Abstract
Neurotrophins play a major role in the regulation of neuronal growth such as neurite sprouting or regeneration in response to nerve injuries. The role of nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor in maintaining the survival of peripheral neurons remains poorly understood. In regenerative medicine, different modalities have been investigated for the delivery of growth factors to the injured neurons, in search of a suitable system for clinical applications. This study was to investigate the influence of nerve growth factor, neurotrophin-3 and brain-derived neurotrophic factor on the growth of neurites using two in vitro models of dorsal root ganglia explants and dorsal root ganglia-derived primary cell dissociated cultures. Quantitative data showed that the total neurite length and tortuosity were differently influenced by trophic factors. Nerve growth factor and, indirectly, brain-derived neurotrophic factor stimulate the tortuous growth of sensory fibers and the formation of cell clusters. Neurotrophin-3, however, enhances neurite growth in terms of length and linearity allowing for a more organized and directed axonal elongation towards a peripheral target compared to the other growth factors. These findings could be of considerable importance for any clinical application of neurotrophic factors in peripheral nerve regeneration. Ethical approval was obtained from the Regione Piemonte Animal Ethics Committee ASLTO1 (file # 864/2016-PR) on September 14, 2016.
Keywords: brain-derived neurotrophic factor, directionality, dorsal root ganglia explant, nerve growth factor, nerve regeneration, neurite growth enhancement, neurotrophic factors, neurotrophin-3, sensory neurons, tortuosity
Introduction
After injury, peripheral nerve repair spontaneously occurs via growth cones located at the tips of axons. A variety of stimuli from the surrounding environment can be detected by these growth cones. The subsequent response to these cues results in axonal outgrowth, possibly over distances of hundreds of microns to reconnect to the end-tissue target (Miller et al., 2001; Neto et al., 2017; Sulaiman et al., 2018; Kornfeld et al., 2019). In some cases, however, there may be a relatively large gap between the severed nerve ends and regenerating axon sprouts fail to reach their end target due to a lack of directionality. The failure of the regenerating axons to quickly re-establish contact with target tissue leads to permanent loss of sensory or motor function. A better understanding of the mechanisms that can guide and direct axons along a desired path are critical for future clinical applications.
Neurotrophic factors (NTs) play a critical role in neuronal survival after nerve injury by protecting neurons from death, thus increasing the potential for axon regeneration. While postnatal and adult sensory neurons do not require NTs for their continued survival, the maintenance of mature phenotypic characteristics and physiologic responsiveness seems to be dependent upon NTs such as nerve growth factor (NGF) and neurotrophin-3 (NT-3) (Kimpinski et al., 1997; Mamounas et al., 2000; Yamamoto and Hanamura, 2005; Santos et al., 2016; Cacialli et al., 2019). Although NGF, NT-3, and brain-derived neurotrophic factor (BDNF) have been described as important factors to promote axonal regrowth through a conserved cell polarity-signalling pathway (Grider et al., 2005; Bagheri et al., 2019; Katebi et al., 2019; Melo et al., 2019), application of these factors in regenerative medicine has seen very poor results thus far (Mitchell et al., 2016). In particular, most of unsuccessful experiments implied a topical application of NTs at the site of lesion. Axons were definitively incentivized to regrow as an unorganized bundle of fibers with no directionality and unable or barely able to reach the periphery, thus impeding functional motor or sensory recovery. At the site of lesion, NGF was found to generate and maintain hypersensitivity by inducing aberrant sprouting and/or neuroma formation in response to tissue and/or nerve injury (Mantyh et al., 2011).
Therefore, the purpose of this study was to investigate the activity of the NT family on neurite growth in vitro with the overall goal of identifying a key player for an organized and oriented fiber regrowth, thus opening possible applications in the reconstructive and regenerative medicine procedures.
The singular effects of NGF, BDNF and NT-3 were studied in two in vitro models: 1) a co-culture of DRG-derived dissociated neuronal and glial cells; 2) dorsal root ganglia (DRG) explants. The effect of BDNF was also tested on sciatic nerve-derived primary culture of Schwann cells.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (Harlan, Barcelona, Spain), weighing 175–200 g, were used in this study. Animals were housed in large cages in a temperature and humidity controlled room with 12-hour light/dark cycles. The animals were fed with standard chow and water ad libitum. Adequate measures were taken to minimize pain and discomfort taking into account humane endpoint criteria for animal suffering and distress. All procedures were performed in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) and approved by the Regione Piemonte Animal Ethics Committee ASLTO1 (file # 864/2016-PR) on September 14, 2016.
DRG explants
Rats were sacrificed by a lethal intramuscular injection of tiletamine + zoletil (3 mg/kg, Virbac, Carros, France) in the back thigh. The vertebral column was surgically dissected and the vertebral body was removed to gain a ventral access to the spinal cord. DRG from all spinal levels were localized, removed, and isolated. The dissecting procedure was completed within 90 minutes. DRG were collected in a 3.5 mL Petri dish with 1.8 mL of F12 medium (Gibco, Carlsbad, CA, USA). The connective-tissue capsules surrounding the ganglia were reduced with fine forceps. The ganglia were adhered onto matrigel-coated coverslips (BD Biosciences, Bedford, MA, USA) and incubated at 37°C for 1 hour (Fornaro et al., 2018). Matrigel was diluted 1:1 in serum- free medium (SFM) (Fornaro et al., 2018). Explants were maintained at 37°C with 5% CO2.
DRG primary culture
DRG were harvested, collected, and then enzymatically dissociated in F12 medium containing 12.5 mg/mL collagenase type IV (Sigma, St. Louis, MO, USA) for 1 hour (37°C, 5% CO2). The incubation in collagenase was repeated for 45 minutes.
DRGs were washed three times with F12 and incubated in bovine pancreatic trypsin 2 × 25 mg/mL (Sigma) for 30 minutes (37°C, 5% CO2). The trypsin was inactivated with 33% fetal bovine serum (FBS; VWR, Radnor, PA, USA) in F12 medium. The medium was removed and DRG were washed three times with F12 medium. Then the ganglia were transferred to a 15 mL falcon with 2 mL F12 medium and mechanically dissociated by gentle trituration using a glass Pasteur pipette. Dissociated cells were filtered through a 70 μm-pore sized filter (BD Biosciences, San Jose, CA, USA), transferred to a 15 mL falcon, and then centrifuged at 25 × g for 5 minutes. Pellets were re-suspended in 0.5 mL of supernatant and 1 mL of F12 medium containing 0.5 mL of F12 and 0.5 mL of 30% bovine serum albumin (Sigma). A differential centrifugation with bovine serum albumen at 75 × g for 10 minutes was employed to separate neurons and a small amount of satellite cells from most of Schwann cells, astrocytes and connective tissue of the ganglionic capsules. The supernatant was completely removed and the pellet containing neurons and satellite cells were re-suspended in F12 medium. The pellet containing Schwann cells and fibroblasts was discarded.
