Abstract
Retinal degenerations are the leading causes of irreversible visual loss worldwide. Many pathologies included under this umbrella involve progressive degeneration and ultimate loss of the photoreceptor cells, with age-related macular degeneration and inherited and ischemic retinal diseases the most relevant. These diseases greatly impact patients’ daily lives, with accompanying marked social and economic consequences. However, the currently available treatments only delay the onset or slow progression of visual impairment, and there are no cures for these photoreceptor diseases. Therefore, new therapeutic strategies are being investigated, such as gene therapy, optogenetics, cell replacement, or cell-based neuroprotection. Specifically, stem cells can secrete neurotrophic, immunomodulatory, and anti-angiogenic factors that potentially protect and preserve retinal cells from neurodegeneration. Further, neuroprotection can be used in different types of retinal degenerative diseases and at different disease stages, unlike other potential therapies. This review summarizes stem cell-based paracrine neuroprotective strategies for photoreceptor degeneration, which are under study in clinical trials, and the latest preclinical studies. Effective retinal neuroprotection could be the next frontier in photoreceptor diseases, and the development of novel neuroprotective strategies will address the unmet therapeutic needs.
Keywords: clinical trials, growth factors, intraocular injection, intravitreal injection, neuroprotection, paracrine properties, photoreceptors, preclinical models, retinal diseases, stem cells
Introduction
The functional and structural complexity of the retina makes this tissue susceptible to multiple types of pathogenic damage (Cuenca et al., 2014). Retinal degeneration results in retinal deterioration caused by different injuries that lead to progressive degeneration and ultimately cell death. This condition is the leading cause of incurable visual loss and blindness worldwide, especially if the diseases affect the macula (Gagliardi et al., 2019). The major retinal disease related to photoreceptor and retinal pigment epithelium (RPE) cell death, and thus, visual impairment, is age-related macular degeneration (AMD). Furthermore, genetic retinal diseases related to photoreceptor degeneration and death, such as retinitis pigmentosa (RP), Leber’s congenital amaurosis, Usher’s syndrome, and Stargardt disease, are other main causes of irreversible visual loss worldwide. Thirty-four million people in the European Union are estimated to have photoreceptor-related degeneration due to conditions such as AMD or inherited retinal degenerations (Li et al., 2019). In addition, ischemic retinal disorders, such as ischemic diabetic maculopathy and ischemic central retinal vein occlusion, cover a range of ocular diseases that affect the blood vessels and that may lead eventually to photoreceptor degeneration within the retina (Kadłubowska et al., 2016). Nevertheless, many different etiologies that cause retinal degeneration often share common mechanistic pathways affecting the cellular response and remodelling, which lead to the same final result, i.e., photoreceptor loss and consequent visual loss (Figure 1) (Gagliardi et al., 2019).
Figure 1.
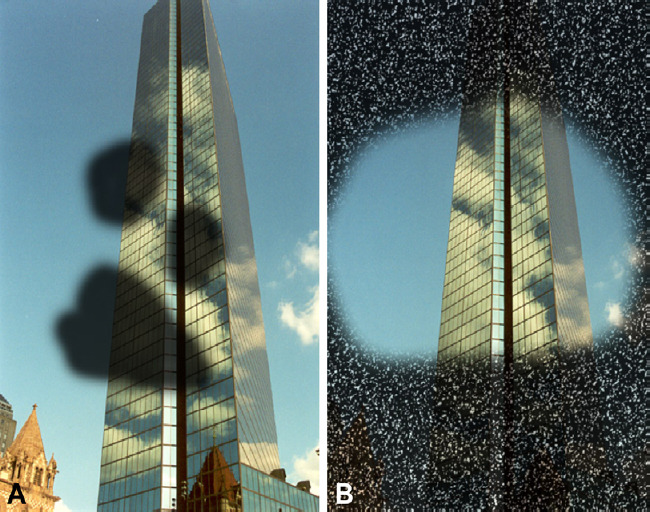
Types of visual impairment.
Central or paracentral scotoma (A), an area of central impairment of the visual field, usually develops in patients with age-related macular degeneration, diabetic retinopathy, or Stargardt disease. While concentric retraction of the visual field (B) occurs in patients with retinitis pigmentosa or glaucoma.
Despite the impact on daily life and the social and economic effects, there is no cure for retinal diseases in which photoreceptor atrophy and death are the main causes of impaired vision. Current therapies are focused primarily on the etiology or specific late consequences, such as neovascularization. The currently available clinical treatments, such as neurotrophic factors or anti-angiogenic agents delivered intraocularly, only delay the onset or slow progression of visual impairment (Gagliardi et al., 2019). Therefore, given the magnitude and severity of this problem, it is not surprising that new therapeutic strategies are being investigated, such as gene therapy, optogenetics, or cellular replacement (Cuenca et al., 2014; Trapani and Auricchio, 2018; Jin et al., 2019; Simunovic et al., 2019).
However, sustained neuroprotection is currently the only potential strategy that seems applicable to different types of retinal degenerative diseases mainly at their initial stages (Cuenca et al., 2014; Kolomeyer and Zarbin, 2014). Considering this, neuroprotection via cell-based therapies offers a new all-encompassing approach. Specifically, the paracrine properties of stem cells injected into the eye allow continuous secretion of neurotrophic, immunomodulatory, and anti-angiogenic factors that could potentially impact deteriorating retinal cells for a considerable period of time (Labrador Velandia et al., 2018; Lejkowska et al., 2019), with the advantage of superior potential than neurotrophic factors with a shorter half-lives and injected in a timely manner (LaVail et al., 1992; Kurokawa et al., 1999; Vidal-Sanz et al., 2001; Takahata et al., 2003; Li et al., 2009; Levkovitch-Verbin et al., 2010, 2019; Kolomeyer and Zarbin, 2014).
The eye is an ideal target for advanced therapies such as cell therapy because of the ease of accessibility and the wealth of surgical expertise dealing with the retina facilitated by the transparent cornea. In addition, the eye is a small, highly compartmentalized organ and fewer cells would be sufficient for potential therapy; which facilitates specific targeting of the neuroretina; and the blood-brain barrier separates the eye from the rest of the body, which ensures ocular immune privilege and avoids systemic dissemination of physiologic conditions (Gagliardi et al., 2019). In addition, many retinal diseases are clinically well characterized. Finally, the availability of high-resolution and non-invasive imaging techniques allow monitoring of retinal changes after treatment (Gagliardi et al., 2019).
