Keywords: beam-walking test, brain-derived neurotrophic factor, cerebral ischemia, correlation analysis, enriched environment, fibronectin type III domain-containing protein 5, Morris water maze task, neural plasticity, neuroprotection, permanent middle cerebral artery occlusion
Abstract
Many studies have shown that fibronectin type III domain-containing protein 5 (FDNC5) and brain-derived neurotrophic factor (BDNF) play vital roles in plasticity after brain injury. An enriched environment refers to an environment that provides animals with multi-sensory stimulation and movement opportunities. An enriched environment has been shown to promote the regeneration of nerve cells, synapses, and blood vessels in the animal brain after cerebral ischemia; however, the exact mechanisms have not been clarified. This study aimed to determine whether an enriched environment could improve neurobehavioral functions after the experimental inducement of cerebral ischemia and whether neurobehavioral outcomes were associated with the expression of FDNC5 and BDNF. This study established ischemic mouse models using permanent middle cerebral artery occlusion (pMCAO) on the left side. On postoperative day 1, the mice were randomly assigned to either enriched environment or standard housing condition groups. Mice in the standard housing condition group were housed and fed under standard conditions. Mice in the enriched environment group were housed in a large cage, containing various toys, and fed with a standard diet. Sham-operated mice received the same procedure, but without artery occlusion, and were housed and fed under standard conditions. On postoperative days 7 and 14, a beam-walking test was used to assess coordination, balance, and spatial learning. On postoperative days 16–20, a Morris water maze test was used to assess spatial learning and memory. On postoperative day 15, the expression levels of FDNC5 and BDNF proteins in the ipsilateral cerebral cortex were analyzed by western blot assay. The results showed that compared with the standard housing condition group, the motor balance and coordination functions (based on beam-walking test scores 7 and 14 days after operation), spatial learning abilities (based on the spatial learning scores from the Morris water maze test 16–19 days after operation), and memory abilities (based on the memory scores of the Morris water maze test 20 days after operation) of the enriched environment group improved significantly. In addition, the expression levels of FDNC5 and BDNF proteins in the ipsilateral cerebral cortex increased in the enriched environment group compared with those in the standard housing condition group. Furthermore, the Pearson correlation coefficient showed that neurobehavioral functions were positively associated with the expression levels of FDNC5 and BDNF (r = 0.587 and r = 0.840, respectively). These findings suggest that an enriched environment upregulates FDNC5 protein expression in the ipsilateral cerebral cortex after cerebral ischemia, which then activates BDNF protein expression, improving neurological function. BDNF protein expression was positively correlated with improved neurological function. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Fudan University, China (approval Nos. 20160858A232, 20160860A234) on February 24, 2016.
Introduction
Ischemic stroke is associated with a high death rate and a lack of effective treatment interventions. Recent studies have shown that enriched-environment (EE) training can have neuroprotective effects for brain function, including the promotion of neural plasticity (Jiang et al., 2016; Wang et al., 2019). Although EE training has been well-documented to improve neurobehavioral functions and to alter brain structures, the potential mechanisms underlying these beneficial effects have not been fully elucidated.
Neurotrophic factors are involved in the survival, proliferation, maturation, and growth of neurons in intact and damaged brains (Miller and Kaplan 2001; Bekinschtein et al., 2008; Cruz et al., 2018; Wagenaar et al., 2018). Previous studies have found that increased levels or the activation of brain-derived neurotrophic factor (BDNF) and related signaling pathways were associated with better recovery after stroke (Ploughman et al., 2007; de la Tremblaye et al., 2017). BDNF levels have been reported to increase in cultured neurons and brains following ischemic events (Zhao et al., 2013). Furthermore, BDNF levels increase in surrounding tissues after stroke (Clarkson et al., 2011), including dentate granule cells and the hippocampus (Wang et al., 2017). Some studies have shown that BDNF promotes nerve regeneration, ischemic penumbra angiogenesis, and the inhibition of inflammation, neurotoxicity, epilepsy, and apoptosis (Clarkson et al., 2011; Balaratnasingam and Janca, 2012; Zhao et al., 2014; Sanna et al., 2017).
