Abstract
Radio amplification by stimulated emission of radiation (RASER) was recently discovered in a low field NMR spectrometer incorporating a highly specialized radio frequency resonator where a high degree of proton spin polarization was achieved by reversible parahydrogen exchange. RASER activity, which results from the coherent coupling between the nuclear spins and the inductive detector, can overcome the limits of frequency resolution in NMR with potentially important opportunities in fundamental and applied physics. Here we show that this phenomenon is not limited to low magnetic fields or the use of resonators with high quality factors. Specifically, we use a commercial, bench-top 1.4 T NMR spectrometer in conjunction with parahydrogen pairwise addition producing proton-hyperpolarized molecules in the Earth’s magnetic field (aka. ALTADENA condition) or in a high magnetic field (aka. PASADENA condition) to induce RASER without any radio-frequency excitation pulses. The results demonstrate that RASER activity can be observed on virtually any NMR spectrometer and measures with high precision most of the important NMR parameters, such as chemical shifts and J-coupling constants. These findings are important for future applications of RASER in many different fields of science and technology, in particular for the development and quality assurance of hyperpolarization techniques such as parahydrogen-induced polarization.
Keywords: RASER, parahydrogen, PHIP, NMR spectroscopy, hyperpolarization
Graphical Abstract
We demonstrate Radio Amplification by Stimulated Emission of Radiation (RASER) activity using 61 MHz NMR spectrometer in conjunction with parahydrogen pairwise addition producing proton-hyperpolarized molecules with NMR signal induction without any radio-frequency excitation pulses. These findings are important for future applications of RASER in many different fields of science and technology. Institute and/or researcher Twitter usernames: Wayne State University
Introduction
Suefke and co-workers reported recently (in 2017) on the first experimental observation of radio amplification by stimulated emission of radiation (RASER) of protons.[1, 2] Unlike lasers and masers, which employ self-organizing systems emitting coherent optical- and micro-waves, RASER is induced by continuous coherent oscillation of radio waves at much lower frequencies through the coupling between nuclear spin magnetization and an LC resonance circuit.[1] Because the required magnetization, i.e., the product of the concentration of nuclear spins and their nuclear spin polarization (P) or the degree of the spin alignment with the static magnetic field of the NMR magnet, is very high, RASER-based NMR spectroscopy is difficult to achieve using thermal nuclear spin polarization. Hyperpolarization techniques allow for enhancing the nuclear spin polarization by several orders of magnitude[3–7] up to unity.[8, 9] Suefke and co-workers thus employed the technique of Signal Amplification by Reversible Exchange (SABRE) to provide a highly magnetized sample in their pioneering RASER demonstration.[10] SABRE relies on the simultaneous exchange between parahydrogen (p-H2, the source of hyperpolarization) and a substrate on a metal complex.[11] With SABRE, the spontaneous polarization of proton spins is efficient in the magnetic field range of 1 to 10 mT.[12] Therefore, Suefke and co-workers employed NMR detection at magnetic fields of several milli-Tesla with corresponding resonance frequencies in the range of 41–512 kHz.[1, 2] These authors also employed SABRE because it allows for continuously regenerating the proton polarization via the sustained delivery of p-H2 gas to the sample placed inside the NMR detector.[1]
RASER activity is initiated when the radiation damping rate 1/τRD satisfies the following condition:
| (1) |
where 1/T2* defines the modified spin-spin relaxation rate and 1/τRD the radiation damping rate, which is given by:
| (2) |
where μ0, η, Q, γ, ℏ, and nS are defined as the vacuum permeability, the filling factor of the resonator, the quality factor of the resonator, the gyromagnetic ratio, Planck’s constant and the spin number density, respectively. The initial magnetization is given as M0 = (1/2)*ℏ*γ*nS*P, where P is the degree of spin polarization.[1] Note that if P > 0, the rate 1/τRD is negative and the associated line is additionally broadened by radiation damping with a total damping rate κtot = (1/T2* - 1/τRD) > 1/T2* . If P < 0, which corresponds to a population inversion, 1/τRD is positive and the line is narrowed due to the decreased total damping rate κtot < 1/T2*. RASER activity starts if κtot < 0, as described by Equation (1). To fulfill this condition for proton spins at low frequencies, Suefke and co-workers employed high-quality (Q ~ 300) resonators,[13] which reduce significantly the RASER threshold requirements for polarization and concentration.[1] Hence, the experimental conditions reunited for this first demonstration RASER, i.e., low magnetic field, high-Q resonator, and continuous regeneration of hyperpolarization, seem very peculiar. As a matter of fact, RASER activity is a much more common phenomenon than one could expect, as we will show below.