For these reasons, much research has been undertaken recently to advance the preclinical knowledge about stem cell management and properties, and, from the clinical standpoint, to confirm their intraocular safety and efficacy to prevent and/or reduce retinal degeneration. All of these steps are necessary for future neuroprotection strategies by using intraocular stem cells in patients with degenerative retinal diseases. We review the recent literature about stem cell-based neuroprotective strategies based on their paracrine properties for photoreceptor cell degeneration, the research about which is currently in clinical trials, and discuss the future direction of this field as described in the latest preclinical research publications.
Search Strategy and Selection Criteria
The review cited the preclinical studies and clinical trials that have been performed recently on the role of intravitreal stem cell paracrine properties in photoreceptor neuroprotection (Figure 2) and searched PubMed, Web of Science, Scopus, Embase, and ClinicalTrials.gov electronic databases from 2013 to September 2019. Potentially relevant articles were sought by using the following search terms in combination as Medical Subject Headings terms and text words: animal models, cell culture, cell therapy, clinical trials, growth factors, in vitro models, in vivo models, intravitreal injection, intraocular injection, neurodegeneration, neuroprotection, organ culture, organotypic culture, paracrine properties, photoreceptor, preclinical studies, retina, retinal diseases, secretome, and stem cell. No language restrictions were applied. English abstracts were used for non-English articles when available. We also scanned the reference lists of the retrieved publications to identify additional relevant articles (cross-reference strategy), and using the MEDLINE option “Related Articles” and consulting review articles on the topic supplemented the search.
Figure 2.
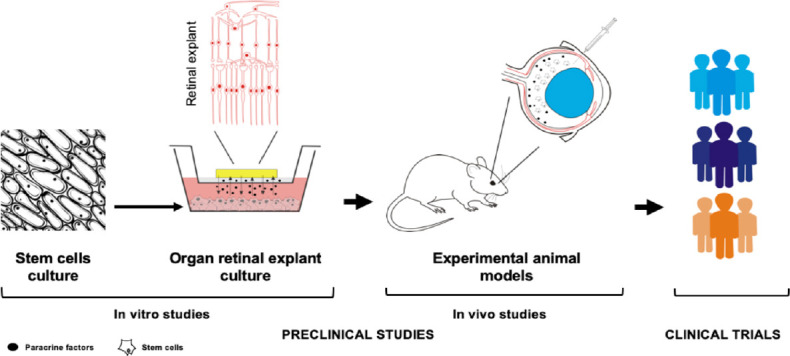
Translational research to evaluate the neuroprotective capacity of the stem cells over photoreceptor cell degeneration.
Organ retinal explant culture is the in vitro model most used to study the neuroprotective processes of stem cells. The organ retinal explant-stem cells co-culture is physically separated by a porous membrane that prevents stem cell migration and integration into the retinal tissue; the membrane also allows molecular exchange between the stem cells and retinal tissue. In vivo preclinical studies have established that the intravitreal injection is the most appropriate route of stem cell administration to evaluate the effects of paracrine neurotrophic factors. The efficacy of stem cells is attributable to production of factors that promote endogenous neuronal growth and angiogenesis, stimulate the synaptic connection and remyelination of damaged axons, diminish apoptosis, and finally regulate inflammation, as observed in preclinical studies. The last step of translational research, before the clinical application of novel therapies, consists of the design and development of clinical trials to confirm the safety and efficacy of intravitreal stem cells to neuropreserve the photoreceptors from degeneration. Some of the most relevant retinal pathologies that could potentially be addressed with cell-based therapies include age-related macular degeneration, retinitis pigmentosa, Stargardt disease or vascular diseases, such as diabetic retinopathy or vein occlusion.
Healthy and Diseased Photoreceptors
Photoreceptors, rods and cones, are highly specialized neurons with a clearly differentiated morphology, that are comprised of an elongated outer segment, connecting cilium, inner segment, cell body, and axon with a synaptic terminal (Cuenca et al., 2014; Bachmann-Gagescu and Neuhauss, 2019) (Figure 3A and C).
Figure 3.
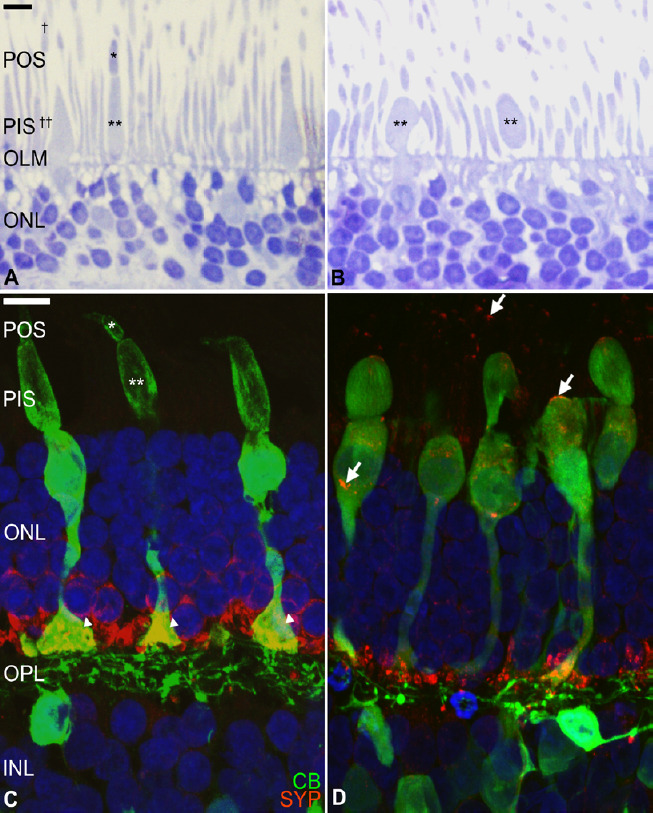
Human photoreceptor degeneration process in an organotypic culture of the neuroretina.