Fibronectin type III domain-containing protein 5 (FDNC5), which was previously described as the peroxisome protein, is believed to be a transmembrane precursor protein that is processed into a soluble, secreted peptide, called irisin, which is highly conserved in mammals. FDNC5 plays a vital role in the neuronal differentiation of embryonic stem cells. Studies have claimed that irisin can regulate hippocampal neurogenesis. In the presence of irisin, the number of hippocampal neurons in the mouse brain increased. FDNC5 can also increase the expression level of BDNF (Zsuga et al., 2016). FDNC5 plays a vital role during the regulation of neuronal development.
In animal studies, EE has been associated with increased activity in the brain. Based on a recent review, EE represents an experienced experimental condition, which can be implemented by exposing experimental animals to improved habitats, raising animals in large cages among social groups, providing access to toys, running cycles, and periodically varying sensory stimuli, and EE exposure in animals increases the abilities of neurons to resist insults (Novkovic et al., 2015). EE is an active rehabilitation training mode that is not associated with stress response and is not harmful to animals. Moreover, this rehabilitation model is very suitable for group therapy. Although this type of rehabilitation therapy has been shown to be associated with curative effects, the specific mechanisms underlying these effects are not clear.
This study investigated the effects of EE exposure on the neurologic behavioral functions and the expression levels of FNDC5 and BDNF in an ischemic mouse model.
Materials and Methods
Animals
A total of 40 healthy, male, C57BL/6 mice, aged 8–12 weeks and weighing 25–28 g, were purchased from Jie Si Jie Lab Animal Co., Ltd., Shanghai, China (Animal license No. SCXK [Hu] 2018-004). Mice were housed at 20°C and 45–50% humidity, in a 12-hour light/dark cycle (lights from 8:00 to 20:00), and allowed free access to water and food. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Fudan University, China (approval Nos. 20160858A232 and 20160860A234) on February 24, 2016. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Experimental design
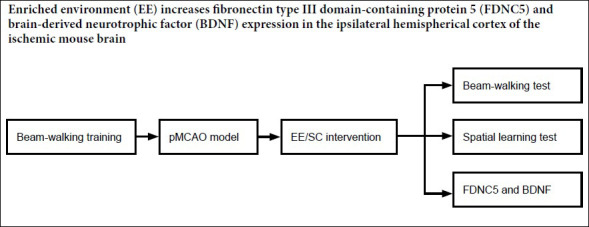
All of the time points used for the subsequent operations and tests are described in Figure 1. A total of 26 mice were subjected to permanent middle cerebral artery occlusion (pMCAO) on the left side. Two mice died after the operation. On postoperative day one, the remaining mice were randomly assigned to either the EE group (n = 12) or the standard housing condition (SC) group (n = 12). The sham group contained 12 sham-operated mice. The EE group and two normal mice were maintained in an EE cage for 14 days. Mice in the SC and sham groups were maintained in cages under standard conditions for 14 days. Beam-walking training was conducted for three consecutive days before surgery. Beam-walking tests were performed on days 7 and 14 after surgery. Western blots were performed on samples collected 15 days after surgery from six mice in each of the EE and SC groups. The Morris water maze task was performed on days 16–20 after surgery in six mice from each of the EE and SC groups.
Figure 1.

A schematic diagram of the experimental design.
Beam-walking training was conducted for 3 consecutive days before surgery. Beam-walking tests were conducted on days 7 and 14 after surgery. Brain samples analyzed by western blots were collected on day 15 after pMCAO. Spatial learning was tested from days 16 to 19 after surgery. A memory test was performed on day 20 after the operation. EE: Enriched environment; pMCAO: permanent middle cerebral artery occlusion; SC: standard housing conditions.
pMCAO models
Model and cerebral blood flow
According to previous studies (Engel et al., 2011; Neher et al., 2011), pMCAO on the left side can cause permanent cerebral ischemia. All mice were anesthetized with isoflurane (3% induction, 1.5–2.0% operation maintenance). To determine the appropriate suture location, cerebral perfusion was monitored using a Moor VMS-LDF2 monitor (Moor. Instruments, Devon, UK). If cerebral blood flow decreased to 30–35% and remained relatively stable, modeling was considered to be successful. pMCAO on the left side was performed, as previously described (Doyle and Buckwalter, 2014) Briefly, a needle probe, with a diameter of 1.9 mm, was placed directly on the skull above the MCA area (1 mm behind and 5 mm outside). The perfusion value of the carotid artery was recorded before and after the operation. The practical method was to perform a midline incision on the ventral surface of the neck. The left common carotid artery was separated and sutured with 6-0 silk threads. Both internal and external carotid arteries were sutured with 6-0 sutures. A 4-0 surgical nylon monofilament with a silicone tip was inserted into the internal carotid artery, through a proximal carotid artery incision. The filaments were then pushed approximately 6.5 cm from the distal end of the proximal carotid artery incision. The mice were allowed to recover from anesthesia on a heating pad at 37.0 ± 0.5°C and returned to their home cages after full recovery from anesthesia. In addition, sham-operated mice underwent the same procedure, without the induction of pMCAO.