Results and Discussion
Here, we show evidence that a commercial, high-field NMR system with standard inductive detection (i.e., without specialized, high-Q resonators) can readily detect RASER when combined with the parahydrogen-induced polarization (PHIP) technique.[14] Specifically, we employ a 1.4 T (61.7 MHz) bench-top NMR spectrometer (SpinSolve Carbon 60, Magritek, New Zealand) with Q = 68 to induce RASER of protons in hyperpolarized (HP) ethyl acetate (EA) and 2-hydroxyethylpropionate (HEP). These HP compounds are formed through the pairwise addition of p-H2 onto the unsaturated C=C chemical bonds of the substrates vinyl acetate (VA) and 2-hydroxyethyl acrylate (HEA), respectively (Figure 1a,b). The symmetry breaking of the nascent p-H2-derived protons allows for the hyperpolarization to become observable.[7, 15] The solutions were prepared with ~0.4 M of substrates and 4 mM of catalyst (Bicyclo[2.2.1]hepta-2,5-diene)[1,4-bis(diphenylphosphino)butane]rhodium(I) tetrafluoroborate (Sigma-Aldrich, P/N 341126–100MG) in CD3OD. Nearly 100% p-H2 was employed using a home-built cryogenic generator.[16] At 75 °C and 100 psi, the substrates were fully converted into their respective HP products via bubbling p-H2 for 15 second with a 150 sccm flow rate controlled by a mass-flow controller, as described previously (Figure 1c).[17] The chemical conversion was monitored by comparing the 1H NMR spectra of thermally polarized samples taken before and after the PHIP reactions (Figures S1 and S2). The polarization of HA and HB was estimated to be between 10% and 20% based on previous study[18] and their T1 relaxation times was measured to be ~16–25 seconds (Figure S3).
Figure 1.
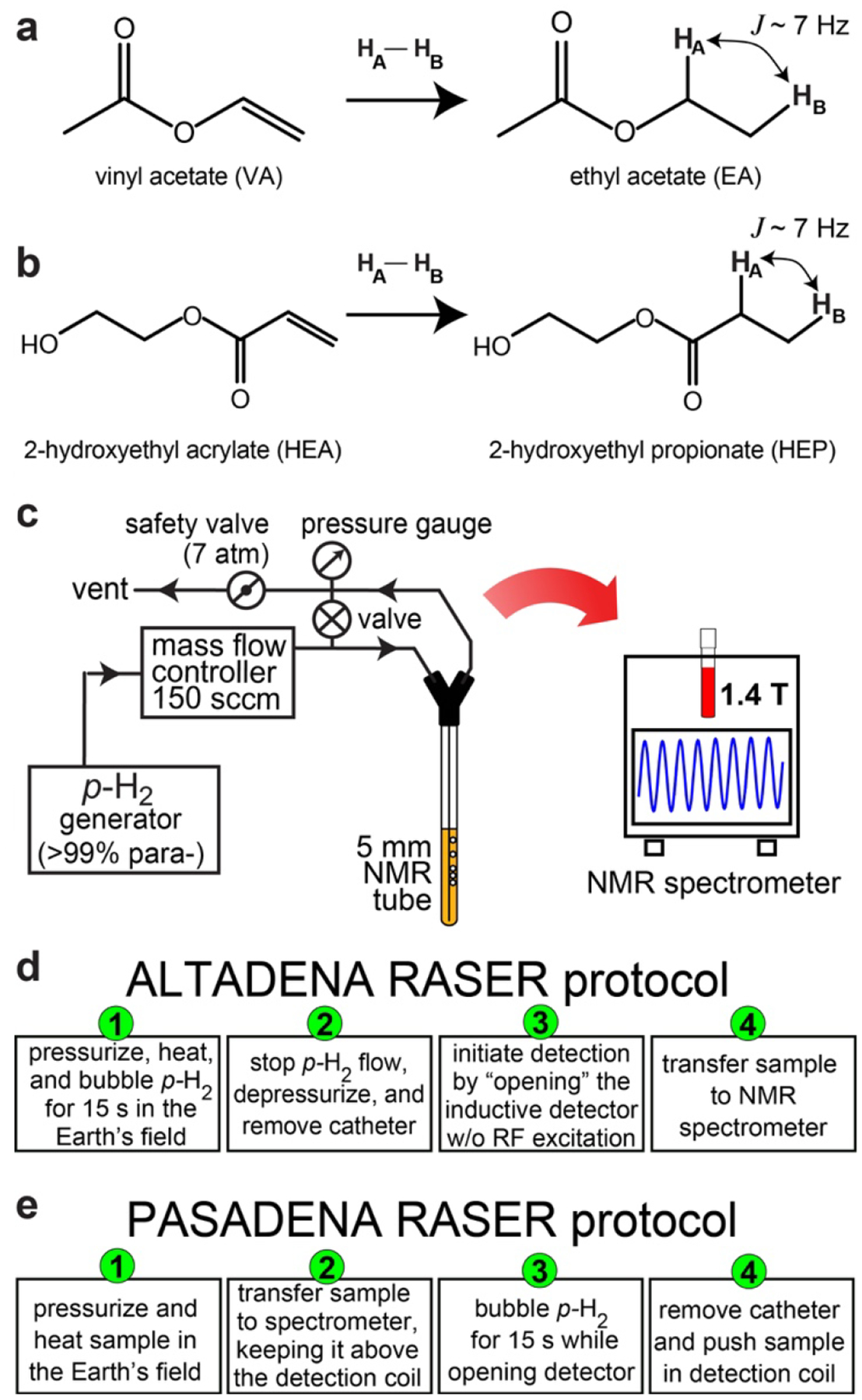
a,b) Reaction schemes for the p-H2 pairwise addition to vinyl acetate (VA) yielding to ethyl acetate (EA) and to 2–2-hydroxyethyl acrylate (HEA) yielding to 2-hydroxyethyl propionate (HEP), respectively. c) Schematic of the experimental setup. d,e) ALTADENA and PASADENA protocols used to evidence parahydrogen-induced RASER activity, respectively.