Organ retinal explant cultures are considered useful tools for cellular and molecular research into retinal degeneration and neuroprotection. Briefly, human neuroretina explants were cultured in Transwell® plates, with the photoreceptor layer facing the supporting membrane. Ultrathin and cryostat sections were evaluated after toluidine blue staining (A, B) and after immunostaining for neuronal markers (C, D). Fresh human neuroretina (A) morphologic organization of the photoreceptors show easily recognizable cone and rod outer (asterisk and dagger, respectively) and inner segments (double asterisk and double dagger, respectively), outer limiting membrane, and highly organized outer nuclear layer. After 6 days of culture (B), the photoreceptor degeneration process is evident with loss of the cone outer segments and swollen cone inner segments (double asterisk) and cell bodies. Immunostaining for calbindin (CB, green), a calcium-binding protein of cones and second-order neurons (C), shows the normal morphology of the cone photoreceptors, including the outer (asterisk) and inner (double asterisk) segments and their terminals (arrowheads). After 9 days of culture (D) some inner segments are swollen, and the cones have degenerated inner and outer segments. Synaptophysin (SYP, red), a synaptic-vesicle protein in the photoreceptors and second-order neurons, was located at photoreceptor axon terminals (C) and after culture, it is identified throughout the photoreceptor cell bodies (D, arrows). Scale bars: 10 µm. These images were obtained in collaboration with Dr. Nicolas Cuenca (Universidad de Alicante, Spain). INL: Inner nuclear layer; OLM: outer limiting membrane; ONL: outer nuclear layer; OPL: outer plexiform layer; PIS: photoreceptor inner segment; POS: photoreceptor outer segment.
The changes in photoreceptors and their synaptic connectivity that lead to dysfunction and cell loss are evident in several human neurodegenerative diseases and animal models of neurodegeneration, which result in visual impairment and eventually blindness (Jones et al., 2012; Cuenca et al., 2014; Gasparini et al., 2019). Photoreceptor degeneration is also accompanied by remodelling of other neuroretinal cells, such as horizontal, bipolar, amacrine, ganglion, and Müller cells (Cuenca et al., 2014; Gasparini et al., 2019). The neural retinal degenerative process usually is divided into different phases (1 to 4) by the scientific community (Marc and Jones, 2003; Marc et al., 2003, 2007; Jones and Marc, 2005; Carr et al., 2009; Vugler, 2010; Jones et al., 2012; Cuenca et al., 2014; Gasparini et al., 2019); therefore, changes at the cellular level can be associated with the stage of retinal degeneration:
Phase 1 is characterized by photoreceptor stress that induces a cascade of events that culminates in molecular changes and eventual cell death. However, the function and morphology of photoreceptor cells and other retinal neurons appear normal as does the retinal layering.
Phase 2 is characterized by continuous photoreceptors stress that leads to truncation of the outer segments, aberrant extension of neurites, dendrite retraction, and progressive photoreceptor death (Figure 3B and D). In parallel, bipolar cell modifications and activation of Müller glia cells are observed. However, before photoreceptor death, several molecular modifications are apparent, such as delocalization of rhodopsin in the rods and transduction in cones, or synaptophysin redistribution and loss (Figure 3D).
Phase 3 is characterized by large-scale photoreceptor cell death followed by retraction of bipolar and horizontal cell dendrites and the beginning of the inner nuclear layer neuronal death. The loss of neuronal cells leads to Müller cell hypertrophy, formation of a glial seal over the neuroretina, and microglial activation.
Phase 4 is characterized by progressive global neuroretinal disorder and neuronal cell migration between the layers and finally death, with rewiring of the retinal circuitries. Furthermore, hypertrophy of Müller cells and epiretinal membrane formation, and retinal invasion by blood vessels and migrating RPE cells can be observed.
In this scenario, therapeutic strategies based on neuroprotection aim to create an adequate environment for preserving the viability of the retinal cells and thus functioning. This can be achieved through delivery to the retinal tissue trophic/growth factors that generally promote cell proliferation, maturation, survival, and/or regeneration to maintain retinal homeostasis. Therefore, neuroprotective compounds seem crucial for maintaining retinal homeostasis (Cideciyan et al., 2013), but the final success of the treatments, understood as the maintenance or improvement of visual function, also relies on appropriate patient selection (age of patient) and the etiology and stage of the disease (Cuenca et al., 2014). Even though treatment of the retinal pathologies must involve a combination of different therapeutic approaches, retinal neuroprotection via delivery of neurotrophic, antioxidative, antiapoptotic and/or anti-inflammatory factors appears essential in phase 1 and necessary in phase 2 to maintain the viability of the retinal cells (Cuenca et al., 2014). Furthermore, neuroprotection also seems crucial in phases 3 and 4, even when vision has been lost completely (Cuenca et al., 2014).
In this sense, several neurotrophic factors have been studied extensively and proven effective for the protection and survival of degenerating photoreceptors in vitro and in vivo (Cuenca et al., 2014; Kolomeyer and Zarbin, 2014). These include brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), ciliary neurotrophic factor (CNTF), pigment epithelium-derived factor, and nerve growth factor. Furthermore, their combination has synergistic neuroprotective effects in photoreceptor rescue (Cuenca et al., 2014; Kolomeyer and Zarbin, 2014). Nevertheless, the neuroprotective effects of factors such as CNTF or bFGF are limited to just over 1 week, and for BDNF 2 weeks after intravitreal administration in experimental animals (LaVail et al., 1992; Kurokawa et al., 1999; Vidal-Sanz et al., 2001; Takahata et al., 2003). Moreover, high doses may damage the retina (Kolomeyer and Zarbin, 2014). Indeed, exogenous administration of these factors is limited by their short half-lives and dose safety (Kolomeyer and Zarbin, 2014). Therefore, to obtain prolonged effects, iterative intravitreal injections may be needed (Cuenca et al., 2014). To address this problem, new strategies such as nanoparticle-containing factors, viral-mediated transference, and implants of genetically modified encapsulated cells have been implanted into the vitreous cavity (Birch et al., 2013) with different success levels.