Housing conditions
All animals that survived surgery on postoperative day 1 were assessed for neurological deficits (Longa et al., 1989). The score criteria for neurological deficit assessment were as follows: 0, no neurological deficit; 1, the right front paw does not fully extend and the grip is reduced when the tail is stretched; 2, voluntarily movement in all directions and turning right when running; 3, walking or hovering to the right, sensitive to nociception when stimulated; 4, no response to stimuli or stroke-related deaths. The mice with scores between 1 and 3 were randomly divided into the EE and SC groups. SC group mice were placed in a standard cage (21 cm wide, 27 cm long, and 16 cm high) (Figure 2A). The EE group mice were placed in the EE home cage (65 cm wide, 75 cm long, and 25 cm high), which contained climbing ladders, sports wheels, plastic tubes, toys, tunnels, running wheels, huts, and various decorations (Figure 2B). Mice in the EE group were provided with enhanced social stimuli. A total of 14 mice were maintained in the EE home cage (12 EE mice and 2 normal mice). The above conditions were changed after 3 days to maintain environmental novelty. SC mice were divided into four groups (n = 3). Sham-operated animals were maintained in standard cages under standard conditions. During the enrichment period, food and water were freely available. Mice in the EE group were fed in cages under EE conditions for 2 weeks.
Figure 2.

SC and EE housing conditions.
(A) SC pMCAO mice; (B) EE pMCAO mice. EE: Enriched environment; pMCAO: permanent middle cerebral artery occlusion; SC: standard housing conditions.
Neurological function tests
Beam-walking test
To assess the coordination and balance of movement, the beam-walking test was performed before cerebral ischemia and 7 and 14 days after cerebral ischemia. The beam-walking apparatus consisted of a square beam (1 cm wide, 30 cm long, 30 cm high) connected to a black box (20 cm × 10 cm × 10 cm). The mouse was placed on one end of the square beam and allowed to walk to the other end of the square beam, which was connected to a black box. The beam-walking test was performed three times and evaluated on a 0–3-point scale (3 points: walking distance > 20 cm; 2 points: walking distance < 20 cm; 1 point: walking distance < 10 cm; and 0 points: falling within a walking distance of 10 cm). The sum of points across the three evaluations was calculated for each mouse, as previously described (Li et al., 2017).
Morris water maze test
The Morris water maze test was used to test the spatial learning and memory of mice. As mentioned earlier, some modifications have been made (Vorhees and Williams, 2006). In short, the Morris water maze was performed in a black circular pool, filled with water, at 21–23°C, which was located in a room with obvious visual clues. Spatial learning was conducted from days 16 to 19 after the operation, for 4 consecutive days. Eight consecutive experiments (using randomly allotted beginning positions) were conducted daily to trace pathways taken by the mice to reach a platform submerged 1.5 cm beneath the water surface. The mice were allowed 60 seconds to locate the platform, which was maintained in a constant location. If they failed to locate the platform within 60 seconds, the mice were manually guided to the platform and maintained on the platform for 10 seconds. The average escape latency of each mouse was recorded and analyzed as an index of spatial learning. One day after the final acquisition training session (day 20 post pMCAO), all of the mice received a probe test with the escape platform removed. The mice were placed into the pool in the location most distal to the target quadrant (with the platform removed). The percent of time spent in the target quadrant was recorded and interpreted as spatial memory (Block, 1999).