Two main experimental protocols were followed. The first protocol corresponds to the ALTADENA condition.[19] In this case, the samples were hydrogenated in the Earth’s magnetic field (~ 50 μT). The p-H2 flow was then interrupted, the sample depressurized, the catheter removed, the “pulse-and-acquire sequence” initiated, and the sample inserted in the NMR spectrometer (Figure 1d). To make sure that the sample does not experience any RF excitation, the sample was inserted several seconds after an RF pulse with a flip angle lower than 0.01° was applied. The detector channel was opened for up to 32 seconds. The second protocol corresponds to the PASADENA condition,[20] in which the hydrogenation reaction was performed at 1.4 T, i.e., inside the spectrometer. During the hydrogenation reaction, the sample was positioned 4–5 cm above the RF coil so the catheter could be removed without interfering with the signal detection, which was initiated before the hydrogenation reaction was completed. Once the reaction was completed and the catheter removed, the sample was pushed inside the RF coil (Figure 1e).
The main results obtained for EA and HEP are illustrated in Figure 2 and Figure 3, respectively. For both ALTADENA and PASADENA experiments, the NMR signal exhibits the characteristic features of RASER activity: Signal persistence for significantly longer periods of time than T2* of ~ 0.6 s.[1, 13] The Fourier transformed spectra of selected regions with defined duration of the RASER active signals, e.g., Figure 2b,c for ALTADENA and Figure 2i–l for PASADENA, clearly show evidence for the enhanced spectral resolution due to RASER activity with sharp peaks with FWHM < 0.2 Hz, whereas the resolution of the spectrometer is ~0.5 Hz after full shimming and ~2 Hz in case of conventional HP experiments. Immediately after these RASER active signals were recorded, additional NMR spectra were acquired using ~3.3° excitation RF pulse. The first of those spectra show partially RASER active NMR lines (see Figure 2e in case of ALTADENA and Figure 3l in case of PASADENA), while the subsequent acquisitions correspond to normal, hyperpolarized ALTADENA (Figures 2g and 3g) and PASADENA (Figures 2n and 3n) spectra.