In this case, the potential of the paracrine neuroprotective properties of intravitreally injected stem cells for the production of neuroprotective factors in a sustained manner over time and allowing a synergistic effect is a potential therapeutic option currently under preclinical and clinical investigation for retinal degenerations, i.e., stem cells injected into the vitreous cavity survived 3 months (Lejkowska et al., 2019), and matured and secreted BDNF, CNTF, and bFGF for at least 4 to 5 weeks (Li et al., 2009; Levkovitch-Verbin et al., 2010).
Stem Cells
Stem cells are those that have self-renewing capabilities and differentiate into multiple cell lineages. The most common way to classify stem cells is based on their source: embryos or adult body tissues. Furthermore, stem cells can be categorized according to their potential to differentiate into different cell types, such as totipotent, pluripotent, multipotent, oligopotent, or unipotent. All stem cells may be useful for medical research, but each type has advantages and limitations. Despite the embryonic stem cell (ESC) therapeutic potential, their use is ethically and politically controversial because of the destruction of human embryos (Lo and Parham, 2009). Conversely, adult stem cells are not associated with this problem but others, i.e., in that pieces of tissue are extracted from any part of the body to obtain the stem cells and some types are difficult to maintain in vitro. Among the adult stem cells and their usefulness for retinal pathologies are the mesenchymal stem cells (MSCs) derived from bone marrow (bmMSC) or adipose tissue (aMSC) (Nauta and Fibbe, 2007; Xu and Xu, 2011; Berglund et al., 2017; Labrador-Velandia et al., 2019). More recently, the induced pluripotent stem cells (iPSC) are being investigated (Alonso-Alonso and Srivastava, 2015).
Stem cell application in retinal diseases
The possible clinical application of stem cells in retinal pathologies is based on two main therapeutic approaches, cell replacement and/or neuroprotection (through its paracrine action). Cellular replacement has limitations; previous studies have suggested that the adult retina does not provide an environment in which transplanted stem cells can easily migrate, integrate, and differentiate (Mellough et al., 2007; Hill et al., 2009; Johnson et al., 2009; Gagliardi et al., 2019; Gasparini et al., 2019). Although some studies have reported that stem cell-derived photoreceptors and photoreceptor precursors may integrate and/or transfer material to the host cells (MacLaren et al., 2006; MacLaren and Pearson, 2007; Waldron et al., 2018), the differentiation of MSCs into functional retinal cells still requires lengthy exploration. Furthermore, the neurologic recovery enhancement demonstrated by stem cells seem to be derived from an indirect paracrine effect rather than direct cell replacement (Seo and Cho, 2012). Therefore, the paracrine ability of stem cells to secrete trophic factors, neurotrophins, cytokines, and signalling molecules that promote angiogenesis and tissue regeneration, inhibit fibrosis and apoptosis, and modulate the immune system and inflammation could improve the survival of degenerating retinal cells (Levkovitch-Verbin et al., 2010). Among factors secreted by the stem cells, which include neurotrophic factors that belong to the family of neurotrophins (molecules involved in tropism and neuronal plasticity), are exhibited by BDNF, NGF and NT3, and other neurotrophic factors such as CNTF, GDNF, insulin-like growth factor 1 and bFGF, which have been proven effective in protecting retinal cells after acute damage in preclinical models (Labouyrie et al., 1999; Lin et al., 2009; Kolomeyer and Zarbin, 2014; Labrador-Velandia et al., 2019; Lejkowska et al., 2019).
Nevertheless, the use of selected and specific stem cell populations seems to have greater therapeutic potential than bone marrow aspirates and also allows allogenic use (Nauta and Fibbe, 2007; Labrador-Velandia et al., 2019). Thus, much work remains to be done in the laboratory and clinical practice to harness the stem cell potential for cell-based therapies to treat retinal diseases.
Pre-Clinical Studies
In vitro cell cultures
Based on our search criteria, no studies were published between 2013 and 2019 that specifically evaluated the stem cell neuroprotective potential in photoreceptor cultures.
In vitro organ cultures
The neuroretinal explant with stem cell co-culture is the most used in vitro model to evaluate the stem cell neuroprotective ability in the photoreceptors. Organ retinal explant cultures are useful for cellular and molecular research into retinal degeneration and neuroprotection due to the relatively low cost and easy development and they are an excellent resource that bridges the gap between cell cultures and in vivo models (Fernandez-Bueno et al., 2012; Di Lauro et al., 2016).
Rodriguez-Crespo et al. (2014), who developed a triple-layered mixed co-culture model of porcine RPE cells and neuroretina with human aMSCs, reported limited neuroprotective effects on the retina. However, the authors hypothesized that various limitations of this triple-layer culture model narrowed the potential positive effects of the stem cells over the retinal parenchyma, such as an insufficient amount of nutrients supplied by the culture medium and/or reduced transmembrane diffusion of necessary nutrients and factors for adequate neuroretina preservation in vitro (Rodriguez-Crespo et al., 2014).
In contrast, Mollick et al. (2016) reported that human neural progenitor cell (NPC)-derived factors slowed the spontaneous degenerative processes of adult porcine retinal explants. The results showed that NPCs limit photoreceptor death through better maintenance of the cone outer segments, reduced opsin mislocalization, and maintained synaptic structural integrity (Mollick et al., 2016). Although the authors could not explain the exact mechanism by which NPCs neuropreserve the photoreceptors from degeneration, they hypothesized that NPCs secrete neurotrophic factors in co-culture with the neuroretina that protect the photoreceptors from degeneration and slow the spontaneous degenerative processes of the retinal explants (Mollick et al., 2016).
Labrador-Velandia et al. (2019) performed another study of the neuroprotective paracrine effects of stem cells over the retina and reported that human bmMSCs have paracrine neuroprotective effects over porcine neuroretinal spontaneous degeneration, including the photoreceptors. The authors reported that the observed neuroprotective effect was associated with elevated concentrations of the neurotrophic factors, BDNF and CNTF (Labrador-Velandia et al., 2019).
Jones et al. (2019) also reported that retinal tissue co-cultured with human NPCs showed less cell death than retinas cultured alone. In this experiment, retinal samples from the Royal College of Surgeons rats, an in vivo model of photoreceptor degeneration, were used. This potential cell therapy is applied to a degeneration model, not “healthy” retinas, as in the previously discussed models. This study also mentioned that NPCs induced nuclear factor (erythroid-derived 2)-like 2 synthesis, which mediates oxidative response signalling and therefore was implicated in the retinal neuroprotection by the NPCs (Jones et al., 2019).