Western blot assay
On postoperative day 15, the ipsilateral cortex surrounding the ischemic zone was harvested. The cortex protein extracts were analyzed by western blot assay. In brief, cortex tissue was homogenized in RIPA lysis buffer. The samples were heated at 95–100°C for 10 minutes, after centrifugation and concentration. Using a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (Biorad, Berkeley, CA, USA), 50 μg of protein were electrophoretically separated and transferred to a nitrocellulose membrane. The membrane was then sealed at room temperature and incubated in a 5% bovine serum albumin liquid (Beyotime, Shanghai, China), for 1 hour. The primary antibodies used were anti-FDNC5 (1:2000; rabbit monoclonal; Abcam, London, UK) and anti-BDNF (1:500; rabbit monoclonal; Abcam). Anti-β-tubulin (1:1000; rabbit polyclonal; Abcam) was used as a loading control. The membranes were incubated overnight with primary antibodies, at 4°C. Within an hour, the membrane was cleaned, washed, and incubated with secondary antibody (anti-mouse IgG, 1:5000; Abcam), at room temperature, for 2 hours. The intensity of protein bands was measured using a western imprinting image system and quantified by ImageJ analysis software (NIH, Bethesda, MD, USA). The ratio of the optical density of the target protein to that of β-tubulin was used as the relative expression level for that protein.
Statistical analysis
The data are presented as the mean ± standard error of the mean (SEM). SPSS 18.0 statistical software (SPSS, Chicago, IL, USA) was used for statistical analysis. Cerebral cortical blood flow value after pMCAO was analyzed using the paired Student’s t-test. The one-way analysis of variance, followed by a least significant difference post hoc test, was used to determine multiple comparisons between groups. The correlations between neurological function outcomes and FDNC5 and BDNF expression levels were analyzed using Pearson’s correlation coefficient. P < 0.05 was considered to be statistically significant.
Results
Alterations of cerebral cortical perfusion in experimental mice
As shown in Figure 3, cerebral cortical blood flow in the sham group showed no significant changes after the operation. In contrast, the cerebral cortical blood flow was decreased to 30–35% and remained stable after surgery in the pMCAO mice. The mean values for cerebral blood flow showed no significant differences between EE and SC pMCAO mice.
Figure 3.

Mean values of cerebral blood flow in EE and SC mice.
No significant difference was found between the EE and SC groups. The cerebral cortical blood flow decreased to 30–35% (n = 6). EE: Enriched environment; SC: standard housing conditions.
Neurological function
Effects of EE on motor coordination and balance
The beam-walking test was chosen to evaluate deficiencies in motor coordination and integration in ischemic mice. As exhibited in Figure 4, beam walking scores were better for ischemic mice in the EE group than those in the SC group.
Figure 4.

Beam-walking test scores in pMCAO mice.
*P < 0.05, vs. SC group; #P < 0.05, vs. sham group. Data are expressed as the mean ± SEM (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). EE: Enriched environment; SC: standard housing conditions; pMCAO: permanent middle cerebral artery occlusion.
Effects of EE on spatial learning and memory
According to the location and severity of the injury, cerebral ischemia may lead not only to motor deficits, but also to spatial learning and memory deficits. Therefore, the protective effects observed for EE on motor function were encouraging. Consequently, the Morris water maze was used to study whether EE has protective effects for learning and memory after ischemic injury. Preliminary assessments of escape latency to identify the underwater platform did not show any significant differences among these three groups during the first day of training (the 16th day after pMCAO). However, mice in the SC group demonstrated a significant increase in the latency to find the platform on days 2, 3 and 4 of training (days 17 18 and 19 post pMCAO, respectively) compared with both the sham and EE groups (Figure 5A). Spatial learning was assessed on the day after the last escape latency test (20 days after pMCAO). During this test, the platform was removed and the time spent by the mouse in the quadrant where the platform had previously been located (the correct quadrant) was measured. The results showed that the EE group spent more time in the correct quadrant than the SC group (Figure 5B).
Figure 5.

Effects of EE on spatial learning and memory of pMCAO mice.
(A) Evaluation of spatial learning, using the Morris water maze test, following pMCAO: The three groups showed no significant differences on day 16 post pMCAO; however, mice in the EE group required less time to find the platform on days 17, 18, and 19 post pMCAO compared with the SC group on those days. (B) Determination of spatial memory using the probe test on day 20 post pMCAO. Compared with the SC group, mice in the EE group spent significantly more time in the correct quadrant. Data are expressed as the mean ± SEM (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. SC group; #P < 0.05, vs. sham group. EE: Enriched environment; pMCAO: permanent middle cerebral artery occlusion; SC: standard housing conditions.
Western blot assay of FDNC5 and BDNF proteins
As displayed in Figure 6, the western blot assay demonstrated that FDNC5 and BDNF protein expression levels increased significantly in the EE group compared with the SC group (P < 0.05). These results indicated that EE promoted neural plasticity in the ischemic cortical zone.