Figure 2.
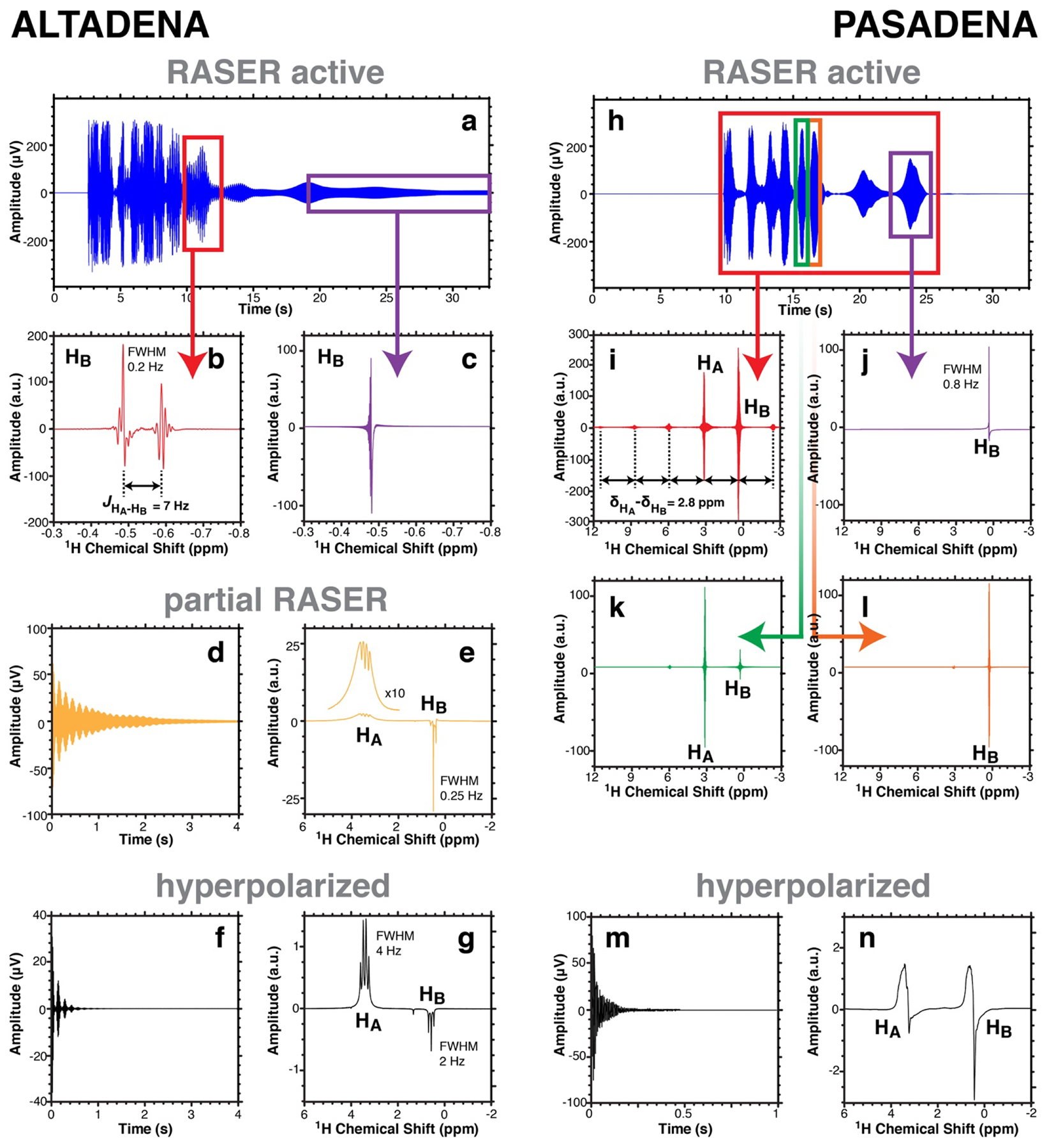
1H NMR spectroscopy of solution-phase PHIP of 0.4 M HP ethyl acetate (EA) probed at 1.4 T. a) ALTADENA RASER signal recorded without RF pulse after hydrogenation in the Earth’s magnetic field. b,c) Fourier spectra of the regions outlined by red and purple boxes in display a), respectively. d,e) Partial ALTADENA RASER signal and Fourier spectrum obtained with ~3° RF pulse. f,g) Canonical ALTADENA (non-RASER) FID and Fourier spectrum recorded after further polarization decay. h) PASADENA RASER signal recorded without RF pulse after hydrogenation at 1.4 T. i,j,k,l) Fourier spectra of the regions outlined by red, purple, green and orange boxes in display h), respectively. m,n) Canonical PASADENA (non-RASER) FID and Fourier spectrum acquired with ~3° RF pulse after further polarization decay (~30 s).
Figure 3.
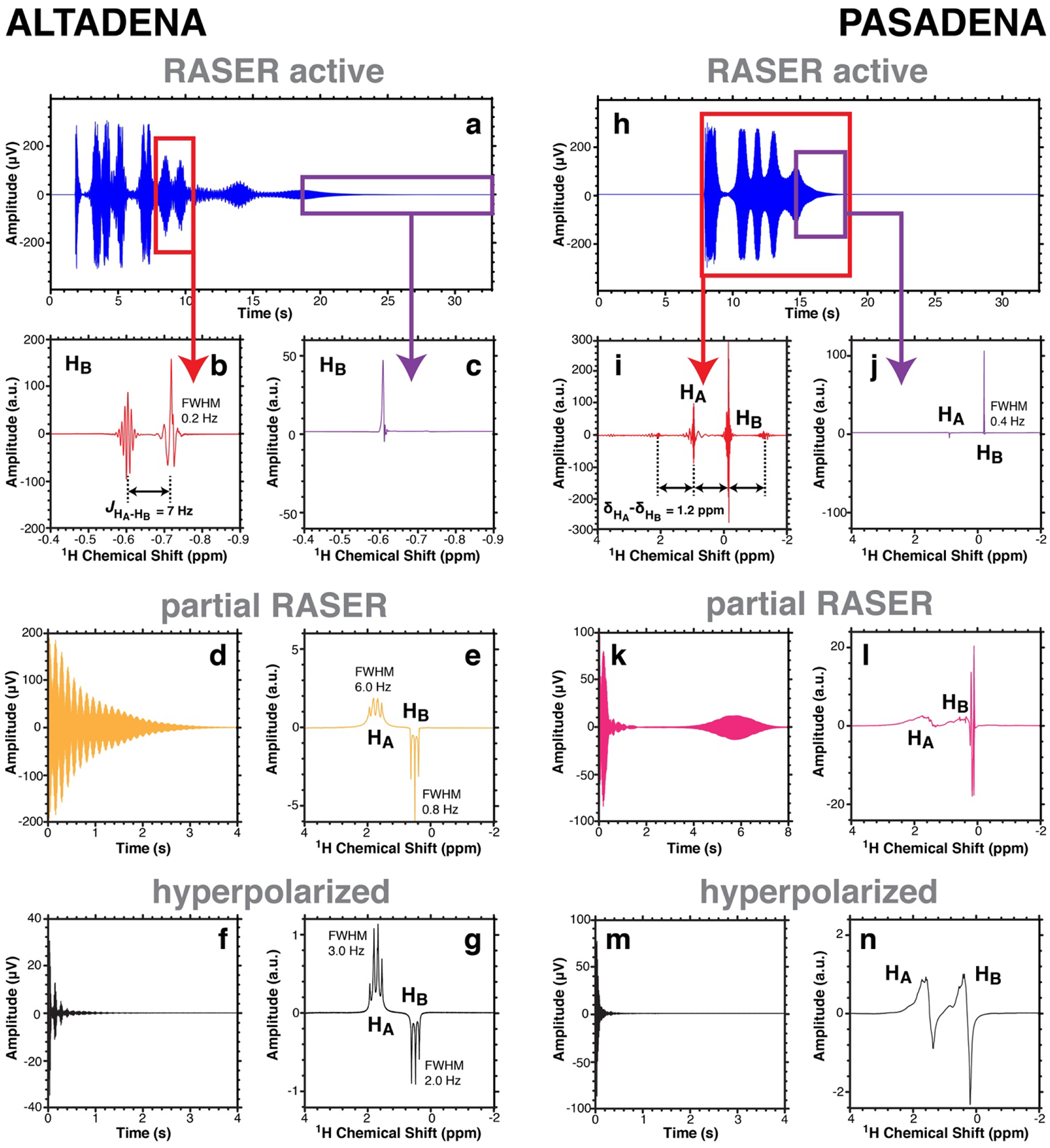
1H NMR spectroscopy of solution-phase PHIP of 0.4 M HP 2-hydroxyethyl propionate (HEP) probed at 1.4 T. a) ALTADENA RASER signal recorded without RF pulse after hydrogenation in the Earth’s magnetic field. b,c) Fourier spectra of the regions outlined by red and purple lines in display b), respectively. d,e) Partial ALTADENA RASER signal and Fourier spectrum acquired using ~3° RF pulse. f,g) Canonical ALTADENA (non-RASER) FID and Fourier spectrum recorded after further polarization decay (~30 s). h) PASADENA RASER signal recorded without RF pulse after hydrogenation at 1.4 T. i,j) Fourier spectra of the regions outlined by red and purple lines in display h), respectively. k,l) Partial PASADENA RASER signal and Fourier spectrum acquired using ~3° RF pulse. m,n) Canonical PASADENA (non-RASER) FID and Fourier spectrum acquired with ~3° RF pulse after further polarization decay (~30 s).