Although an ideal study would use human tissues, the in vitro models described here are based on the use of porcine neuroretina because of the anatomic and histologic similarities of the porcine and human retinas (Sanchez et al., 2011) or the use of neuroretina from animal models of retinal diseases, and offer several advantages over dependency on the availability of human sample for research purposes.
In summary, the in vitro results showed that stem cells can retard photoreceptor layer degeneration through the paracrine secretion of neurotrophic factors. Those studies provide in vitro evidence for the possible application of stem cell paracrine properties in diseases of the retinal photoreceptors.
In vivo experimental animals
Studies in animal models of retinal pathologies have compared intravenous (systemic) injection, subretinal transplantation and intravitreal injection of stem cells (Xu and Xu, 2011). According to these studies, intravitreal injection is the most appropriate route of administration to investigate the effect of paracrine neurotrophic factors, because secreted neurotrophins can cross the inner limiting membrane and diffuse into the retina, while it would also be a natural barrier that prevents stem cells to pass through and, therefore, integrated into the neural retina (Xu and Xu, 2011). Although, according to the search criteria previously defined, no studies between 2013 and 2019 specifically evaluated the paracrine effects of intravitreally administered stem cells on photoreceptor degeneration in laboratory animal models. Conversely, several experiments have evaluated the intravitreal MSCs in different murine models of retinal pathologies, such as retinal degeneration, RP, retinal ischemia, and glaucoma. Those studies attributed the efficacy of MSC therapy to secretion of factors, such as BDNF, NGF, NT3, CNTF, GDNF, insulin-like growth factor 1, or bFGF, that promote endogenous neuronal growth and angiogenesis, stimulate the synaptic connection and remyelination of damaged axons, diminish apoptosis, and finally regulate inflammation (Li et al., 2009; Johnson et al., 2010; Wang et al., 2010; Williams and Hare, 2011; Seo and Cho, 2012; Emre et al., 2015; Lejkowska et al., 2019). Among those studies, Emre et al. (2015) reported that bmMSCs and aMSCs exhibit a neuroprotective effect over retinal degeneration in a rat model of ocular hypertension. The authors attributed the neuroprotective effects to secretion of neurotrophic factors for modulating the inflammatory processes and inhibitory signals that regulate axonal regrowth along with neuronal repair and activation of endogenous repair mechanisms (Emre et al., 2015), as also previously described (Zhang et al., 2007; Johnson et al., 2011). Besides, Lejkowska et al. (2019) showed that BDNF overexpression by genetically modified intravitreally bmMSCs could rescue retinal cells from degenerative processes and also enhance the neuroprotective properties in the rd6 mouse, a model of retinal degeneration. Those investigators observed that bmMSCs survived for at least 3 months after transplantation and described that the bmMSCs rescued damaged retinal cells, associated with anti-apoptotic signaling (Lejkowska et al., 2019).
Concerning other experimental animals, Labrador Velandia et al. (2018) concluded that human bmMSC cells administered by intravitreal injection are safe and well-tolerated in pigmented immunocompetent rabbits at 2 and 6 weeks after injection, since no signs of infection and/or inflammation or changes in the cells and tissues at the histologic level were observed. Furthermore, the bmMSCs remained inside the vitreous cavity and showed special tropism for the vitreous regions near the posterior capsule of the lens and optic nerve head at 2 weeks after injection. It has not been observed that human bmMSCs pass through the lens capsule or the inner limiting membrane of the rabbit retina to integrate into any ocular tissue other than the vitreous and do not migrate to the main hematopoietic or gonadal organs (Labrador Velandia et al., 2018). Furthermore, bmMSCs survive at least 2 weeks after intravitreal injection (Labrador Velandia et al., 2018), thus allowing the bmMSCs to develop prolonged paracrine action. No other non-murine animal studies that evaluate the neuroprotective properties of intraocular stem cells were found in the databases based on our search, probably because there are only a few animal models of retinal degeneration. Thus, adequate animal models that are easily reproducible and that adequately develop the pathophysiology of photoreceptor diseases are needed to evaluate the safety, bioavailability, and efficacy of the potential cell therapy techniques before being transferred to clinical practice.
In summary, preclinical studies such as those presented here and others performed before 2013, have been the basis for the approach and development of the current clinical trials that apply intraocular stem cell therapy to treat retinal degenerative diseases.
Clinical Trials
A number of completed current clinical trials or those underway have been designed to specifically treat retinal conditions by using intravitreal stem cells through their neuroprotective paracrine properties (Additional Table 1).
Table 1.
