Consciousness is controlled by the ascending reticular activating system (ARAS) (Teasdale and Jennett, 1974; Jang and Lee, 2015; Jang et al., 2016a). Scientific medical strategies for recovery in patients with impaired consciousness are relatively few compared with other diseases. Therefore, research to clarify the neural structures in the ARAS involved in the recovery of impaired consciousness should be encouraged (Teasdale and Jennett, 1974; Schiff, 2010; Jang and Lee, 2015; Jang et al., 2016a). Several studies revealed that recovery of an injured ARAS contributed to recovery of impaired consciousness in several brain injuries (Teasdale and Jennett, 1974; Laureys et al., 2000; Giacino et al., 2004; Schiff, 2010; Jang et al., 2015, 2016a; Jang and Lee, 2015). However, there are no related diffusion tensor tractography (DTT) reports.
In our study, we report on DTT findings of an injured ARAS during recovery from a minimally conscious state (MCS) to normal consciousness in a stroke patient.
A 33-year-old male patient underwent extraventricular draainage and craniectomy for management of right putaminal intracerebral and intraventricular hemorrhage which occurred because of the rupture of the arteriovenous malformation in the right basal ganglia at 8 weeks after onset. The patient also underwent ventriculoperitoneal shunt operation for hydrocephalus (Figure 1A). He was transferred to the rehabilitation department from other university hospital at 5 months after onset. He had impaired consciousness, with a Glasgow Coma Scale score (GCS) of 9 (full GCS score 15: covering eye opening: 3, best verbal response: 2, and best motor response: 4; Teasdale and Jennett, 1974) and a Coma Recovery Scale-Revised (CRS-R) score of 8 (full CRS-R score 23: covering auditory: 1, visual: 2, motor: 3, verbal: 1, communication: 0, and arousal: 1; Giacino et al., 2004). He underwent rehabilitative therapy (physical therapy and occupational therapy). He took neurotropic drugs (methylphenidate, amantadine, levodopa, bromocriptine, venlafaxine, and baclofen) (Jang et al., 2014). After 3 months of rehabilitation at the university hospital, his consciousness was improved to full recovery (clearly distinct consciousness) on both GCS score 15 and CRS-R score 23 (Folstein et al., 1975; Yeo et al., 2013). The Mental State Exam (MMSE) score was 0 at 5 months after onset when he started rehabilitation and it increased to 18 after 3 months of rehabilitation (Jang and Kwon, 2015). He underwent rehabilitation at two other local rehabilitation hospitals and his consciousness level was maintained. The patient provided signed informed consent. This study was conducted retrospectively, and approval for the study was obtained from the Institutional Review Board of Yeungnam University Hospital (YUMC-2018-10-042) on October 22, 2018.
Figure 1.
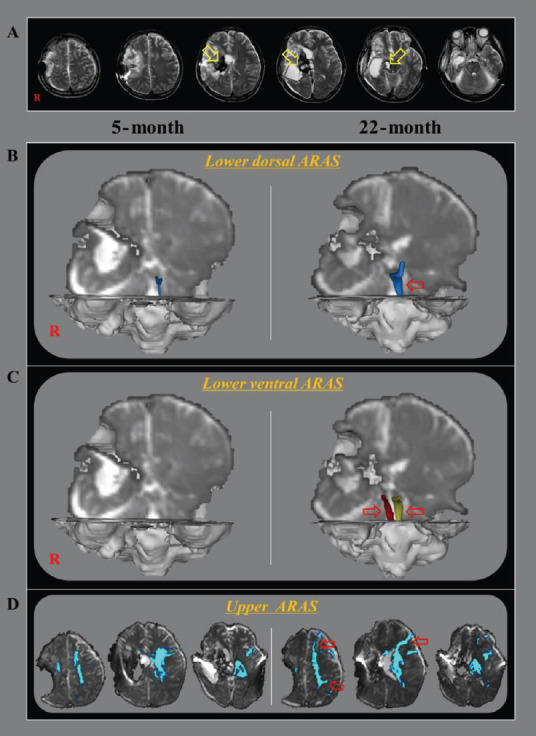
DTT for the ARAS of a 33-year-old male stroke patient.
(A) DTT images taken at 5 months after onset reveal leukomalactic lesions in the right basal ganglia and thalamus, and midbrain (yellow arrows). (B) On 5-month and 22-month DTT images, the right lower dorsal ARAS was not reconstructed on both hemispheres, while thin left lower dorsal ARAS observed on the 5-month DTT image had become thicker on the 22-month DTT image (red arrow). (C) On 5-month DTT image, the lower ventral ARAS was not reconstructed in the both hemispheres, however, it was well-reconstructed on both sides on 22-month DTT image (red arrows). (D) On 5-month DTT image, the neural connectivity of the upper ARAS between the ILN and cerebral cortex was decreased in bilateral prefrontal cortex, parietal cortex, bilateral basal forebrain, and the right thalamus. By contrast, the neural connectivity of the upper ARAS was increased in the left prefrontal cortex and parietal cortex on the 22-month DTT image (red arrows). ARAS: Ascending reticular activating system; DTT: diffusion tensor tractography; ILN: intralaminar nucleus; R: right.