With ALTADENA, the Fourier spectra of the time slices of the RASER active signals (displayed in Figures 2a and 3a) show a doublet (Figures 2b and 3b). These two RASER resonances can be attributed to lines in the PHIP spectra depicted in Figures 2g and 3g respectively. In particular, each of the three triplet lines corresponding to proton HB are population inverted (have negative sign) and its two most intense lines are RASER active. The doublet is separated by splitting corresponding to the spin-spin coupling JHA-HB of 7.0 Hz between proton HA and HB in EA and HEP (Figure 2b and 3b respectively).[21] While the HP state decays, the number of RASER active lines changes, for example from two RASER active lines in Figures 2b and 3b to one single line in Figures 2c and 3c correspondingly. This could be explained by different transverse relaxation rates and multiplicities of each RASER line. For instance, at low polarization only one line with the highest amplitude in the NMR spectrum and with the smallest line-width overcomes the RASER threshold and is RASER active.
All ALTADENA-hyperpolarized RASER spectra from Figures 2 and 3 differ significantly from the corresponding PHIP spectra in Figures 2g and 3g. The latter feature the HP resonances of HA and HB with the lines of the quartet and the triplet spectrum of opposite signs. These quartet and triplet are separated by ~2.8 ppm (~174 Hz) for EA and ~1.2 ppm (~74 Hz) for HEP. Specifically, the linewidth of the quartet FWHM of ~4 Hz in Figure 2g is significantly broader compared to the linewidth of each of the triplet lines with FWHM of ~2 Hz. This is more pronounced in Figure 2e, where the difference in linewidth is more than one order of magnitude. The same trend is observed in Figures 3e and 3g. The reason for this is the sign and the magnitude of the HP state, which introduce a broadening with κtot >1/T2* for the quartet lines and a narrowing with κtot <1/T2* of the triplet lines. For the RASER lines in Figures 2b and 3b κtot is negative and the linewidth in principal is only limited by the finite measurement time and ultimately by the Cramér–Rao condition.[22] We conclude that the RASER spectra of VA and HEP hyperpolarized by ALTADENA allow the J-coupling constant JHA-HB to be determined with enhanced precision but the chemical shift difference between HA and HB is not measurable in this RASER experiment.