Summary of the current clinical trials completed or in progress designed to evaluate intravitreal stem cells on retinal photoreceptor neurodegenerative conditions
| Reference | Disease | Cell type | Administration route | Status | Sponsor |
|---|---|---|---|---|---|
| NCT02320812 | Retinitis pigmentosa | ESC | Intravitreal injection | Phase I/II | jCyte, Inc. |
| NCT03073733 | Retinitis pigmentosa | ESC | Intravitreal injection | Phase II | jCyte, Inc. |
| NCT03944239 | Retinitis pigmentosa | ESC | Subretinal injection | Phase I | Qi Zhou |
| NCT02464436 | Retinitis pigmentosa | ESC | Subretinal injection | Phase I/II | ReNeuron Limited |
| Jonas et al. 2008 | Diabetic retinopathy | BMMSC | Intravitreal injection | ||
| Jonas et al. 2009 | Diabetic retinopathy | BMMSC | |||
| AMD | Intravitreal injection | ||||
| Glaucoma | |||||
| Siqueira et al. 2011 | Retinitis pigmentosa Cone-rod dystrophy | BMMSC | Intravitreal injection | Phase I | University of Sao Paulo |
| Siqueira et al. 2015b (NCT01560715) | Retinitis pigmentosa | BMMSC | Intravitreal injection | Phase II | University of Sao Paulo |
| Cotrim et al. 2017 (NCT01518127) | Atrophic AMD Stargardt disease |
BMMSC | Intravitreal injection | Phase I/II | University of Sao Paulo |
| NCT01518842 | Ischemic retinopathy | bmMSC | Intravitreal injection | University of Sao Paulo | |
| Park et al. 2015 (NCT01736059) | Atrophic AMD | BMMSC | Intravitreal injection | Phase I | University of California |
| Diabetic retinopathy | |||||
| Retinal vein occlusion | |||||
| Retinitis pigmentosa | |||||
| Hereditary macular disease | |||||
| NCT02709876 | Retinitis pigmentosa | BMMSC | Intravitreal injection | Phase I/II | Stem Cells Arabia |
| NCT02280135 | Retinitis pigmentosa | BMMSC | Intravitreal injection | Phase I | Spanish National Health System |
| NCT03981549 | Central retinal vein occlusion | BMMSC | Intravitreal injection | Phase I/II | The Emmes Company, LLC |
| Weiss et al. 2015a, b, 2016a, b (NCT01920867) | Retinal disease | BMMSC | Retrobulbar injection | MD Stem Cells | |
| Macular degeneration | Sub-Tenon injection | ||||
| Hereditary retinal dystrophy | Intravenous injection | ||||
| Optic nerve disease | Intravitreal injection | ||||
| Glaucoma | Intraocular | ||||
| Weiss et al. 2015a, b, 2016a, b, 2017, 2018, 2019a, b (NCT03011541) | AMD | BMMSC | MD Stem Cells | ||
| Retinitis pigmentosa | |||||
| Stargardt | |||||
| Optic neuropathy | |||||
| Non-arteritic ischemic optic | |||||
| neuropathy | |||||
| Optic atrophy | |||||
| Optic nerve disease | Retrobulbar injection | ||||
| Glaucoma | Subtenon injection | ||||
| Leber hereditary optic neuropathy | Intravenous injection | ||||
| Blindness | Intravitreal injection | ||||
| Night vision loss | Intraocular | ||||
| Partial vision loss | |||||
| Retinopathy | |||||
| Maculopathy | |||||
| Macular degeneration | |||||
| Retina atrophy | |||||
| NCT02024269 | Dry macular degeneration | aMSC | Intravitreal injection | Bioheart, Inc. | |
| NCT03403699 | Diabetic retinopathy | iPSC | Intravitreal injection | The University of Alabama at Birmingham |
AMD: Age-related macular degeneration; aMSC: adipose MSC; BMMSC: bone marrow MSC; ESC: embryonic stem cells; iPSC: induced pluripotent stem cells; MSC: mesenchymal stem cells.
Among the clinical studies using ESCs, in the clinical trial performed by jCyte, human retinal progenitor cells were injected into the vitreous cavity of 28 patients with RP to potentially rescue and reactivate diseased photoreceptors before cell death (NCT02320812). Furthermore, because many patients reported improved vision, reading ability and better mobility, in a follow-on extension study 22 of the initial 28 patients also were treated in the contralateral eye. Based on those promising results, a phase IIb study (NCT03073733) is ongoing to compare visual function and functional visual changes between one retinal progenitor cell intravitreal injection and a sham-treated control group. Two other interesting trials are recruiting patients. In the first, 10 patients with RP are being recruited to test the safety and efficacy of human ESC-derived RPE (NCT03944239), and in the second, 50 patients with RP are being recruited to evaluate the safety and tolerability of human retinal progenitor cells in a phase I/IIa study (NCT02464436). However, both studies involved subretinal transplantation of stem cells for neuroprotective purposes, not the intravitreal route, and thus, they are outside of the search criteria used in the current review.
Regarding MSCs, the first studies on intravitreal stem cell therapy in humans published by Jonas et al. (2008, 2009) demonstrated the safety and feasibility of an intravitreal injection of autologous bone marrow aspirate as a potential MSC source in three patients with advanced degenerative retinopathies.
Subsequently, Siqueira et al. (2011) reported the safety of an intravitreal injection of autologous mononuclear cells from bone marrow in 5 patients with advanced retinal dystrophies in a phase I clinical trial. This group also reported a time-dependent improvement in the quality of life after intravitreal bmMSC injection in patients with RP (Siqueira et al., 2015b); and improvement in the best-corrected visual acuity after intravitreal injection of bmMSCs containing CD34+ cells in patients with atrophic AMD (Cotrim et al., 2017).
Other active clinical trials are using an intravitreal injection of specifically selected autologous stem cells, i.e., one in patients with several retinal degenerative conditions or retinal vascular diseases that are treated with intravitreal CD34+- from autologous bone marrow aspirate (NCT01736059) (Park et al., 2015), and another in patients with RP will be that injected intravitreally with autologous bone marrow-derived CD34+, CD133+, and CD271+ (NCT02709876).
Among other clinical studies that use autologous bmMSCs intravitreally as a potential neuroprotective therapy, a clinical trial includes 8 patients with RP (NCT02280135) and a prospective phase I/II study on AMD and patients with Stargardt disease (NCT01518127) have been completed, but results are not available yet.
Furthermore, concerning vascular retinopathies, use of intravitreal autologous bmMSCs resulted in decreased macular edema and improved retinal function in 2 patients, one with ischemic diabetic maculopathy and another with ischemic central retinal vein occlusion (NCT01518842) (Siqueira et al., 2015a). A phase I/II clinical trial (NCT03981549) is ongoing to determine whether intravitreal autologous CD34+ is safe, feasible, and potentially beneficial for patients with central retinal vein occlusion. Another active trial is evaluating intravitreal injection of bmMSCs in 30 patients with ischemic retinopathy (NCT01518842).
It is also worth mentioning two studies, the Stem Cell Ophthalmology Treatment Study (SCOTS) and the SCOTS 2 (NCT01920867 and NCT03011541, respectively), that have evaluated the use of autologous bmMSC injections by different routes to treat several retinal and optic nerve diseases (Weiss et al., 2015a, b, 2016a, b, 2017; Weiss and Levy, 2018, 2019a, b). Although the safety of bmMSCs was confirmed, in our opinion these clinical trials include many different diseases and routes of administration that affect the robustness of the published efficacy results (Weiss and Levy, 2018). Thus, there is variability in treated conditions including degenerative, ischemic, or physical damage of the retina and/or optic nerve. Besides, the eyes were treated by injection of bmMSCs using the following routes of administration: retrobulbar; sub-Tenon and intravenous together, or a combination of retrobulbar, sub-Tenon, intravitreal, and intravenous.