Dissociated neurons and satellite cells were seeded on glass coverslips pre-coated with laminin (2 µg/cm2, Sigma) in 24-well culture dishes with SFM and incubated at 5% CO2 at 37°C for 24, 48 and 72 hours. The primary culture of glial cells with a high representation of satellite cells was maintained in vitro for several passages.
Schwann cell primary culture
Primary Schwann cells cultures were obtained from sciatic nerves harvested from adult Sprague Dawley rats. The nerves were collected in cold DMEM plus glutamax (Gibco, Carlsbad, CA, USA) containing 100 U/mL penicillin (R&D system, Minneapolis, MN, USA) and 100 g/mL Streptomycin (R&D system, Minneapolis, MN, USA). Nerves were then gently dissected and the epineurium removed with fine forceps. Fragments of nerves were then plated in 35 mm petri dishes and incubated with glial permissive medium (DMEM plus glutamax, 100 U/mL penicillin and 100 g/mL streptomycin, 14M forskolin and 100 ng/mL LNRG11; R&D System, Minneapolis, MN, USA) for 2 weeks under culture conditions (37°C, 5% CO2). The medium was replenished every 72 hours. The culture medium was then enriched with 0.125% collagenase type IV (ThermoFisher, Lafayette, CO, USA) and 117 U/mg dispase (Gibco) for 24 hours and mechanically dissociated with a Pasteur glass pipette. The cell suspension was filtered through a 70 µm cell strainer (BD Biosciences) and centrifuged at 100 × g for 5 minutes. The cell pellet was resuspended in glial permissive medium, seeded on 35 mm petri dishes pre-coated with poly-D-lysine (Sigma) and incubated under culture conditions. An antibody complement method was adopted to purify Schwann cells from fibroblasts (Tohill et al., 2004; Kaewkhaw et al., 2012; Pascal et al., 2014).
NT treatments
To evaluate the direct effects of NTs on neurite outgrowth, primary cultures and DRG explants were treated with SFM with or without neurotrophic factors NGF (50 ng/mL, 2.5S beta-NGF, Sigma) (Malcangio et al., 1997; Turney et al., 2016; Naletova et al., 2019), BDNF (10 ng/mL, Sigma) (Santos et al., 2016) and NT-3 (10 ng/mL, Sigma) (Malcangio et al., 1997; Turney et al., 2016) for 24, 48 and 72 hours. To test for the lack of dependence on these neurotrophic factors neutralizing antibody against NGF (1 µg/mL, Sigma), BDNF (1 µg/mL, Sigma) and NT-3 (1 µg/mL, Sigma) were added to culture medium. To evaluate whether BDNF specifically activates satellite cells or both satellite and Schwann cells, the two glial populations were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (1 g/L glucose), 10% FBS, 1% penicillin/streptomycin with 63 ng/mL of glial growth factor and 10 µM of forskolin with or without BDNF. The cultures were maintained in a humidified incubator at 37°C and 5% CO2 for 72 hours. The cell cultures were then processed for immunofluorescence.
Immunocytochemistry
To evaluate the morphology and neurite outgrowth, cell cultures and explants were fixed with 4% paraformaldehyde for 20 minutes. After rinsing with phosphate buffered saline (PBS pH 7.2), the cells were incubated in 0.1% triton X-100, 10% normal goat serum (NGS, Thermo Scientific, Bedford, MA, USA)/0.1% NaN3, for 1 hour. Samples were incubated for 1 hour at room temperature in primary antibodies and processed for single or double immunofluorescence. The primary antibodies used in this study were: mouse monoclonal anti-βIII Tubulin (1:1000, Thermo Scientific), rabbit polyclonal anti-S-100 (1:600, Abcam, Cambridge, MA, USA) and rabbit polyclonal anti-Peripherin (1:1000, Millipore, Temecula, CA, USA). After rinsing with PBS, the specimens were incubated for 1 hour at room temperature with secondary antibodies Alexa Fluor 488 anti-mouse IgG (1:200, Molecular Probes, Eugene, Oregon, USA) and Cy3 anti-rabbit IgG (1:200, Jackson Immunoresearch, West Grove, PA, USA). Finally, samples were mounted with a fluorescent mounting medium (Dako, carpinteria, CA, USA), imaged using the Nikon Eclipse Ti A1R confocal microscope (Nikon, Melville, NY, USA), and analyzed using the NIS-Elements software (Nikon).
Quantitative analysis of neurite length and tortuosity
Confocal images of dissociated cells cultures were analyzed with the Leica image-pro Premier software (Leika Microsystems, Buffalo Grove, IL, USA). The image 3D-mode was utilized to better resolve the fibers. The same software was utilized to measure the neurite length (L) and total distance (vector, v) from the tip of the neurite to its origin at the cell body. The GraphPad Prism 8.2.1 software (GraphPad Software, San Diego, CA, USA) was utilized for statistical analysis and graph preparation.
Confocal images of DRG explants were processed by image analysis software (ImageJ, NIH, Bethesda, MD, USA) to evaluate the spatial distribution and extension of neurites, using a modified Sholl analysis (Sholl, 1953). In Sholl analysis, the number of dendrite branches of a neuron is represented as a function of the distance from the cell body, by counting the number of intersections of neuritic processes with concentric circles centered in the cell soma. In this study, the perimeter of the ganglion was manually drawn on the image (Polygon selection tool) and then progressively enlarged (Edit-Selection-Enlarge tool) to create concentric curves at fixed distance (7 µm) between each other, which were used as coordinates of reference for neurite analysis. The number of intersections of neuritic processes (both primary neurites and branches) with the different curves of DRG grown in the presence of each factor was blindly counted and represented in terms of mean values and standard deviation as a function of the distances from the ganglion perimeter.