Figure 6.

Western blot assay for FDNC5 (A) and BDNF (B) proteins in the cortex surrounding the ischemic border zone, using samples collected on day 15 after pMCAO.
The optical density values were normalized to their corresponding tubulin loading controls. Data are expressed as the mean ± SEM (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. SC group, #P < 0.05, vs. sham group. BDNF: Brain-derived neurotrophic factor; EE: enriched environment; FDNC5: fibronectin type III domain-containing protein 5; pMCAO: permanent middle cerebral artery occlusion; SC: standard housing conditions.
Relationship between neurological function outcomes and BDNF protein expression
To evaluate the possible relationship between BDNF protein expression and neurological function, Pearson’s correlation coefficient (r) was used to compare beam-walking test scores and BDNF protein expression levels 15 days after cerebral ischemia, as shown in Figure 7A. The beam-walking test scores were positively correlated with BDNF protein expression levels.
Figure 7.

Correlation between the beam-walking test scores and the expression levels of BDNF (A) and the correlation between the expression levels of FDNC5 and BDNF (B) in the EE group.
r represents Pearson’s correlation coefficient. P < 0.05 was considered to have a significant correlation. BDNF: Brain-derived neurotrophic factor; FDNC5: fibronectin type III domain-containing protein 5.
Correlation between the protein expression levels of FDNC5 and BDNF
Pearson’s correlation coefficient (r) was used to analyze the relationship between the relative expression levels of FDNC5 and BDNF proteins in the EE group. As shown in Figure 7B, the FDNC5 protein expression level was positively correlated with the BDNF expression level.
Discussion
BDNF is a member of the neurotrophin family and is involved in the differentiation, development, and survival of neurons. Some studies have suggested that elevated levels of BDNF may be beneficial during the recovery from acute and chronic stroke (Ploughman et al., 2005; Vaynman and Gomez-Pinilla, 2005; Rosenblum et al., 2015). The most probable mechanisms underlying these benefits involve the prevention of acute ischemic injury (Ferrer et al., 2001), increased angiogenesis (Kermani and Hempstead 2007; Pikula et al., 2013), increased brain repairs (Wang et al., 2013; Luo et al., 2015) and neurogenesis (Schabitz et al., 2007), and enhanced synaptic plasticity (Kuczewski et al., 2010; Balaratnasingam and Janca, 2012). Ischemic brain injury, a primary cause of mortality and morbidity, results from reduced oxygen and blood flow to the brain. EE training can reduce nerve injury and promote BDNF protein expression. Furthermore, some studies have suggested that FDNC5 expression is associated with BDNF expression, and FDNC5 plays a vital role during neuronal development (Lecker et al., 2012; Hashemi et al., 2013; Forouzanfar et al., 2015) and is expressed in the brain (Dun et al., 2013). A study observed significantly higher levels of BDNF and FNDC5 in quercetin-treated integrated synapses, as observed by transmission electron microscopy, and the improved performance by rats during the Morris water maze test highlighted the maintenance of neuronal function in quercetin-treated rats (Liu et al., 2015). Compared with SC rats, EE training was able to promote the expression of BDNF and FDNC5 after pMCAO. The levels of BDNF and FDNC5 in the brains of EE mice increased significantly, and these mice were able to maintain nerve function, as demonstrated by improved Morris water maze test results.
The direct injection of exogenous BDNF into the damaged brain is unsafe and impractical because it may cause additional damage. The intravenous infusion of exogenous BDNF is a relatively non-invasive treatment method. However, the permeability of BDNF through the blood-brain barrier is weak, dramatically reducing its application prospects and clinical value (Zhao et al., 2014). Consequently, determining safe, efficient and clinically applicable strategies for the administration of BDNF is necessary. Activating the expression of endogenous BDNF in the area surrounding the cerebral infarction may represent a better solution (Peng et al., 2008; He et al., 2018) than exogenous BDNF administration.