Finally, a study is assessing the safety and effects of aMSCs obtained during liposuction procedures and injected intravitreally in dry macular degeneration (NCT02024269). However, the study was discontinued.
iPSCs have been used to generate CD34+ and CD45+ cells in combination with iPSC-derived mesoderm (vascular wall-derived progenitor cells) or with endothelial colony-forming cells that are intravitreally administered in a clinical trial to evaluate their potentially beneficial effect to prevent development of microvascular complications in diabetic retinopathy (NCT03403699) due to their antioxidative and anti-inflammatory effects.
In summary, clinical trials evaluating intravitreal stem cell potential to treat photoreceptor degenerative diseases have reported adequate tolerance and safety for patients. Nevertheless, future studies are needed to expand the current knowledge about the stem cell neuroprotective potential and to reinforce efficacy.
Conclusion
After considerable initial concerns about the use of human stem cells intravitreally as therapeutic agents due to their potential to form benign tumors or trigger an immune response, which would have deleterious effects on the retina, most clinical trials reviewed in this report share the conclusion that the intraocular stem cell approach is generally well tolerated and safe for patients. However, the data from a few completed studies do not yet have sufficient power to demonstrate a significant difference regarding efficacy in humans (Terrell and Comander, 2019). Therefore, it is necessary to continue developing preclinical studies and clinical trials that facilitate broadening of the current knowledge base about the neuroprotective potential of intravitreal stem cells in retinal pathologies that mainly affect the photoreceptors.
In conclusion, adequate retinal neuroprotection is one of the next challenges to overcome in retinal diseases, in which advanced therapies, mainly through the paracrine properties of stem cells, will play a fundamental role in the development of novel neuroprotective strategies. Although it is necessary to continue advancing the basic understanding of retinal diseases and their clinical implications in patients’ visual health, we consider that continuous advances in the clinical and preclinical fields will facilitate application of stem cell-based neuroprotection as a therapeutic strategy to reduce the visual loss that affects millions of people worldwide.
Additional files
Additional file 1: Open peer review report 1 (82.6KB, pdf) .
Additional Table 1: Summary of the current clinical trials completed or in progress designed to evaluate intravitreal stem cells on retinal photoreceptor neurodegenerative conditions
Acknowledgments
The authors acknowledge Jose Carlos Pastor, MD, PhD (Instituto Universitario de Oftalmobiología Aplicada, University of Valladolid, Spain), for critical reading of this manuscript and insightful comments/suggestions.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: KPN, RUM, and IFB were supported by Fundación Carolina, Madrid, Spain; Fondo Europeo de Desarrollo Regional, Fondo Social Europeo, and Consejería de Educación (Grant VA077P17), Junta de Castilla y León, Spain; and Centro en Red de Medicina Regenerativa y Terapia Celular, Junta de Castilla y León, Spain, respectively.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Yuntao Hu, Beijing Tsinghua Changgung Hospital, Beijing, China.
Funding: KPN, RUM, and IFB were supported by Fundación Carolina, Madrid, Spain; Fondo Europeo de Desarrollo Regional, Fondo Social Europeo, and Consejería de Educación (Grant VA077P17), Junta de Castilla y León, Spain; and Centro en Red de Medicina Regenerativa y Terapia Celular, Junta de Castilla y León, Spain, respectively.
P-Reviewer: Hu Y; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Alonso-Alonso ML, Srivastava GK. Current focus of stem cell application in retinal repair. World J Stem Cells. 2015;7:641–648. doi: 10.4252/wjsc.v7.i3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann-Gagescu R, Neuhauss SC. The photoreceptor cilium and its diseases. Curr Opin Genet Dev. 2019;56:22–33. doi: 10.1016/j.gde.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:288. doi: 10.1186/s13287-017-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W. Ciliary Neurotrophic Factor Retinitis Pigmentosa Study Groups (2013) Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol. 156:283–292e1. doi: 10.1016/j.ajo.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK, Semo M, Gias C, da Cruz L, Moore HD, Walsh J, Coffey PJ. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–295. [PMC free article] [PubMed] [Google Scholar]
- 6.Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komáromy AM, Hauswirth WW, Aguirre GD. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;10:E517–525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotrim CC, Toscano L, Messias A, Jorge R, Siqueira RC. Intravitreal use of bone marrow mononuclear fraction containing CD34+ stem cells in patients with atrophic age-related macular degeneration. Clin Ophthalmol. 2017;11:931–938. doi: 10.2147/OPTH.S133502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca N, Fernández-Sánchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Di Lauro S, Rodriguez-Crespo D, Gayoso MJ, Garcia-Gutierrez MT, Pastor JC, Srivastava GK, Fernandez-Bueno I. A novel coculture model of porcine central neuroretina explants and retinal pigment epithelium cells. Mol Vis. 2016;22:243–253. [PMC free article] [PubMed] [Google Scholar]
- 10.Emre E, Yüksel N, Duruksu G, Pirhan D, Subaşi C, Erman G, Karaöz E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow–derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy. 2015;17:543–559. doi: 10.1016/j.jcyt.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Bueno I, Fernández-Sánchez L, Gayoso MJ, García-Gutierrez MT, Pastor JC, Cuenca N. Time course modifications in organotypic culture of human neuroretina. Exp Eye Res. 2012;104:26–38. doi: 10.1016/j.exer.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardi G, Ben M’Barek K, Goureau O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog Retin Eye Res. 2019;7:1–25. doi: 10.1016/j.preteyeres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Gasparini SJ, Llonch S, Borsch O, Ader M. Transplantation of photoreceptors into the degenerative retina: Current state and future perspectives. Prog Retin Eye Res. 2019;69:1–37. doi: 10.1016/j.preteyeres.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Hill AJ, Zwart I, Tam HH, Chan J, Navarrete C, Jen LS, Navarrete R. Human umbilical cord blood-derived mesenchymal stem cells do not differentiate into neural cell types or integrate into the retina after intravitreal grafting in neonatal rats. Stem Cells Dev. 2009;18:399–410. doi: 10.1089/scd.2008.0084. [DOI] [PubMed] [Google Scholar]