For DRG explants treated with NGF and NT-3 (explants treated with BDNF did not differ significantly from control), primary neurites were traced on the image (segmented line tool). Their length (L) and the distance (d) between the neurite termination and its starting point from the ganglion were measured (Analyze-Measure tool). The neurite tortuosity (t) was calculated using the following formula:
t = 1–d/L
The neurite tortuosity was reported as mean values and standard deviation. One-way analysis of variance was conducted to assess statistical significance.
Results
Neurotrophic effect on DRG-derived dissociated cells culture
Semi-purified co-culture of sensory neurons and satellite cells was seeded in vitro in a 2-dimensional system. After 48–72 hours in SFM, cells and their projections spontaneously formed clusters (Figure 1A–C). To study the effect of NTs on axonal regrowth, each singular factor was added to the culture medium at day-in-vitro (div) 0 and the effect of specific NT on cell morphology (both neuron and glia), cell-cell interaction, axonal growth and linearity/tortuosity of the projections was analyzed. To assess the effect of each single factor, antibodies against the other two factors were added to the culture medium. Moreover, the effect of the single factor was null when added together with the antibody against the factor itself (negative control). DRG dissociated cells in the presence of NGF (Figure 1A1–C1) have a high tendency to cluster together, anticipating the normal aggregations of cells observed in SFM (Figure 1A–C). In the presence of NGF, a round, well-defined cluster of β-tubulin-positive neurons and S-100-positive glial cells formed in half of the time (24 hours instead of 48 hours). At 72 hours, the double immunocytochemistry showed a complex bundle of β-tubulin-positive neurites surrounding and defining the cluster of neuronal and S-100-positive glial cells. These neurites did not leave the cluster or barely elongate outside of it. The cell aggregation was inhibited by adding anti-NGF to the culture medium (Figure 1C1 inset), confirming that NGF plays a role in favoring the cell-cell interaction and cluster formation. A cluster formation was observed also when cells were grown in the presence of NGF, anti-BDNF, and anti-NT-3 alone and in combination.
Figure 1.
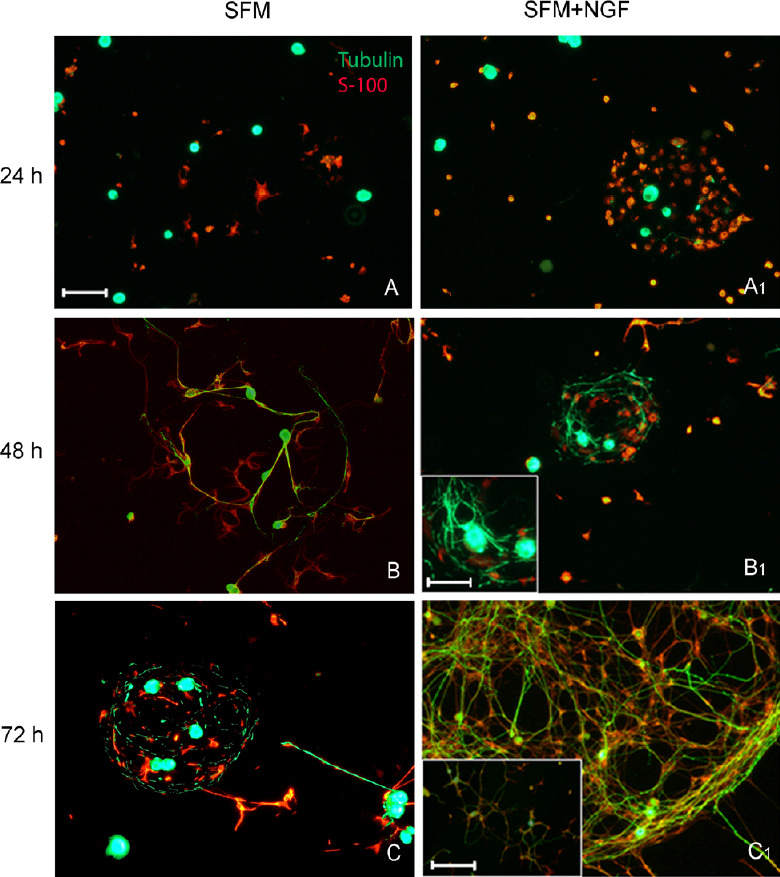
Dorsal root ganglia-derived dissociated cells spontaneously aggregate in vitro forming cell clusters.
Adding NGF to the culture medium expedited the cluster formation. (A) β-Tubulin-positive sensory neuronal bodies (green) and S-100-positive dissociated glial cells (red) cultured in SFM were still separated after 24 hours in vitro. (B) At 48 hours, both β-tubulin-positive neurons (green) and S-100-positive glial cells started approaching each other to form a spherical-shaped cluster. (C) At 72 hours, a well-defined bundle of both neurons and glial cells was seen. The cluster was defined and surrounded by β-tubulin-positive neurites. (A1) In the presence of NGF, spherical cell aggregation was observed earlier at 24 hours. Very few neurites could be detected at this time. (B1) A bundle of β-tubulin-positive neurites surrounded the cell cluster after 48 hours. B1 inset shows a higher magnification of a β-tubulin-positive neuron and its sprouted neurites. (C1) At 72 hours, cell cluster was well defined by the growing neurites. NGF was principally responsible for cell cluster aggregation. C1 inset: As control, cell aggregation was inhibited by adding anti-NGF antibody to the culture medium, confirming that NGF plays a role in cell-cell interaction. Scale bars: 100 µm for A–C and A1–C1; 50 µm for B1 inset; 200 µm for C1 inset. NGF: Nerve growth factor; SFM; serum-free medium.
Similarly to what was seen for NGF, also in the presence of BDNF, cluster formation and presence of tortuous fibers were observed. However, the aggregation was delayed (72 hours) compared to cultures treated with NGF. The neurites of sensory neurons did not elongate far out from the round-shaped cluster (Figure 2A). No cell aggregation was observed when anti-BDNF antibody was added to the culture medium (data not shown). Interestingly, adding anti-NGF antibody to the cultures treated with BDNF prevented cell cluster formation (Figure 2B), while the same effect was not obtained after adding anti-NT-3 antibody (data not shown). The effect of BDNF was further evaluated in two different primary cultures of glial cells: DRG-derived satellite cells (Figure 2C and D) and sciatic nerve-derived Schwann cells (Figure 2E and F). BDNF-dependent morphological changes of the two cell populations were observed in confocal microscopy after cells were immunolabelled with the glial marker S-100. More significant morphological changes were seen in the satellite cell population when BDNF was added to the culture medium (Figure 2C and its inset). Cells looked more confluent, possibly more numerous, bigger in size and displayed more and well developed processes branching out of the cell body compared to the same population grown in the presence of BDNF and anti-BDNF antibody (Figure 2D) and its inset). On the other hand, no morphological changes were detected in sciatic nerve-derived Schwann cells culture in the presence of BDNF (Figure 2E) compared to cells grown with BDNF plus anti-BDNF antibody (Figure 2F), suggesting that BDNF may specifically induce morphological changes in satellite cells but not in Schwann cells.