EE is an effective rehabilitation program for rodents and animals, implemented by exposing the animals to larger social groups, in larger cages, with many new objects to promote increased social interactions, activities, and exploration (Xie et al., 2013). EE provides various sensory, motor, and cognitive stimuli, improving the morphological and biochemical changes in the adult brain, and promotes the functional recovery of brain-injured animals (Yu et al., 2014). Scholars have suggested that EE stimuli may induce some varieties of plasticity in the brains of adults, which range from biochemical parameters to dendritic growth, gliogenesis, and neurogenesis (Nithianantharajah and Hannan 2006; Zhang et al., 2017). An enriched environment was shown to induce significant increases in the cell body and nucleus sizes, dendritic branch numbers, dendritic spine densities, and synapse numbers (Mataga et al., 2004; Leggio et al., 2005; Alvarez and Sabatini, 2007; Karpova, 2014; Moreno-Jimenez et al., 2019).
Some studies have shown that EE can enhance brain plasticity and promote the release of BDNF (Nithianantharajah and Hannan, 2006; Shono et al., 2011; Moradi-Kor et al., 2019). Some experimental data have suggested that the protective effects of EE are associated with increased BDNF expression levels (Lu, 2003), which are consistent with our results. Therefore, this treatment strategy for cerebral ischemia treatment may be more promising than neurotrophic therapy alone.
In summary, EE treatment should translate into a rehabilitation strategy for poststroke patients in the clinical setting. However, many factors must be examined prior to the routine clinical use of this therapy. Many studies include multiple stimulation modalities during EE, and no quantitative indicator exists for EE. In our previous study, we only quantified the amount of exercise received by mice in an EE environment (Yu et al., 2013), whereas this study used multiple stimuli. Therefore, most research must be performed to elaborate on the specific role played by EE. The mechanisms underlying the EE training effects observed for neuroprotection after cerebral ischemia require further investigations.
Additional file: Open peer review reports 1 (87.5KB, pdf) and 2 (88.8KB, pdf) .
Acknowledgments
We are grateful for the financial and equipment supports by the Med-X Research Institute of Shanghai Jiao Tong University, China.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This study was supported by the National Natural Science Foundation of China, Nos. 81601961 (to KWY), 81672242 (to YW); the Key Construction Projects of Shanghai Health and Family Planning on Weak Discipline, China, No. 2015ZB0401 (to YW). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The experimental protocols were approved by the Institutional Animal Care and Use Committee of Fudan University, China (approval Nos. 20160858A232, 20160860A234) on February 24, 2016. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Peng Luo, Fourth Military Medical University, China; Fabricio Ferreira de Oliveira, Universidade Federal de Sao Paulo, Brazil.
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81601961 (to KWY), 81672242 (to YW); the Key Construction Projects of Shanghai Health and Family Planning on Weak Discipline, China, No. 2015ZB0401 (to YW).
P-Reviewers: Luo P, de Oliveira FF; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Giles L, de Souza M, Qiu Y, Song LP; T-Editor: Jia Y
Chinese Library Classification No. R494.9; R363; R364
References
- 1.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 2.Balaratnasingam S, Janca A. Brain derived neurotrophic factor: a novel neurotrophin involved in psychiatric and neurological disorders. Pharmacol Ther. 2012;134:116–124. doi: 10.1016/j.pharmthera.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block F. Global ischemia and behavioural deficits. Prog Neurobiol. 1999;58:279–295. doi: 10.1016/s0301-0082(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31:3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz Y, García EE, Gálvez JV, Arias-Santiago SV, Carvajal HG, Silva-García R, Bonilla-Jaime H, Rojas-Castañeda J, Ibarra A. Release of interleukin-10 and neurotrophic factors in the choroid plexus: possible inductors of neurogenesis following copolymer-1 immunization after cerebral ischemia. Neural Regen Res. 2018;13:1743–1752. doi: 10.4103/1673-5374.238615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Tremblaye PB, Benoit SM, Schock S, Plamondon H. CRHR1 exacerbates the glial inflammatory response and alters BDNF/TrkB/pCREB signaling in a rat model of global cerebral ischemia: implications for neuroprotection and cognitive recovery. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:234–248. doi: 10.1016/j.pnpbp.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Doyle KP, Buckwalter MS. A mouse model of permanent focal ischemia: distal middle cerebral artery occlusion. Methods Mol Biol. 2014;1135:103–110. doi: 10.1007/978-1-4939-0320-7_9. [DOI] [PubMed] [Google Scholar]