DTT data were acquired twice (5 and 22 months after onset) using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Amsterdam, the Netherlands) with single-shot echo-planar imaging. Imaging scanning conditions were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192, field of view = 240 × 240 mm2, repetition time = 10,398 ms, echo time = 72 ms, parallel imaging reduction factor (SENSE factor) = 2, echo-planar imaging factor = 59, b = 1000 s/mm2, slice gap = 0 mm, and slice thickness = 2.5 mm.
For the lower dorsal ARAS, the first region of interest (ROI) was placed at the reticular formation of the pons and the second ROI was placed at the thalamic intralaminar nucleus. For the lower ventral ARAS, the first ROI was placed at the reticular formation of the pons, and the second ROI was placed at the hypothalamus. For the connectivity of thalamic intralaminar nucleus, the first ROI was placed at the thalamic intralaminar nucleus (Motofei and Rowland, 2014; Jang et al., 2016b, c).
The right lower dorsal ARAS was not reconstructed on 5- and 22-month DTT images. The thin left lower dorsal ARAS observed on the 5-month DTT image had become thicker on 22-month DTT image. The lower ventral ARAS on both sides was not reconstructed on the 5-month DTT image; however, it was well-reconstructed on both sides on the 22-month DTT image. On the 5-month DTT image, the neural connectivity of the upper ARAS between the thalamic intralaminar nucleus and the cerebral cortex was decreased in bilateral prefrontal cortex, parietal cortex, bilateral basal forebrain, and the right thalamus. By contrast, on 22-month DTT image, the neural connectivity of the upper ARAS was increased in the left prefrontal cortex and parietal cortex (Figure 1B).
In this study, we evaluated the change of an injured ARAS in a stroke patient who showed good recovery of severely impaired consciousness after 3 months of comprehensive rehabilitation. The change of the injured ARAS on DTT images indicates recovery of the injured neural tracts as follows: 1) the left lower dorsal ARAS (Figure 1B), 2) both lower ventral ARAS (Figure 1C), and 3) the upper ARAS to left prefrontal cortex (especially), parietal cortex (Figure 1D). Consequently, in this patient, the recovery of the injured neural tracts in the prefrontal cortex, parietal cortex, lower dorsal ARAS, and lower ventral ARAS contributed to the recovery of impaired consciousness. Because the upper ARAS is responsible for conscious awareness and the lower dorsal and ventral ARAS are responsible for arousal and sleep, respectively, the recovery of the injured upper ARAS mainly contributes to the recovery of impaired consciousness in this patient (Paus, 2000; Parker and Alexander, 2005; Cavanna et al., 2011; Goldfine and Schiff, 2011; Jang, 2016).
Several studies have demonstrated the change of injured ARAS during recovery of impaired consciousness in patients with several brain injuries (Teasdale and Jennett, 1974; Laureys et al., 2000; Giacino et al., 2004; Schiff, 2010; Jang et al., 2015, 2016a). Laureys et al. (2000) reported resolution of the metabolic decrement in the posterior associative cortices during recovery from a vegetative state to a normal state of consciousness in a patient with CO intoxication. Jang et al. (2015) demonstrated that the recovery of an injured lower dorsal ARAS in a traumatic brain injury patient who showed recovery of the impaired consciousness from a minimally conscious state to a normal conscious state. Jang and Lee (2015) reported the recovery of the injured lower dorsal ARAS and the injured upper ARAS of the patient who recovered from a vegetative state to a minimally conscious state following hypoxic/ischemic brain injury. Jang et al. (2016d) reported recovery of an injured ARAS in a stroke patient who recovered from a minimally conscious state to a normal state over a period of 3 weeks in the early stage of hypoxic/ischemic brain injury. During the same year, Jang et al. (2016e) reported that in a stroke patient, the recovery of the injured upper ARAS was concurrent with the recovery from a vegetable state to a minimally conscious state. Also in 2016, Jang et al. demonstrated changes in the upper ARAS were concurrent with the recovery from a vegetable state to a minimally conscious state in a traumatic brain injury patient (Jang et al., 2016a). Recently, Jang and Lee (2017) reported that in a stroke patient, the recovery of consciousness from a vegetable state to a normal conscious state was concurrent with the recovery of the injured ARAS. Our results in this patient appear to support those in the aforementioned studies (Teasdale and Jennett, 1974; Laureys et al., 2000; Giacino et al., 2004; Schiff, 2010; Jang et al., 2015, 2016a).
