Epidemiology and physiopathology of ischemic stroke: Every year, around 15 million of people suffer a stroke event all around the world. Among those, around 6.7 million will die, and most of the survivors will suffer some grade of disability. Due to the increase in the life expectancy of population and the stagnation in birthrates (especially in developed countries), the trend towards new cases is considered to be alarming (Béjot et al., 2016; Rodríguez-Castro et al., 2018). From a physiological point of view, stroke can be defined as a cerebrovascular disease in which the central nervous system blood flow is interrupted due to an obstruction (ischemic stroke represents about 85% of total events) or rupture (hemorrhagic stroke) of a vessel, causing oxygen and nutrients deprivation in the surrounding tissue. It is important to note that ischemic stroke is a dynamic process, consisting in a fast-developing acute phase, followed by a slow or delayed process of reparation. This time-dependent deprivation sustained over time causes cell damage, often resulting in cell death, with the consequent loss of function in the neural tissue. The number of deaths from this cerebrovascular disease was significantly reduced in the last decades, mostly due to improvements in patient management with the creation of specialized Stroke Units, the use of recombinant tissue plasminogen activator as a thrombolytic therapy and advances in mechanical thrombectomy. However, long-term morbidity remains as a significant problem since many patients are dependent in daily life activities (Rodríguez-Castro et al., 2018). In this context, new strategies implemented in the different phases of ischemic stroke, such as photobiomodulation (PBM) or hypothermia therapy have recently emerged as an opportunity to reduce the associated adverse effects (Vieites-Prado et al., 2016; Hamblin, 2018). In this endeavor, the main objective of this research work is to summarize the recent findings in PBM therapy for ischemic stroke and to answer the question of whether light-based therapy could become a real therapy for patients.
Brain PBM therapy: The processes by which light energy can induce chemical changes in cells, causing biological reactions in benefit of a neuroprotective response, inflammation reduction and neurogenesis have been extensively studied in different preclinical research works (Hamblin, 2017; Hennessy et al., 2017; Salehpour et al., 2018). However, the mechanisms of action underlying the interaction between light energy and tissues are complex and not completely understood, mainly due to the different cellular chromophores involved (water, oxyhemoglobin, deoxyhemoglobin, myoglobin, melanin, cytochromes, and flavins). In particular, a detailed review of light-based therapy summarizing many of these in vitro and in vivo studies developed in the last years has been recently published (Salehpour et al., 2018).
Currently, the positive effects of light therapy at cellular level seem to be explicit and non-speculative. It is clear that red/near-infrared (NIR) light is a way to stimulate the cytochrome c oxidase of the mitochondrial respiratory chain, thus increasing ATP production. This mechanisms modulates the activation of transcription factors and signaling mediators such as nuclear factor-κB, resulting in long-lasting effects on cells mainly due to the production of reactive oxygen species and the release of Ca2+ as versatile second messengers (Figure 1A). Other positive implications of light-based therapy include stimulation of cell survival and enhanced dendrite growth through up-regulation of brain-derived neurotrophic factor mediated by the activation of extracellular signal regulated kinase/cAMP-responsive element binding signaling pathway; promotion of axonal protection via nerve growth factor-induced neurite outgrowth; or neurite outgrowth and synaptogenesis stimulation promoted by mitogen-activated protein kinase activation.
Figure 1.
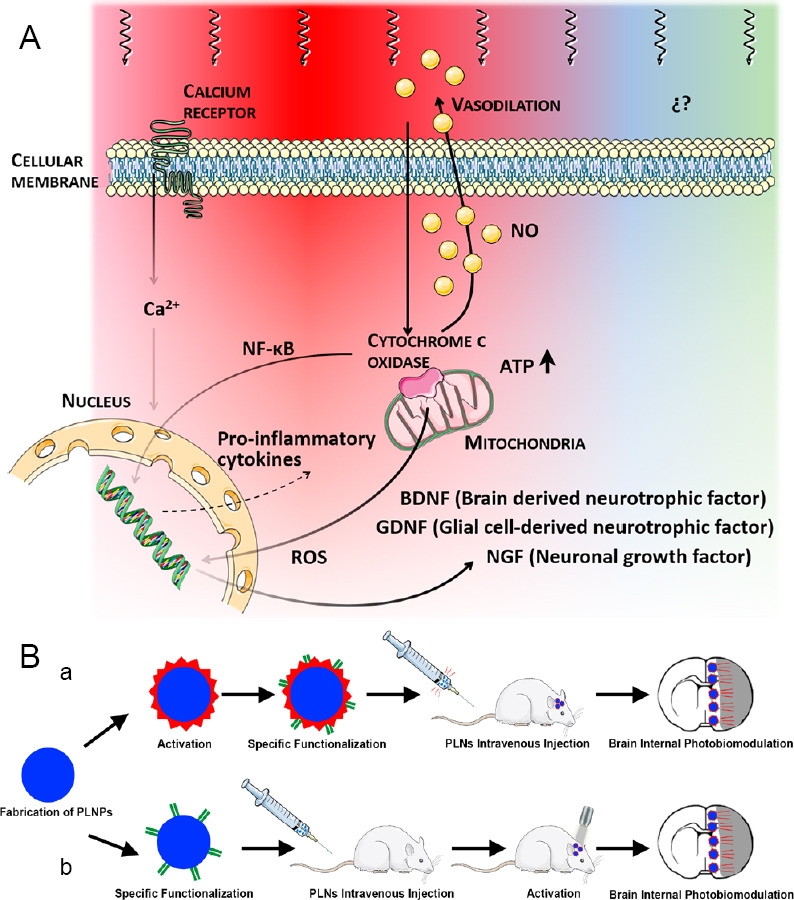
PBM underlying mechanisms at cellular level and example of internal PBM therapy application.