Figure 2.
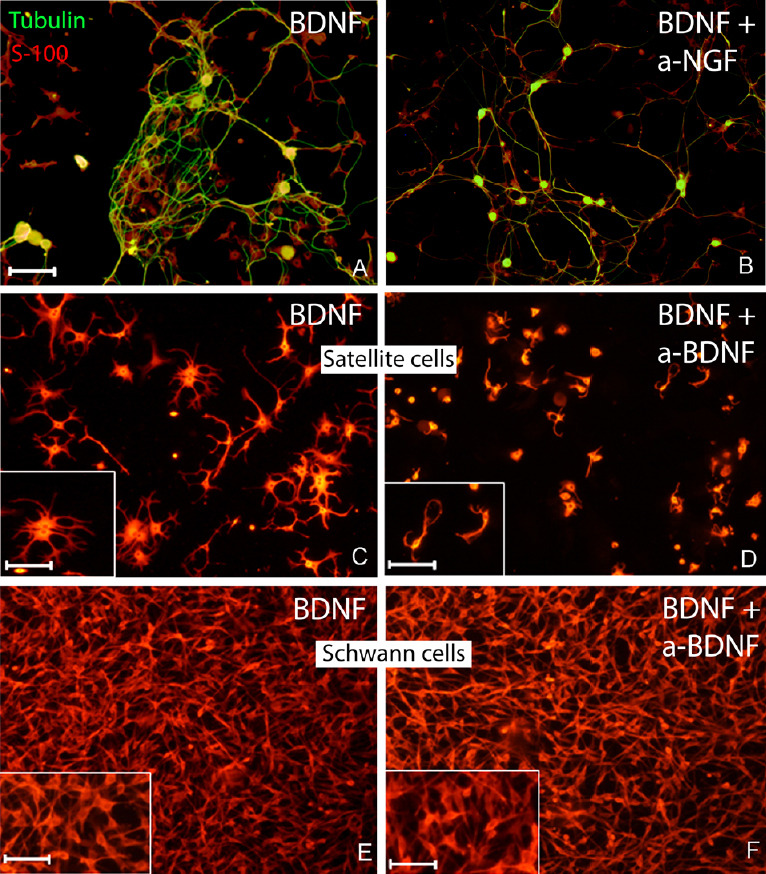
BDNF enhances cell cluster formation via activation of the satellite cells.
(A) In the presence of BDNF at 72 hours, a semi-defined cluster of β-tubulin-positive neurons (green) and S-100-positive satellite cells (red) was seen. (B) The cell cluster formation was avoided when BDNF and anti-nerve growth factor antibody were simultaneously added to the medium. BDNF specifically activated satellite cells and did not affect Schwann cells. (C, D) At 72 hours, S-100-positive satellite cells derived from dissociated DRG featured larger cell bodies and well-developed processes (C) as compared to the same culture with anti-BDNF antibody added to the medium (D). Both insets in C and D are higher magnifications to appreciate the more extensive cellular processes branching in the presence of BDNF compared to BDNF + anti-BDNF antibody. The immunostaining using S100 antibody did not reveal any morphological differences (in number, size, maturation features) in sciatic nerve-derived Schwann cell population cultured in the presence of BDNF (E) or without BDNF (F). Scale bars: 100 µm for A, F; 50 µm for all insets. BDNF: Brain-derived neurotrophic factor.
Contrary to the other two factors, when NT-3 was singularly added to the medium (in combination with anti-NGF and anti-BDNF antibodies) of DRG-derived cell cultures, no cell-cell aggregation was observed. Instead, NT-3 elicited more linear growth of neuronal processes (Figure 3). The effect of NT-3 was reverted when anti-NT-3 was added to the medium, allowing the formation of cell cluster and confused bundle of neuritis (Figure 3 inset).
Figure 3.
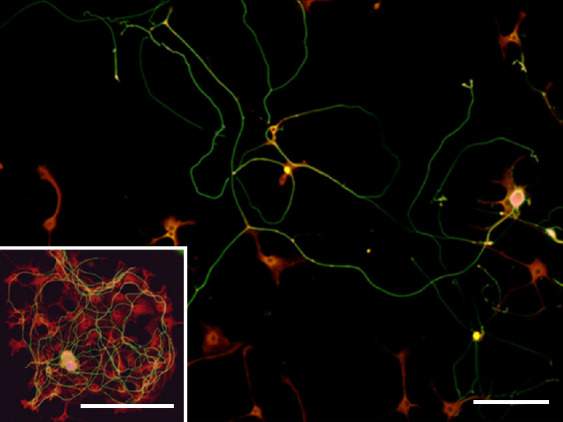
NT-3 enhances ordinate and oriented axonal elongation.
A double immunofluorescence for both neurons (β-tubulin, green) and satellite cells (S-100, red) showed linear and oriented axon growth in the presence of NT-3. When anti-NT-3 antibody was added to the medium, the linearity of axonal growth was lost and the feature of a cell cluster was depicted again (inset). Scale bars: 100 µm for and inset. NT-3: Neurotrophin-3.
The effect of each factor on neurite growth was evaluated in dissociated sensory neurons (Figure 4). Confocal images of β-tubulin and S-100 double immunolabelled cells were imported to the imaging software Leica Image-Pro Premiere and converted in 3D view to obtain a better resolution of the neurites (Figure 4A–C). The two parameters blindly measured were: (1) neurite’s length (L) and (2) linear growth resulting from the vector distance (V) between the cell body and the distal tip of the process (Figure 4D). A comparative analysis of the neurites grow in the presence of the growth factors showed a significant increase in the length of neurites in the presence of either NT-3 or NGF compared to in the presence of BDNF, and there was no significant statistical difference between the NT-3 and NGF groups (Figure 4E). However, analysis of the vector lengths showed a significant increase in the linear extension of neurites treated with NT-3 compared to that treated with NGF or BDNF, and there was no significant difference in linear extension of neurites between the NGF and BDNF groups (Figure 4F).