- 9.Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel O, Kolodziej S, Dirnagl U, Prinz V. Modeling stroke in mice-middle cerebral artery occlusion with the filament model. J Vis Exp. 2011 doi: 10.3791/2423. doi: 103791/2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer I, Krupinski J, Goutan E, Marti E, Ambrosio S, Arenas E. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 2001;101:229–238. doi: 10.1007/s004010000268. [DOI] [PubMed] [Google Scholar]
- 12.Forouzanfar M, Rabiee F, Ghaedi K, Beheshti S, Tanhaei S, Shoaraye Nejati A, Jodeiri Farshbaf M, Baharvand H, Nasr-Esfahani MH. Fndc5 overexpression facilitated neural differentiation of mouse embryonic stem cells. Cell Biol Int. 2015;39:629–637. doi: 10.1002/cbin.10427. [DOI] [PubMed] [Google Scholar]
- 13.Hashemi MS, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, Nasr-Esfahani MH, Baharvand H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. doi: 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 14.He JX, Gao Y, Wu G, Lei XM, Zhang Y, Pan WK, Yu H. Molecular mechanism of estrogen-mediated neuroprotection in the relief of brain ischemic injury. BMC Genet. 2018;19:46. doi: 10.1186/s12863-018-0630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang C, Yu K, Wu Y, Xie H, Liu G, Wu J, Jia J, Kuang S. Enriched environment enhances poststroke neurological function recovery on rat: involvement of p-ERK1/2. J Stroke Cerebrovasc Dis. 2016;25:1590–1598. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Karpova NN. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology. 2014;76(Pt C):709–718. doi: 10.1016/j.neuropharm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczewski N, Porcher C, Gaiarsa JL. Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. Eur J Neurosci. 2010;32:1239–1244. doi: 10.1111/j.1460-9568.2010.07378.x. [DOI] [PubMed] [Google Scholar]
- 19.Lecker SH, Zavin A, Cao P, Arena R, Allsup K, Daniels KM, Joseph J, Schulze PC, Forman DE. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812–818. doi: 10.1161/CIRCHEARTFAILURE.112.969543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Li DJ, Li YH, Yuan HB, Qu LF, Wang P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017;68:31–42. doi: 10.1016/j.metabol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Zou D, Yi L, Chen M, Gao Y, Zhou R, Zhang Q, Zhou Y, Zhu J, Chen K, Mi M. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1 alpha pathway. Restor Neurol Neurosci. 2015;33:143–157. doi: 10.3233/RNN-140446. [DOI] [PubMed] [Google Scholar]
- 23.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral-artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo L, Liu XL, Li J, Mu RH, Liu Q, Yi LT, Geng D. Macranthol promotes hippocampal neuronal proliferation in mice via BDNF-TrkB-PI3K/Akt signaling pathway. Eur J Pharmacol. 2015;762:357–363. doi: 10.1016/j.ejphar.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moradi-Kor N, Ghanbari A, Rashidipour H, Yousefi B, Bandegi AR, Rashidy-Pour A. Beneficial effects of Spirulina platensis, voluntary exercise and environmental enrichment against adolescent stress induced deficits in cognitive functions, hippocampal BDNF and morphological remolding in adult female rats. Horm Behav. 2019;112:20–31. doi: 10.1016/j.yhbeh.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Jimenez EP, Jurado-Arjona J, Avila J, Llorens-Martin M. The social component of environmental enrichment is a pro-neurogenic stimulus in adult c57bl6 female mice. Front Cell Dev Biol. 2019;7:62. doi: 10.3389/fcell.2019.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- 31.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 32.Novkovic T, Mittmann T, Manahan-Vaughan D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus. 2015;25:1–15. doi: 10.1002/hipo.22342. [DOI] [PubMed] [Google Scholar]