(A) Mechanisms involved in photobiomodulation in the red/near-infrared range. (B) Representative diagram of internal light-based therapy by means of PLNPs probes activated before (a) or after (b) being intravenously injected in an animal model of stroke. NF-κB: Nuclear factor kappa B; PBM: photobiomodulation; PLNPs: persistent luminescence nanoparticles; ROS: reactive oxygen species.
Non-invasive delivery of light from an external light-device to the head and then into the brain is denominated transcranial PBM. To implement light-based therapies in vivo, it is crucial to know the depth of light penetration through the scalp, skull, and brain tissues. Different reports have been conducted ex vivo (animals and human), in vivo (animals), and in simulations (Monte Carlo) in order to evaluate the total light energy that is able to reach the damaged tissue inside the brain. However, up to this date there is no consensus about the real light penetration into brain tissues as it is dependent on the optical parameters, tissue characteristics and protocol performed (wavelength, irradiance, exposure time, irradiated area, coherence and light source). Briefly, literature reports regarding light preclinical studies show that PBM treatment for ischemic stroke involves the use of light from the visible to NIR portions of the spectrum (wavelength ~600–1100 nm) with a relatively low fluence or energy density (1–30 J/cm2) to avoid thermal effects or burns in the tissue. In addition, other parameters and methodologies have been taken into account to evaluate the possible effectiveness of this novel technique, such as the irradiation location/protocol (at the injury site or whole brain; holding probe in direct contact with the shaved skull or through a burr hole), timepoint to start the light-based treatment (usually within the first 24 hours of the stroke onset), exposure time and number of repetitions (usually less than 60 minutes with more than one irradiation session), light operation mode (continuous or pulsed), and coherence (low level lasers and light emitting diodes (LED) are the main devices used). Germane to light properties, an average transmission of ~1–20% with a penetration length of ~3–30 mm has been reported (Salehpour et al., 2018).
Despite the variability in the protocols implemented in animal models of ischemic stroke, all results seem to indicate that PBM therapy applied < 24 hours from stroke onset reduces neurological damage evaluated by functional tests, final infarct volume assessment, and mortality rate or cell neurogenesis. However, the majority of PBM in vivo studies for stroke tend to be mainly limited by size and light penetration capacity across different brain tissues. In this line, we have recently shown that LED-PBM treatment, initiated 24 hours after the stroke, does not achieve significant recovery results analyzed by means of lesion reduction, behavioral tests and functional resonance imaging (Argibay et al., 2019).
The established consensus on the benefits of light-based therapy disappears when we examine the PBM clinical trials conducted. The first clinical trial (NEST-1) demonstrated the safety and effectiveness of transcranial low-level laser therapy for acute ischemic stroke (within 24 hours of onset). In this study, 120 patients between 40–85 years of age with a diagnosis of ischemic stroke and a measurable neurological deficit were included. Light energy (808 nm, 1 J/cm2 at cortical surface) was delivered to the whole head in 20 sequential spots each lasting for 2 minutes. However, the second clinical trial, called NEST-2; ClinicalTrials.gov Identifier: NCT00419705 (660 patients between 40–90 years of age) and low-level laser therapy within 24 hours of stroke onset demonstrated safety but the efficacy did not achieve statistical significance. A third clinical trial (NEST-3; ClinicalTrials.gov Identifier: NCT01120301) was conducted with the intention of enrolling 1000 patients with acute ischemic stroke treated ≤ 24 hours after the onset and who did not undergo thrombolytic therapy. Results were not satisfactory, and a neuroprotective effect was not found taking into account the 90-day functional outcome of the patients (Lampl et al., 2007; Hacke et al., 2014).
