine in 3-week-old calves experimentally challenged with PI3V. Vet. J. 2009;179:101–108. doi: 10.1016/j.tvjl.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Vierron E., Giraudeau B. Design effect in multicenter studies: gain or loss of power? BMC Med. Res. Methodol. 2009;9:39. doi: 10.1186/1471-2288-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierron E., Giraudeau B. Sample size calculation for multicenter randomized trial: taking the center effect into account. Contemp. Clin. Trials. 2007;28:451–458. doi: 10.1016/j.cct.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Walz P.H., Newcomer B.W., Riddell K.P., Scruggs D.W., Cortese V.S. Virus detection by PCR following vaccination of naive calves with intranasal or injectable multivalent modified-live viral vaccines. J. Vet. Diagn. Investig. 2017;29:628–635. doi: 10.1177/1040638717709039. [DOI] [PubMed] [Google Scholar]
- Wang M., Schneider L.G., Hubbard K.J., Smith D.R. Cost of bovine respiratory disease in preweaned calves on US beef cow-calf operations (2011–2015) J. Am. Vet. Med. Assoc. 2018;253:624–631. doi: 10.2460/javma.253.5.624. [DOI] [PubMed] [Google Scholar]
- Wildman B.K., Perrett T., Abutarbush S.M., Guichon P.T., Pittman T.J., Booker C.W., Schunicht O.C., Fenton R.K., Jim G.K. A comparison of 2 vaccination programs in feedlot calves at ultra-high risk of developing undifferentiated fever/bovine respiratory disease. Can. Vet. J. 2008;49:463–472. [PMC free article] [PubMed] [Google Scholar]
- Windeyer M.C., Gamsjäger L. Vaccinating calves in the face of maternal antibodies: challenges and opportunities. Vet. Clin. N. Am.: Food Anim. Pract. 2019;35:557–573. doi: 10.1016/j.cvfa.2019.07.004. [DOI] [PubMed] [Google Scholar]