The analysis of the RASER active signals in the PASADENA case (Figure 2h and 3h) renders other interesting observations in addition to the anticipated line narrowing. In particular, the Fourier spectra of the RASER active signals (Figures 2i and 3i) exhibit two large central RASER lines separated by the chemical shift difference δHA − δHB between the HA and HB protons, i.e., δHA − δHB = 2.8 ppm (~174 Hz) for EA and 1.2 ppm (~74 Hz) for HEP. The two central lines are accompanied by evenly spaced small sidebands, and the distance between two consecutive lines is exactly δHA − δHB. This can be explained by the non-linear interaction between different RASER active modes (here two), leading to a frequency comb like spectrum.[2] We also found even frequency comb like spectra in the case of the ALTADENA pumped RASER where the two central modes and all sidebands are spaced by JHA-HB. Moreover, the resonance frequencies of the RASER active protons (Figures 2b, 2c, 3b, and 3c) are sometimes shifted by about 1 ppm when compared with the partial RASER and hyperpolarized ones. We speculate that this is due to the magnetic field fluctuations induced by RASER. A detailed evaluation of these and other nonlinear phenomena will be published elsewhere.
A series of additional experiments were performed, demonstrating further that the experimental conditions necessary for observing RASER through PHIP reactions are not stringent at all. RASER bursts can indeed be observed with more dilute samples, as illustrated by the NMR signal shown in Figure S4 and obtained with a 40 mM VA solution. This indicates that PHIP RASER occurs at relatively low concentrations of HP substrate; in particular lower concentrations than those reported previously for spontaneous emission of NMR signals with the dissolution Dynamic Nuclear Polarization (d-DNP) technique.[23] ALTADENA RASER activity was also observed while leaving the catheter (1/16” outer diameter, 1/32” inner diameter) inside the NMR tube, thus creating more stringent conditions for its occurrence because the presence of the catheter leads to significant susceptibility induced B0 gradient and effectively shorter T2* (see Figures S5 and S6). Note that in additional PASADENA experiments, the hydrogenation reactions were performed within the RF coil and RASER was detected immediately after the cessation of p-H2 bubbling because the bubbles induce a significant degradation of T2* (likely below 1 ms) that prevents RASER activity at this magnetic field.
These findings are crucial in the context of PHIP studies and biomedical applications. The HP substrates used here can indeed be employed as in vivo contrast agents. For example, HP HEP has been extensively studied as a potential contrast agent in angiography studies.[24–26] Because some of the PHIP techniques rely on the application of RF pulses, especially in the case of polarization transfer from protons to 13C nuclei,[27–35] the RF coils may interact with the highly proton-hyperpolarized compounds resulting in complicated nonlinear effects and depletion of hyperpolarization via RASER activity. Therefore, RASER effects may be considered as an obstacle in this context. The use of untuned RF coils[36, 37] may help mitigate the occurrence of RASER, so that the hyperpolarized proton pool is not depleted prior its utilization during contrast agent preparation. Moreover, the recent advent of PHIP via side-arm hydrogenation (SAH) significantly expanded the range of biomolecules (including ethyl acetate) that can be hyperpolarized via PHIP.[17, 38] With this technique, a wide range of carboxylic acids have been hyperpolarized and employed in vivo for metabolism tracking[21, 27, 38–42]
Amid the peer-review evaluation of the present work, another interesting study regarding PHIP-RASER by Pravdivtsev and co-workers was reported.[43] In contrast with the common and somewhat ordinary conditions we have presented here, Pravdivtsev et al. designed a specific experiment dedicated to observe parahydrogen-induced RASER activity under PASADENA conditions at a magnetic field of 14 T (600 MHz) and with a cryogenically-cooled coil (Q~500).[43] The catalyst activity was tuned for building up polarization throughout an extended period of time (about 10 min) with a continuous delivery of p-H2. The p-H2 pairwise addition was performed with two substrates incorporating C≡C triple bonds. Without RF pulse excitation, RASER activity was detected only after bubbling p-H2 for about 90 s., Only chemical shifts but no J-couplings could be determined in these very high field experiments and the reported line-widths (in the order of 1 Hz) of the RASER active lines do not differ significantly from the linewidth obtained by a corresponding standard NMR spectrum (a few ppb at 600 MHz). The work presented in this article differs in many regards. First and foremost, the equipment employed here is a commercially available NMR spectrometer with unaltered room-temperature RF coil with Q of ~68—such instrumentation is widely available making our observations possible and easy to replicate by others interested in using PHIP-RASER effect. Second, we demonstrated PHIP-RASER under both PASADENA and ALTADENA conditions and showed intriguing J-coupling and chemical-shift controlled dynamics of RASER signal evolution. No RF stimulation was required to induced RASER effect. Third, we employed two molecular moieties (acetate[44, 45] and propionate[25] via pairwise p-H2 addition to double C=C bond) that have been previously employed in in vivo bio-imaging studies of perfusion and metabolism. Importantly, we employed the process of batch hyperpolarization,[34, 35] when a bolus of material is hyperpolarized over a short period of time (ca. 10 s), and such bolus could be employed for in vivo imaging applications—paving the way to potential future use of RASER in bio-imaging applications, which have the potential to revolutionize MRI and medical imaging.