- 33.Peng CH, Chiou SH, Chen SJ, Chou YC, Ku HH, Cheng CK, Yen CJ, Tsai TH, Chang YL, Kao CL. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol. 2008;18:128–140. doi: 10.1016/j.euroneuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Pikula A, Beiser AS, Chen TC, Preis SR, Vorgias D, DeCarli C, Au R, Kelly-Hayes M, Kase CS, Wolf PA, Vasan RS, Seshadri S. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke. 2013;44:2768–2775. doi: 10.1161/STROKEAHA.113.001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136:991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 36.Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, Corbett D. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblum S, Smith TN, Wang N, Chua JY, Westbroek E, Wang K, Guzman R. BDNF Pretreatment of human embryonic-derived neural stem cells improves cell survival and functional recovery after transplantation in hypoxic-ischemic stroke. Cell Transplant. 2015;24:2449–2461. doi: 10.3727/096368914X679354. [DOI] [PubMed] [Google Scholar]
- 38.Sanna MD, Ghelardini C, Galeotti N. HuD-mediated distinct BDNF regulatory pathways promote regeneration after nerve injury. Brain Res. 2017;1659:55–63. doi: 10.1016/j.brainres.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 40.Shono Y, Yokota C, Kuge Y, Kido S, Harada A, Kokame K, Inoue H, Hotta M, Hirata K, Saji H, Tamaki N, Minematsu K. Gene expression associated with an enriched environment after transient focal ischemia. Brain Res. 2011;1376:60–65. doi: 10.1016/j.brainres.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 41.Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- 42.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagenaar N, de Theije CGM, de Vries LS, Groenendaal F, Benders M, Nijboer CHA. Promoting neuroregeneration after perinatal arterial ischemic stroke: neurotrophic factors and mesenchymal stem cells. Pediatr Res. 2018;83:372–384. doi: 10.1038/pr.2017.243. [DOI] [PubMed] [Google Scholar]
- 44.Wang CJ, Wu Y, Zhang Q, Yu KW, Wang YY. An enriched environment promotes synaptic plasticity and cognitive recovery after permanent middle cerebral artery occlusion in mice. Neural Regen Res. 2019;14:462–469. doi: 10.4103/1673-5374.245470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G, Su J, Li L, Feng J, Shi L, He W, Liu Y. Edaravone alleviates hypoxia-acidosis/reoxygenation-induced neuronal injury by activating ERK1/2. Neurosci Lett. 2013;543:72–77. doi: 10.1016/j.neulet.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Zhang S, Ma H, Yang S, Liu Z, Wu X, Wang S, Zhang Y, Liu Y. Chronic intermittent hypobaric hypoxia pretreatment ameliorates ischemia-induced cognitive dysfunction through activation of ERK1/2-CREB-BDNF pathway in anesthetized mice. Neurochem Res. 2017;42:501–512. doi: 10.1007/s11064-016-2097-4. [DOI] [PubMed] [Google Scholar]
- 47.Xie H, Wu Y, Jia J, Liu G, Zhang F, Zhang Q, Yu K, Hu Y, Bai Y, Hu R. Enriched environment preconditioning induced brain ischemic tolerance without reducing infarct volume and edema: the possible role of enrichment-related physical activity increase. Brain Res. 2013;1508:63–72. doi: 10.1016/j.brainres.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 48.Yu K, Wu Y, Hu Y, Zhang Q, Xie H, Liu G, Chen Y, Guo Z, Jia J. Neuroprotective effects of prior exposure to enriched environment on cerebral ischemia/reperfusion injury in rats: the possible molecular mechanism. Brain Res. 2013;1538:93–103. doi: 10.1016/j.brainres.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 49.Yu K, Wu Y, Zhang Q, Xie H, Liu G, Guo Z, Li F, Jia J, Kuang S, Hu R. Enriched environment induces angiogenesis and improves neural function outcomes in rat stroke model. J Neurol Sci. 2014;347:275–280. doi: 10.1016/j.jns.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Chen XP, Lin JB, Xiong Y, Liao WJ, Wan Q. Effect of enriched environment on angiogenesis and neurological functions in rats with focal cerebral ischemia. Brain Res. 2017;1655:176–185. doi: 10.1016/j.brainres.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Zhao F, Qu Y, Liu H, Du B, Mu D. Umbilical cord blood mesenchymal stem cells co-modified by TERT and BDNF: a novel neuroprotective therapy for neonatal hypoxic-ischemic brain damage. Int J Dev Neurosci. 2014;38:147–154. doi: 10.1016/j.ijdevneu.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J, Xu H, Tian Y, Hu M, Xiao H. Effect of electroacupuncture on brain-derived neurotrophic factor mRNA expression in mouse hippocampus following cerebral ischemia-reperfusion injury. J Tradit Chin Med. 2013;33:253–257. doi: 10.1016/s0254-6272(13)60135-1. [DOI] [PubMed] [Google Scholar]
- 53.Zsuga J, Tajti G, Papp C, Juhasz B, Gesztelyi R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med Hypotheses. 2016;90:23–28. doi: 10.1016/j.mehy.2016.02.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