Figure 4.
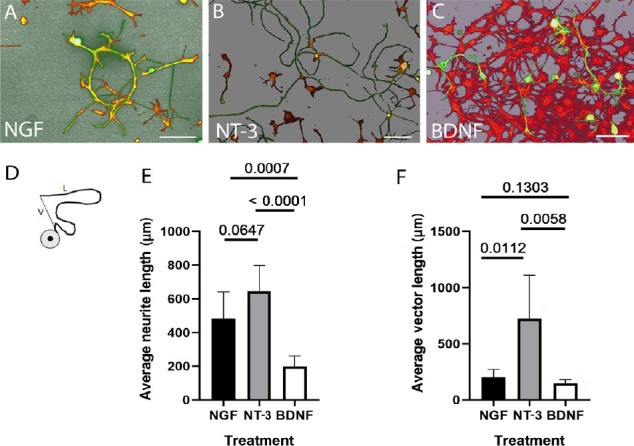
Quantification of neurite growth in dissociated cells.
Double immunofluorescence with β-tubulin (green) and S-100 (red) showed the morphology of DRG-dissociated cells grown for 72 hours in the presence of NGF (A), NT-3 (B), or BDNF (C). The confocal images were converted in 3D-mode using the Leica image-pro premier software for a better resolution of neuronal processes. The same imaging software was used to measure 2 parameters: 1, the total length of the neurite (L) and 2, the vector (V) distance from the tip of the neurite to the cell body (D). Quantification of the averaged neurite length for cells growth in the presence of NGF (n = 16 neurites), NT-3 (n = 14) or BDNF (n = 14) shows a significant increase in length for neurites grown in the presence of either NGF or NT-3 compared to BDNF. No significance was detected between NGF (483.36 ± 158.90 µm) and NT-3 (645.64 ± 151.13 µm) groups (E). The linearity of growth (vector distance) shows a significant increase in neurite elongation in the presence of NT-3 (725.67 ± 384.9 µm) compared to NGF (203.92 ± 68.19 µm) and BDNF (146.44 ± 34.07 µm) groups. There was no significant difference in average vector length between NGF and BDNF groups (F). The P values are reported in the graphs. Scale bars: 100 µm for A–C. BDNF: Brain-derived neurotrophic factor; NGF: nerve growth factor; NT-3: neurotrophin-3.
Neurotrophic effect on DRG explants
The activity of the NTs was also studied in DRG explants (Figure 5). The data obtained in the explants supported and confirmed the results shown in dissociated cells cultures. For a clear resolution of the neurite sprouts, all the measures were conducted on DRG grown for 3 days ex vivo (Figure 5A–C). A more obvious linear growth in the presence of NT-3 (Figure 5D) compared to NGF (Figure 5E) can be seen often 7 days in vitro. However, in this study, the effect of NGF, BDNF and NT-3 on axonal growth was quantified in terms of length and linearity/tortuosity of 3 days in vitro. Images of DRG explants treated with a singular growth factor were imported and analyzed and over-imposed with a grid of concentrical lines to quantify the total number of intersections between the grid and the neurite (Figure 5F). Moreover, length of neurites (L), and vector distance (d) were measured (Figure 5G). A quantitative analysis showed a significant increase in tortuosity (t = d/L) of neurites grown in the presence of NGF compared to NT-3, suggesting that NT-3 allows for a more linear growth (Figure 5H).
Figure 5.
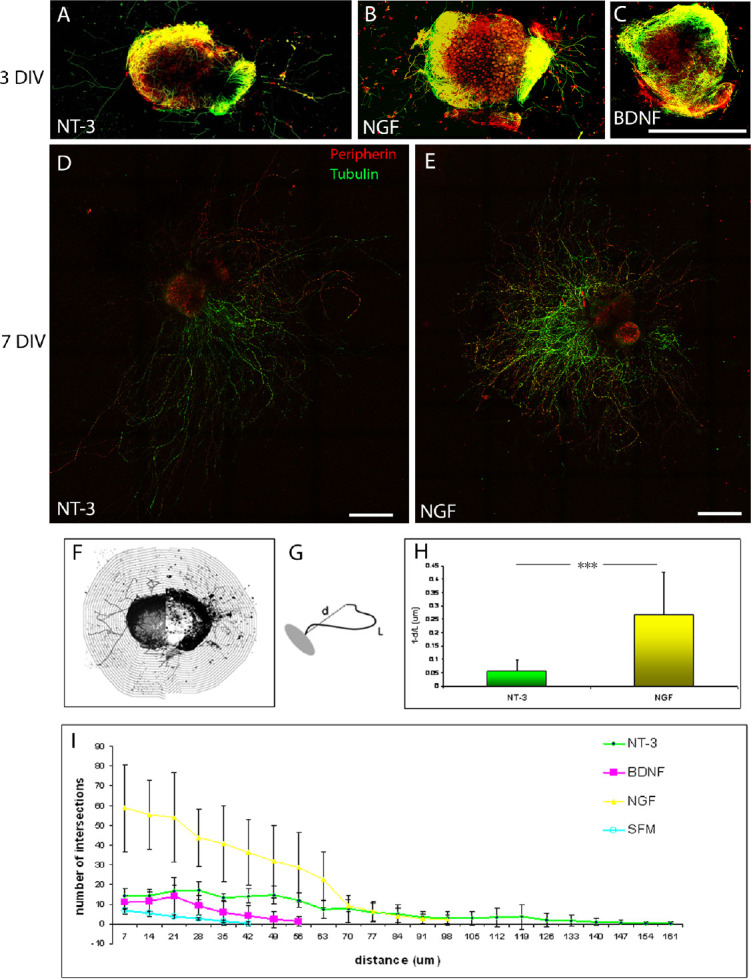
Quantification of neurite growth in DRG explants.