Conclusion
To summarize, RASER activity of two PHIP-hyperpolarized compounds is reported here using standard NMR hardware at concentrations as low as 40 mM and at estimated proton polarization values of over 10% at the time of the detection.[17] RASER activity is observed with and without applications of RF excitation pulses and under both ALTADENA and PASADENA conditions. J-coupling constants as well as chemical shift differences could be measured with increased precision. As the field of PHIP continues to grow, researchers using standard, commercial NMR spectrometers are therefore expected to experience RASER activity and radiation damping or line narrowing phenomena routinely. Appropriate considerations must be made when performing NMR experimentations with such highly polarized compounds. Our observations are especially important for PHIP studies that aim at providing highly polarized contrast agents for imaging of metabolism, i.e., where high levels of polarization at high substrate concentrations are desired.[46] Despite its complications the parahydrogen-induced RASER phenomenon described here could enable new applications in magnetic resonance, quantum computing, data encryption and beyond.[1, 2, 13] Further studies are underway in our laboratories to provide additional theoretical and experimental insights about this intriguing phenomenon and its applications.
Supplementary Material
Acknowledgements
This work was supported by NSF under Grant CHE-1904780, National Cancer Institute under 1R21CA220137, and by DOD CDMRP under BRP W81XWH-12-1-0159/BC112431 and under W81XWH-15-1-0271. Research reported in this publication was also supported by the National Institute of Biomedical Imaging and Bioengineering of the NIH under R21EB025313. In addition, T.T. and S.L. acknowledge funding from the North Carolina Biotechnology Center as well as the Mallinckrodt Foundation.
References
- [1].Suefke M, Lehmkuhl S, Liebisch A, Blumich B, Appelt S, Nat. Phys 2017, 13, 568–572. [Google Scholar]
- [2].Appelt S, Kentner A, Lehmkuhl S, Blümich B, Prog. Nucl. Mag. Res. Spectrosc 2019, 114–115, 1–32. [DOI] [PubMed] [Google Scholar]
- [3].Nikolaou P, Goodson BM, Chekmenev EY, Chem. Eur. J 2015, 21, 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K, Proc. Natl. Acad. Sci. U. S. A 2003, 100, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ardenkjaer-Larsen J-H, Boebinger GS, Comment A, Duckett S, Edison AS, Engelke F, Griesinger C, Griffin RG, Hilty C, Maeda H, Parigi G, Prisner T, Ravera E, van Bentum J, Vega S, Webb A, Luchinat C, Schwalbe H, Frydman L, Angew. Chem. Int. Ed 2015, 54, 9162–9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Navon G, Song YQ, Room T, Appelt S, Taylor RE, Pines A, Science 1996, 271, 1848–1851. [Google Scholar]
- [7].Kovtunov KV, Pokochueva EV, Salnikov OG, Cousin S, Kurzbach D, Vuichoud B, Jannin S, Chekmenev EY, Goodson BM, Barskiy DA, Koptyug IV, Chem. Asian J 2018, 13, 1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nikolaou P, Coffey AM, Walkup LL, Gust BM, Whiting N, Newton H, Barcus S, Muradyan I, Dabaghyan M, Moroz GD, Rosen M, Patz S, Barlow MJ, Chekmenev EY, Goodson BM, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rayner PJ, Burns MJ, Olaru AM, Norcott P, Fekete M, Green GGR, Highton LAR, Mewis RE, Duckett SB, Proc. Natl. Acad. Sci. U. S. A 2017, 114, E3188–E3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J, Williamson DC, Science 2009, 323, 1708–1711. [DOI] [PubMed] [Google Scholar]
- [11].Adams RW, Duckett SB, Green RA, Williamson DC, Green GGR, J. Chem. Phys 2009, 131, 194505. [DOI] [PubMed] [Google Scholar]
- [12].Cowley MJ, Adams RW, Atkinson KD, Cockett MCR, Duckett SB, Green GGR, Lohman JAB, Kerssebaum R, Kilgour D, Mewis RE, J. Am. Chem. Soc 2011, 133, 6134–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suefke M, Liebisch A, Blumich B, Appelt S, Nature Phys. 2015, 767–771. [Google Scholar]
- [14].Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL, J. Am. Chem. Soc 1987, 109, 8089–8091. [Google Scholar]
- [15].Bowers CR, Weitekamp DP, Phys. Rev. Lett 1986, 57, 2645–2648. [DOI] [PubMed] [Google Scholar]