Future outlook: The overall results from extensive in vitro and in vivo studies of the PBM therapy show the benefits of light-based strategies in the acute stroke phase in terms of neuroprotection and neurorecovery. Nevertheless, negative results from the clinical studies halt the possible viability of this therapy in patients at this moment. We consider that the greatest limitation of this light-based technique lies in the impossibility of delivering non-invasive effective light energy into the damaged area inside the patient’s brain able to induce the corresponding biological effect. In this regard, light irradiation via the nasal or oral cavity has demonstrated beneficial effects in Alzheimer’s disease, depression and anxiety (Hennessy et al., 2017). These non-invasive techniques may be an alternative option to deliver light energy into the brain, but we consider that they would not be suitable for all stroke subtypes due to the human brain structure. Hereafter, we propose a new perspective to solve this technical challenge; we consider that novel functionalized persistent luminescence nanoparticles could be an alternative way to irradiate deep, damaged brain tissue from inside the brain itself (internal PBM). NIR persistent luminescence nanoparticles are optical materials that emit long-lasting luminescence (for minutes or hours) even after the excitation ceases, and that allow for reactivation of the persistent luminescence by NIR light or X-ray (Wang et al., 2017; Sun et al., 2018). Although internal PBM protocols have been carried out in cancer preclinical in vivo studies (Sun et al., 2018) as new therapeutic and diagnostic strategies (theranostic), PBM has never been proposed or used in stroke before. The fabrication process of these PLNPs nanoprobes for biological applications has been optimized in the last years; appropriate size (< 250 nm), specific functionality (antibody), appropriate luminescence, long afterglow and repeated renewability. Functionalized NIR-emitting persistent luminescence nanoparticles with antibodies against peri-infarct brain region such as anti-heat shock protein 72 (or inducible form of anti-heat shock protein 70) could allow successful damage-targeted light exposure in vivo via intravenous injection. Figure 1B details the two protocols by which nanoparticles could be designed, specifically functionalized and intravenous injected to reach the brain and internally irradiate the damaged region; probes could be active before being injected or after being injected in an animal model of stroke. We conclude that clinic PBM therapy using laser or LED sources could become a promising tool in stroke therapy, but further preclinical studies are necessary in order to open, develop and optimize novel but indirect approaches.
This work was supported by Instituto de Salud Carlos III (PI17/01103), Spanish Research Network on Cerebrovascular Diseases RETICS-INVICTUS PLUS (RD16/0019). Spanish Ministry of Economy and Competitiveness (SAF2017-84267-R), Xunta de Galicia (Consellería Educación: GRC2014/027 and IN607A2018/3).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Argibay B, Campos F, Perez-Mato M, Vieites-Prado A, Correa-Paz C, López-Arias E, Da Silva-Candal A, Moreno V, Montero C, Sobrino T, Castillo J, Iglesias-Rey R. Light-emitting diode photobiomodulation after cerebral ischemia. Front Neurol. 2019;10:911. doi: 10.3389/fneur.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Béjot Y, Bailly H, Duriewr J, Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016;45:e391–398. doi: 10.1016/j.lpm.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Schellinger PD, Albers GW, Bornstein NM, Dahlof BL, Fulton R, Kasner SE, Shuaib A, Richieri SP, Dilly SG, Zivin J, Lees KR. NEST 3 Committees and Investigators (2014) Transcranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke. 45:3187–3193. doi: 10.1161/STROKEAHA.114.005795. [DOI] [PubMed] [Google Scholar]
- 4.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4:337–361. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamblin MR. Photobiomodulation for traumatic brain injury and stroke. J Neurosci Res. 2018;96:731–743. doi: 10.1002/jnr.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennessy M, Hamblin MR. Photobiomodulation and the brain: a new paradigm. J Opt. 2017;19:013003. doi: 10.1088/2040-8986/19/1/013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Castro E, López-Dequit I, Santamaría-Cadavid M, Arias-Rivas S, Rodríguez-Yáñez M, Pumar JM, Hervella P, López-Arias E, da Silva-Candal A, Estany A, Piñeiro-Lamas M, Sobrino T, Campos F, Portela M, Vázquez-Lima M, Castillo J, Iglesias-Rey R. Trends in stroke outcomes in the last ten years in a European tertiary hospital. BMC Neurol. 2018;18:164. doi: 10.1186/s12883-018-1164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR. Brain photobiomodulation therapy: a narrative review. Mol Neurobiol. 2018;55:6601–6636. doi: 10.1007/s12035-017-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun SK, Wang HF, Yan XP. Engineering persistent luminescence nanoparticles for biological applications: from biosensing/bioimaging to theranostics. Acc Chem Res. 2018;51:1131–1143. doi: 10.1021/acs.accounts.7b00619. [DOI] [PubMed] [Google Scholar]
- 11.Vieites-Prado A, Iglesias-Rey R, Fernández-Susavila H, da Silva-Candal A, Rodríguez-Castro E, Gröhn OH, Wellmann S, Sobrino T, Castillo J, Campos F. Protective effects and magnetic resonance imaging temperature mapping of systemic and focal hypothermia in cerebral ischemia. Stroke. 2016;47:2386–2396. doi: 10.1161/STROKEAHA.116.014067. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Li Y, Mao R, Wang Y, Yan X, Liu J. Persistent luminescent nanoparticles as energy mediators for enhanced photodynamic therapy with fractionated irradiation. J Mater Chem B. 2017;5:5793–5805. doi: 10.1039/c7tb00950j. [DOI] [PubMed] [Google Scholar]