Double immunofluorescence with β-tubulin (green) and peripherin (red) showed the morphology of DRG explants at 3 DIV in the presence of NT-3 (A), NGF (B), and BDNF (C). A more dramatic neurite growth and more linear oriented fibers in the presence of NT-3 was detected at DIV 7 (D) compared to a more random growth in the presence of NGF at the same time point (E). Example of co-ordinates of reference for neurite analysis and counting obtained by progressively enlarging the ganglion perimeter curve using ImageJ software is shown (F). A schematic representation of a ganglion (in grey) and a neuritic process (black line) with the indication of neurite length (L) and vector distance (d) between its termination and the starting point (dotted line) shows the parameters used for the quantification of neurite growth and tortuosity (G). Quantification of the tortuosity (t = 1–d/L) was conducted on primary neurites for explants treated with NGF and NT-3 after 3 DIV. The graph shows that in the presence of NT-3 (n = 23, mean ± SD: 0.2672 ± 0.16), DRGs fibers grew significantly more linearly (***P < 0.001) than in the presence of NGF (n = 14, mean ± SD: 0.0551 ± 0.043) (H). The distance of neuritic processes and the number of intersections of each process with the over-imposed grid were quantified for explants treated with NGF (n = 14), BDNF (n = 8), NT-3 (n = 23) or simply grown in SFM (n = 6). The graph is a representation of mean values and SD. The effect of each single factor was tested on the growing fibers by adding to the culture antibody against the other factors. Scale bars: 1000 µm for A–C; 500 µm for D and E.
A different behavior of growing fibers in the presence of different factors is also shown in terms of the number of intersections with over-imposed grid counted for each condition (Figure 5I). In the presence of NGF, fibers wound around and have a higher percentage of intersections compared to fibers in the presence of NT-3 which grew more linearly and reached longer distances. Finally, explants treated with BDNF were not different from control (SFM) and showed a limited neurite growth.
Discussion
This study compared the contribution of NT family members in the regeneration of peripheral fibers in in vitro DRG models. This comparison highlights not only the different behavior of growing fibers in the presence of each single factor but also which factor is the key to facilitating a more organized and oriented neurite elongation under the regenerative medicine principle that a more linear and guided axonal growth results in a more effective nerve regeneration and increased function recovery after peripheral nerve lesion.
In this study, NGF, BDNF, and NT-3 were tested on two different in vitro models: DRG-derived dissociated primary cell cultures and DRG organotypic cultures. BDNF was also tested on the primary culture of Schwann cells derived from rat sciatic nerves. In serum-free medium condition, dissociated DRG cells spontaneously aggregated in cell clusters. The neurites sprouted and grew in a tortuous way instead of linearly. The growing fibers enwrapped the cell cluster and did not extend far from it. The cluster formation was observed after 48 hours.
Adding NGF to the medium expedited the formation of more structured cell clusters and undoubtedly enhanced neurite sprouting and elongation. Fibers grew with no directionality and the more tortuous neurites did not extend far from the neuronal cell bodies.
The role of NGF as a chemo-attractant in fiber regeneration when released by a target has been well documented (Petruska and Mendell, 2004; Campbell, 2008). However, when NGF is released in the milieu in which ganglionic cells are growing, it results in random and disorganized growth. Therefore, from a regenerative medicine perspective, NGF impedes the axon of ganglionic sensory neurons from growing linearly and effectively towards a peripheral target.
The effect of BDNF on the neurite growth was similar to that of NGF. Dissociated cells were able to spontaneously aggregate and the neurite growth on both DRG models showed a lack of linearity. The 24-hour delay in the cluster formation together with the ability of anti-NGF antibody to null the effect of BDNF may suggest an indirect effect of BDNF on the growth of sensory fibers. Although we know that BDNF may have a preferential motor profile as previously suggested by Santos and colleagues (2016), we speculate that BDNF may enhance the release of NGF in the milieu via activation of the satellite glial cells (SGC). BDNF acting upstream to NGF would explain the delayed formation of cell cluster and the tortuosity of fiber growth. Little is known about SGC physiology and their interactions with neurons. Quantitative studies on several species showed that the number of SGC per neuron increases in proportion to the neuron’s volume (Pannese, 1981; Ledda et al., 2004; Haberberger et al., 2019), which is consistent with the idea that SGC support the neurons metabolically.
The re-clustering behavior of ganglionic cells in vitro has been mentioned before (Pannese and Procacci, 2002; Whitlon et al., 2009) and the consensual conclusion of these descriptive studies was that the formation of these clusters can be reconducted to the cross-talking between neurons and glia. Interestingly, the cross-talking between the sensory neurons and its supportive SGC modulates the morphology of both cells phenotypes, thus enhancing the outgrowth of slender projections from the nerve cell body surface and promoting neurite growth (Pannese and Procacci, 2002; Whitlon et al., 2009; Retamal et al., 2017). Like other glial cells, such as Schwann cells and astrocytes, SGC have numerous receptors for neurotransmitters and other bioactive molecules (Hanani, 2005; Jabs et al., 2005; Del Rio et al., 2015; Retamal, et al., 2017). Thus, it is possible that the cross-talk between sensory ganglia (neurons-neurons, neurons-SGC, and SGC-SGC) serves as a site for the modification of neuronal signals in the sensory pathway (Takeda et al., 2009; Huang et al., 2013).
Satellite cells express a high level of p75 receptor (Pannese, 1981; Dublin and Hanani, 2007; Koike et al., 2019) and TrkA (Pannese and Procacci 2002; Neto et al., 2017; Haberberger et al., 2019; Koike et al., 2019). Therefore, if BDNF enhances the metabolism of satellite cells and these cells express a high level of p75 as well as the NGF-specific receptor TrkA, then the satellite cells may be able to internalize NGF intracellularly as previously suggested by Pannese and Procacci (2002). The satellite cell itself or cross-talking with neurons (Deng et al., 2000; Chan et al., 2004; Huang et al., 2013; Del Rio et al., 2015; Retamal et al., 2017) may then release NGF in the milieu creating the environment that favors cell re-clustering formation and tortuous axonal growth. Ultimately, the neuron-supporting activity of the satellite cells may impede sensory axons to grow and extend outside of the ganglia.