In conclusion, changes of an injured ARAS were demonstrated in a stroke patient who showed good recovery of consciousness. The increased neural connectivity in the upper ARAS (prefrontal cortex and parietal cortex) mainly contributed to the recovery of impaired consciousness in this patient. Further studies regarding the contributions of other portions of the ARAS to the recovery of impaired consciousness should be warrented. The limitation of DTT studies should be considered. DTT may result in false negative results due to fiber complexity and crossing of the regions (Parker and Alexander, 2005).
Footnotes
Conflicts of interest: The authors report no disclosures relevant to the manuscript.
Financial support: This work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (MSIP) (2018R1A2B6000996) (to SHJ).
Institutional review board statement: Approval for the study was obtained from the Institutional Review Board of Yeungnam University Hospital (YUMC-2018-10-042) on October 22, 2018.
Declaration of patient consent: The authors certify that they have obtained the appropriate patient consent form. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that his name and initial will not be published and due efforts will be made to conceal his identity.
Reporting statement: This manuscript was prepared in accordance with the CAse REport (CARE) guidelines.
Biostatistics statement: No statistical method was used in this study.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Bo Li, General Hospital of Jinan Military Command, China; Masaaki Hori, Juntendo University School of Medicine, Japan; Byung G. Kim, Anjou University School of Medicine, Republic of Korea.
R-Reviewers: Li B, Hori M, Kim BG; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Cavanna AE, Shah S, Eddy CM, Williams A, Rickards H. Consciousness: a neurological perspective. Behav Neurol. 2011;24:107–116. doi: 10.3233/BEN-2011-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 3.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Goldfine AM, Schiff ND. Consciousness: its neurobiology and the major classes of impairment. Neurol Clin. 2011;29:723–737. doi: 10.1016/j.ncl.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang S, Kim S, Lee H. Recovery from vegetative state to minimally conscious state: a case report. Am J Phys Med Rehabil. 2016a;95:e63–66. doi: 10.1097/PHM.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 6.Jang SH, Lee HD. Ascending reticular activating system recovery in a patient with brain injury. Neurology. 2015;84:1997–1999. doi: 10.1212/WNL.0000000000001563. [DOI] [PubMed] [Google Scholar]
- 7.Jang SH, Kwon HG. The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: a diffusion tensor imaging study. Neurosci Lett. 2015;590:58–61. doi: 10.1016/j.neulet.2015.01.071. [DOI] [PubMed] [Google Scholar]
- 8.Jang SH, Lim HW, Yeo SS. The neural connectivity of the intralaminar thalamic nuclei in the human brain: a diffusion tensor tractography study. Neurosci Lett. 2014;579:140–144. doi: 10.1016/j.neulet.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Jang SH, Kim SH, Lim HW, Yeo SS. Recovery of injured lower portion of the ascending reticular activating system in a patient with traumatic brain injury. Am J Phys Med Rehabil. 2015;94:250–253. doi: 10.1097/PHM.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 10.Jang SH, Chang CH, Jung YJ, Seo JP. Post-stroke hypersomnia. Int J Stroke. 2016b;11:NP5–6. doi: 10.1177/1747493015607502. [DOI] [PubMed] [Google Scholar]
- 11.Jang SH, Lee HD, Chang CH, Jung YJ. Recovery of hypersomnia concurrent with recovery of an injured ascending reticular activating system in a stroke patient: a case report. Medicine. 2016c;95:e2484. doi: 10.1097/MD.0000000000002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang SH, Hyun YJ, Lee HD. Recovery of consciousness and an injured ascending reticular activating system in a patient who survived cardiac arrest: A case report. Medicine. 2016d;95:e4041. doi: 10.1097/MD.0000000000004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang SH, Chang CH, Jung YJ, Seo YS. Change of ascending reticular activating system with recovery from vegetative state to minimally conscious state in a stroke patient. Medicine. 2016e;95:e5234. doi: 10.1097/MD.0000000000005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang SH, Lee HD. Recovery of multiply injured ascending reticular activating sytems in a stroke patient. Neural Regen Res. 2017;12:671–672. doi: 10.4103/1673-5374.205109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 16.Motofei IG, Rowland DL. The ventral-hypothalamic input route: a common neural network for abstract cognition and sexuality. BJU Int. 2014;113:296–303. doi: 10.1111/bju.12399. [DOI] [PubMed] [Google Scholar]
- 17.Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005;360:893–902. doi: 10.1098/rstb.2005.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paus T. Functional anatomy of arousal and attention systems in the human brain. Prog Brain Res. 2000;126:65–77. doi: 10.1016/S0079-6123(00)26007-X. [DOI] [PubMed] [Google Scholar]
- 19.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 21.Yeo SS, Chang PH, Jang SH. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci. 2013;7:416. doi: 10.3389/fnhum.2013.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]