- [16].Feng B, Coffey AM, Colon RD, Chekmenev EY, Waddell KW, J. Magn. Reson 2012, 214, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Salnikov OG, Chukanov NV, Shchepin RV, Manzanera Esteve IV, Kovtunov KV, Koptyug IV, Chekmenev EY, J. Phys. Chem. C 2019, 123, 12827–12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Joalland B, Schmidt A, Kabir MSH, Chukanov NV, Kovtunov KV, Koptyug IV, Hennig J, Hövener J-B, Chekmenev EY, Anal. Chem 2020, 92, 1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pravica MG, Weitekamp DP, Chem. Phys. Lett 1988, 145, 255–258. [Google Scholar]
- [20].Bowers CR, Weitekamp DP, J. Am. Chem. Soc 1987, 109, 5541–5542. [Google Scholar]
- [21].Cavallari E, Carrera C, Boi T, Aime S, Reineri F, J. Phys. Chem. B 2015, 119, 10035–10041. [DOI] [PubMed] [Google Scholar]
- [22].Heil W, Gemmel C, Karpuk S, Sobolev Y, Tullney K, Allmendinger F, Schmidt U, Burghoff M, Kilian W, Knappe-Grüneberg S, Schnabel A, Seifert F, Trahms L, Annalen der Physik 2013, 525, 539–549. [Google Scholar]
- [23].Chen H-Y, Lee Y, Bowen S, Hilty C, J. Magn. Reson 2011, 208, 204–209. [DOI] [PubMed] [Google Scholar]
- [24].Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD, Magn. Reson. Mater. Phy 2005, 18, 245–256. [DOI] [PubMed] [Google Scholar]
- [25].Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS, Magn. Reson. Med 2001, 46, 1–5. [DOI] [PubMed] [Google Scholar]
- [26].Goldman M, Johannesson H, Axelsson O, Karlsson M, Magn. Reson. Imaging 2005, 23, 153–157. [DOI] [PubMed] [Google Scholar]
- [27].Korchak S, Mamone S, Glöggler S, ChemistryOpen 2018, 7, 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Korchak S, Yang S, Mamone S, Glöggler S, ChemistryOpen 2018, 7, 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Waddell KW, Coffey AM, Chekmenev EY, J. Am. Chem. Soc 2011, 133, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cai C, Coffey AM, Shchepin RV, Chekmenev EY, Waddell KW, J. Phys. Chem. B 2013, 117, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goldman M, Johannesson H, Physique CR 2005, 6, 575–581. [Google Scholar]
- [32].Goldman M, Johannesson H, Axelsson O, Karlsson M, Chimie CR 2006, 9, 357–363. [Google Scholar]
- [33].Schmidt AB, Berner S, Schimpf W, Müller C, Lickert T, Schwaderlapp N, Knecht S, Skinner JG, Dost A, Rovedo P, Hennig J, von Elverfeldt D, Hövener JB, Nat. Commun 2017, 8, 14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Coffey AM, Shchepin RV, Truong ML, Wilkens K, Pham W, Chekmenev EY, Anal. Chem 2016, 88, 8279–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Coffey AM, Shchepin RV, Feng B, Colon RD, Wilkens K, Waddell KW, Chekmenev EY, J. Magn. Reson 2017, 284, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hövener J-B, Chekmenev EY, Harris KC, Perman W, Robertson L, Ross BD, Bhattacharya P, Magn. Reson. Mater. Phy 2009, 22, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hövener J-B, Chekmenev EY, Harris KC, Perman W, Tran T, Ross BD, Bhattacharya P, Magn. Reson. Mater. Phy 2009, 22, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Reineri F, Boi T, Aime S, Nat. Commun 2015, 6, 5858. [DOI] [PubMed] [Google Scholar]
- [39].Cavallari E, Carrera C, Sorge M, Bonne G, Muchir A, Aime S, Reineri F, Sci. Rep 2018, 8, 8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cavallari E, Carrera C, Aime S, Reineri F, Chem. Eur. J 2017, 23, 1200–1204. [DOI] [PubMed] [Google Scholar]
- [41].Cavallari E, Carrera C, Aime S, Reineri F, J. Magn. Reson 2018, 289, 12–17. [DOI] [PubMed] [Google Scholar]
- [42].Shchepin RV, Barskiy DA, Coffey AM, Manzanera Esteve IV, Chekmenev EY Angew. Chem. Int. Ed 2016, 55, 6071–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pravdivtsev AN, Sönnichsen FD, Hövener J-B, ChemPhysChem 2020, 10.1002/cphc.201901056. [DOI] [Google Scholar]
- [44].Bastiaansen JAM, Cheng T, Mishkovsky M, Duarte JMN, Comment A, Gruetter R, Biochim. Biophys. Acta-Gen. Subj 2013, 1830, 4171–4178. [DOI] [PubMed] [Google Scholar]
- [45].Mikkelsen EFR, Mariager CØ, Nørlinger T, Qi H, Schulte RF, Jakobsen S, Frøkiær J, Pedersen M, Stødkilde-Jørgensen H, Laustsen C, Sci. Rep 2017, 7, 16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hövener J-B, Pravdivtsev AN, Kidd B, Bowers CR, Glöggler S, Kovtunov KV, Plaumann M, Katz-Brull R, Buckenmaier K, Jerschow A, Reineri F, Theis T, Shchepin RV, Wagner S, Bhattacharya P, Zacharias NM, Chekmenev EY, Angew. Chem. Int. Ed 2018, 57, 11140–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