Even though satellite cells and Schwann cells share many characteristics and both function as attendant cells to the neurons (Pannese et al., 1994; Huang et al., 2013), Schwann cells do not seem to be affected by BDNF. The expression of neurotrophin receptors is different between the two glial cell populations. Schwann cells in contact with axonal processes express very few if any p75 receptors. However, p75 is highly expressed in the absence of axons (Taniuchi et al., 1986; DiStefano and Johnson, 1988; Yamamoto et al., 1993; Tomita et al., 2007; Perez et al., 2019). It seems fair to speculate that in the case of peripheral nerve regeneration, Schwann cells which act as a conduit for axon regrowth, start expressing higher levels of p75 to bind and internalize NGF which would act as the target chemo-attractant source to guide and enhance the axonal growth. To our knowledge, no one thus far has reported the expression of TrkA receptors in Schwann cells of adult mammals (Althaus and Richter-Landsberg, 2000) which may affect the ability to internalize NGF and explain the different behaviors of these cells in the presence of BDNF.
Finally, out of the three members of the neurotrophin family considered in this study, our data identified NT-3 as the growth factor that plays a role in a linear and oriented axonal elongation. Both the dissociated ganglionic cells and the DRG explant models showed that NT-3 and NGF promoted axonal growth, however, NT-3 only confers linear orientation to growing neurites.
Conclusions
In agreement with Allodi et al., (2012), our data suggest a more prosensory role of NT-3 and NGF compared to BDNF. The most novel and clinically relevant aspect of our data is the effect of each factor on the linearity of growth. While NT-3 allows for a more linear elongation of growing neurites, NGF results in convoluted bundles of neurites winding around the neuronal cell body with less direction of growth. BDNF does not seem to have a direct effect on the linearity of sensory fiber growth. This finding not only adds some more to the level of understanding on the biochemistry of different growth factor members but also provides evidence to support possible applications in the field of regenerative medicine.
Acknowledgments
We sincerely thank Dr. Maria Giuseppina Robecchi for her help and support in experimental procedures, data analysis, and proof-reading the paper. We thank Ms. Kathryn Eggert and Mrs. Jennifer Lee for proof-reading the paper. We also thank the imaging core facility at Midwestern University for the technical support.
Footnotes
Chinese Library Classification No. R453; R364; R741
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: This study was supported by the research start-up and the MWU’s intramural grant (to MF) and the Italian MURST-MIUR foundation (to SG and IP).
Institutional review board statement: Ethical approval was obtained from the Regione Piemonte Animal Ethics Committee ASLTO1 (file # 864/2016-PR) on September 14, 2016.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the research start-up and the MWU’s intramural grant (to MF) and the Italian MURST-MIUR foundation (to SG and IP).
C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98:16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Althaus HH, Richter-Landsberg C. Glial cells as targets and producers of neurotrophins. Int Rev Cytol. 2000;197:203–277. doi: 10.1016/s0074-7696(00)97005-0. [DOI] [PubMed] [Google Scholar]
- 3.Bagheri A, Habibzadeh P, Razavipour SF, Volmar CH, Chee NT, Brothers SP, Wahlestedt C, Mowla SJ, Faghihi MA. HDAC inhibitors induce BDNF expression and promote neurite outgrowth in human neural progenitor cells-derived neurons. Int J Mol Sci. 2019 doi: 10.3390/ijms20051109. doi: 103390/ijms20051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacialli P, Gatta C, D’Angelo L, Leggieri A, Palladino A, de Girolamo P, Pellegrini E, Lucini C. Nerve growth factor is expressed and stored in central neurons of adult zebrafish. J Anat. 2019;235:167–179. doi: 10.1111/joa.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Rio R, Quintanilla RA, Orellana JA, Retamal MA. Neuron-glia crosstalk in the autonomic nervous system and its possible role in the progression of metabolic syndrome: a new hypothesis. Front Physiol. 2015;6:350. doi: 10.3389/fphys.2015.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng YS, Zhong JH, Zhou XF. Effects of endogenous neurotrophins on sympathetic sprouting in the dorsal root ganglia and allodynia following spinal nerve injury. Exp Neurol. 2000;164:344–350. doi: 10.1006/exnr.2000.7432. [DOI] [PubMed] [Google Scholar]
- 9.DiStefano PS, Johnson EM., Jr Nerve growth factor receptors on cultured rat Schwann cells. J Neurosci. 1988;8:231–241. doi: 10.1523/JNEUROSCI.08-01-00231.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Fornaro M, Sharthiya H, Tiwari V. Adult mouse DRG explant and dissociated cell models to investigate neuroplasticity and responses to environmental insults including viral infection. J Vis Exp. 2018 doi: 10.3791/56757. doi: 103791/56757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grider MH, Mamounas LA, Le W, Shine HD. In situ expression of brain-derived neurotrophic factor or neurotrophin-3 promotes sprouting of cortical serotonergic axons following a neurotoxic lesion. J Neurosci Res. 2005;82:404–412. doi: 10.1002/jnr.20635. [DOI] [PubMed] [Google Scholar]
- 13.Haberberger RV, Barry C, Dominguez N, Matusica D. Human dorsal root ganglia. Front Cell Neurosci. 2019;13:271. doi: 10.3389/fncel.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Huang LY, Gu Y, Chen Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia. 2013;61:1571–1581. doi: 10.1002/glia.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabs R, Pivneva T, Huttmann K, Wyczynski A, Nolte C, Kettenmann H, Steinhauser C. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci. 2005;118:3791–3803. doi: 10.1242/jcs.02515. [DOI] [PubMed] [Google Scholar]
- 17.Kaewkhaw R, Scutt AM, Haycock JW. Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nat Protoc. 2012;7:1996–2004. doi: 10.1038/nprot.2012.118. [DOI] [PubMed] [Google Scholar]
- 18.Katebi S, Esmaeili A, Ghaedi K, Zarrabi A. Superparamagnetic iron oxide nanoparticles combined with NGF and quercetin promote neuronal branching morphogenesis of PC12 cells. Int J Nanomedicine. 2019;14:2157–2169. doi: 10.2147/IJN.S191878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimpinski K, Campenot RB, Mearow K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. J Neurobiol. 1997;33:395–410. doi: 10.1002/(sici)1097-4695(199710)33:4<395::aid-neu5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Koike T, Tanaka S, Hirahara Y, Oe S, Kurokawa K, Maeda M, Suga M, Kataoka Y, Yamada H. Morphological characteristics of p75 neurotrophin receptor-positive cells define a new type of glial cell in the rat dorsal root ganglia. J Comp Neurol. 2019;527:2047–2060. doi: 10.1002/cne.24667. [DOI] [PubMed] [Google Scholar]
- 21.Kornfeld T, Vogt PM, Radtke C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med Wochenschr. 2019;169:240–251. doi: 10.1007/s10354-018-0675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledda M, De Palo S, Pannese E. Ratios between number of neuroglial cells and number and volume of nerve cells in the spinal ganglia of two species of reptiles and three species of mammals. Tissue Cell. 2004;36:55–62. doi: 10.1016/j.tice.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Malcangio M, Garrett NE, Cruwys S, Tomlinson DR. Nerve growth factor- and neurotrophin-3-induced changes in nociceptive threshold and the release of substance P from the rat isolated spinal cord. J Neurosci. 1997;17:8459–8467. doi: 10.1523/JNEUROSCI.17-21-08459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115:189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo Z, Castillo X, Moreno-Carranza B, Ledesma-Colunga MG, Arnold E, López-Casillas F, Ruíz-Herrera X, Clapp C, Martínez de la Escalera G. Vasoinhibin suppresses nerve growth factor-induced differentiation and survival of PC12 pheochromocytoma cells. Neuroendocrinology. 2019;109:152–164. doi: 10.1159/000499507. [DOI] [PubMed] [Google Scholar]
- 27.Miller C, Shanks H, Witt A, Rutkowski G, Mallapragada S. Oriented Schwann cell growth on micropatterned biodegradable polymer substrates. Biomaterials. 2001;22:1263–1269. doi: 10.1016/s0142-9612(00)00278-7. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell AC, Briquez PS, Hubbell JA, Cochran JR. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1–12. doi: 10.1016/j.actbio.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naletova I, Satriano C, Pietropaolo A, Gianì F, Pandini G, Triaca V, Amadoro G, Latina V, Calissano P, Travaglia A, Nicoletti VG, La Mendola D, Rizzarelli E. The copper(II)-assisted connection between NGF and BDNF by means of nerve growth factor-mimicking short peptides. Cells. 2019 doi: 10.3390/cells8040301. doi: 103390/cells8040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neto E, Alves CJ, Leitao L, Sousa DM, Alencastre IS, Conceicao F, Lamghari M. Axonal outgrowth, neuropeptides expression and receptors tyrosine kinase phosphorylation in 3D organotypic cultures of adult dorsal root ganglia. PLoS One. 2017;12:e0181612. doi: 10.1371/journal.pone.0181612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pannese E, Rigamonti L, Ledda M, Arcidiacono G. Perikaryal projections of spinal ganglion neurons: quantitative differences between membrane domains in contact with different microenvironments. J Anat. 1994;185(Pt 3):497–502. [PMC free article] [PubMed] [Google Scholar]
- 32.Pannese E, Procacci P. Ultrastructural localization of NGF receptors in satellite cells of the rat spinal ganglia. J Neurocytol. 2002;31:755–763. doi: 10.1023/a:1025708132119. [DOI] [PubMed] [Google Scholar]
- 33.Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- 34.Pascal D, Giovannelli A, Gnavi S, Hoyng SA, De Winter F, Morano M, Fregnan F, Dell’Albani P, Zaccheo D, Perroteau I, Pellitteri R, Gambarotta G. Characterization of glial cell models and in vitro manipulation of the neuregulin1/ErbB system. Biomed Res Int. 2014;2014:310215. doi: 10.1155/2014/310215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez V, Bermedo-Garcia F, Zelada D, Court FA, Perez MA, Fuenzalida M, Abrigo J, Cabello-Verrugio C, et al. The p75(NTR) neurotrophin receptor is required to organize the mature neuromuscular synapse by regulating synaptic vesicle availability. Acta Neuropathol Commun. 2019;7:147. doi: 10.1186/s40478-019-0802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett. 2004;361:168–171. doi: 10.1016/j.neulet.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Retamal MA, Riquelme MA, Stehberg J, Alcayaga J. Connexin43 hemichannels in satellite glial cells, can they influence sensory neuron activity. Front Mol Neurosci. 2017;10:374. doi: 10.3389/fnmol.2017.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos D, Gonzalez-Perez F, Navarro X, Del Valle J. Dose-dependent differential effect of neurotrophic factors on in vitro and in vivo regeneration of motor and sensory neurons. Neural Plast. 2016;2016:4969523. doi: 10.1155/2016/4969523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 40.Sulaiman W, Dreesen T, Nguyen D. Single local application of TGF-beta promotes a proregenerative state throughout a chronically injured nerve. Neurosurgery. 2018;82:894–902. doi: 10.1093/neuros/nyx362. [DOI] [PubMed] [Google Scholar]
- 41.Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33:784–792. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Taniuchi M, Clark HB, Johnson EM., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tohill MP, Mann DJ, Mantovani CM, Wiberg M, Terenghi G. Green fluorescent protein is a stable morphological marker for schwann cell transplants in bioengineered nerve conduits. Tissue Eng. 2004;10:1359–1367. doi: 10.1089/ten.2004.10.1359. [DOI] [PubMed] [Google Scholar]
- 44.Tomita K, Kubo T, Matsuda K, Fujiwara T, Yano K, Winograd JM, Tohyama M, Hosokawa K. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia. 2007;55:1199–1208. doi: 10.1002/glia.20533. [DOI] [PubMed] [Google Scholar]
- 45.Turney SG, Ahmed M, Chandrasekar I, Wysolmerski RB, Goeckeler ZM, Rioux RM, Whitesides GM, Bridgman PC. Nerve growth factor stimulates axon outgrowth through negative regulation of growth cone actomyosin restraint of microtubule advance. Mol Biol Cell. 2016;27:500–517. doi: 10.1091/mbc.E15-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitlon DS, Tieu D, Grover M, Reilly B, Coulson MT. Spontaneous association of glial cells with regrowing neurites in mixed cultures of dissociated spiral ganglia. Neuroscience. 2009;161:227–235. doi: 10.1016/j.neuroscience.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto M, Sobue G, Li M, Arakawa Y, Mitsuma T, Kimata K. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and low-affinity nerve growth factor receptor (LNGFR) mRNA levels in cultured rat Schwann cells; differential time- and dose-dependent regulation by cAMP. Neurosci Lett. 1993;152:37–40. doi: 10.1016/0304-3940(93)90477-3. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto N, Hanamura K. Formation of the thalamocortical projection regulated differentially by BDNF- and NT-3-mediated signaling. Rev Neurosci. 2005;16:223–231. doi: 10.1515/revneuro.2005.16.3.223. [DOI] [PubMed] [Google Scholar]