- 15.Jin ZB, Gao ML, Deng WL, Wu KC, Sugita S, Mandai M, Takahashi M. Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res. 2019;69:38–56. doi: 10.1016/j.preteyeres.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Johnson TV, Bull ND, Martin KR. Stem cell therapy for glaucoma: possibilities and practicalities. Expert Rev Ophthalmol. 2011;6:165–174. doi: 10.1586/eop.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson TV, Bull ND, Martin KR. Transplantation prospects for the inner retina. Eye (Lond) 2009;23:1980–1984. doi: 10.1038/eye.2008.376. [DOI] [PubMed] [Google Scholar]
- 19.Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Jones BW, Marc RE, Pfeiffer RL. Retinal degeneration, remodeling and plasticity. In: Kolb H, Fernandez E, Nelson R, editors. SourceWebvision: The Organization of the Retina and Visual System [Internet] Salt Lake City (UT): University of Utah Health Sciences Center; 2012. [PubMed] [Google Scholar]
- 21.Jones MK, Lu B, Chen DZ, Spivia WR, Mercado AT, Ljubimov AV, Svendsen CN, Van Eyk JE, Wang S. In vitro and in vivo proteomic comparison of human neural progenitor cell‐induced photoreceptor survival. Proteomics. 2019;19:e1800213. doi: 10.1002/pmic.201800213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadłubowska J, Malaguarnera L, Wąż P, Zorena K. Neurodegeneration and neuroinflammation in diabetic retinopathy: potential approaches to delay neuronal loss. Curr Neuropharmacol. 2016;14:831–839. doi: 10.2174/1570159X14666160614095559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolomeyer AM, Zarbin MA. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv Ophthalmol. 2014;59:134–165. doi: 10.1016/j.survophthal.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa T, Katai N, Shibuki H, Kuroiwa S, Kurimoto Y, Nakayama C, Yoshimura N. BDNF diminishes caspase-2 but not c-Jun immunoreactivity of neurons in retinal ganglion cell layer after transient ischemia. Investig Ophthalmol Vis Sci. 1999;40:3006–3011. [PubMed] [Google Scholar]
- 25.Labouyrie E, Dubus P, Groppi A, Mahon FX, Ferrer J, Parrens M, Reiffers J, de Mascarel A, Merlio JP. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol. 1999;154:405–415. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labrador-Velandia S, Alonso-Alonso ML, Di Lauro S, García-Gutierrez MT, Srivastava GK, Pastor JC, Fernandez-Bueno I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp Eye Res. 2019;185:107671. doi: 10.1016/j.exer.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Labrador Velandia S, Di Lauro S, Alonso-Alonso ML, Tabera Bartolomé S, Srivastava GK, Pastor JC, Fernandez-Bueno I. Biocompatibility of intravitreal injection of human mesenchymal stem cells in immunocompetent rabbits. Graefes Arch Clin Exp Ophthalmol. 2018;256:125–134. doi: 10.1007/s00417-017-3842-3. [DOI] [PubMed] [Google Scholar]
- 28.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lejkowska R, Kawa MP, Pius-Sadowska E, Rogińska D, Łuczkowska K, Machaliński B, Machalińska A. Preclinical evaluation of long-term neuroprotective effects of BDNF-engineered mesenchymal stromal cells as intravitreal therapy for chronic retinal degeneration in Rd6 mutant mice. Int J Mol Sci. 2019 doi: 10.3390/ijms20030777. doi: 103390/ijms20030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Investig Opthalmol Vis Sci. 2010;51:6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- 31.Li JQ, Terheyden JH, Welchowski T, Schmid M, Letow J, Wolpers C, Holz FG, Finger RP. Prevalence of retinal vein occlusion in Europe: a systematic review and Meta-analysis. Ophthalmologica. 2019;241:183–189. doi: 10.1159/000494224. [DOI] [PubMed] [Google Scholar]
- 32.Li N, Li XR, Yuan JQ. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009;247:503–514. doi: 10.1007/s00417-008-1009-y. [DOI] [PubMed] [Google Scholar]
- 33.Lin N, Hu K, Chen S, Xie S, Tang Z, Lin J, Xu R. Nerve growth factor-mediated paracrine regulation of hepatic stellate cells by multipotent mesenchymal stromal cells. Life Sci. 2009;85:291–295. doi: 10.1016/j.lfs.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLaren RE, Pearson RA. Stem cell therapy and the retina. Eye (Lond) 2007;21:1352–359. doi: 10.1038/sj.eye.6702842. [DOI] [PubMed] [Google Scholar]
- 36.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 37.Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003;28:139–147. doi: 10.1385/MN:28:2:139. [DOI] [PubMed] [Google Scholar]
- 38.Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 40.Mellough C, Cui Q, Harvey A. Treatment of adult neural progenitor cells prior to transplantation affects graft survival and integration in a neonatal and adult rat model of selective retinal ganglion cell depletion. Restor Neurol Neurosci. 2007;25:177–190. [PubMed] [Google Scholar]
- 41.Mollick T, Mohlin C, Johansson K. Human neural progenitor cells decrease photoreceptor degeneration, normalize opsin distribution and support synapse structure in cultured porcine retina. Brain Res. 2016;1646:522–534. doi: 10.1016/j.brainres.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 42.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 43.Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, Zawadzki RJ, Werner JS, Nolta J. Intravitreal autologous bone marrow cd34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Investig Ophthalmol Vis Sci. 2015;56:81–89. doi: 10.1167/iovs.14-15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Crespo D, Di Lauro S, Singh AK, Garcia-Gutierrez MT, Garrosa M, Pastor JC, Fernandez-Bueno I, Srivastava GK. Triple-layered mixed co-culture model of RPE cells with neuroretina for evaluating the neuroprotective effects of adipose-MSCs. Cell Tissue Res. 2014;358:705–716. doi: 10.1007/s00441-014-1987-5. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez I, Martin R, Ussa F, Fernandez-Bueno I. The parameters of the porcine eyeball. Graefes Arch Clin Exp Ophthalmol. 2011;249:475–482. doi: 10.1007/s00417-011-1617-9. [DOI] [PubMed] [Google Scholar]
- 46.Seo JH, Cho SR. Neurorestoration induced by mesenchymal stem cells: potential therapeutic mechanisms for clinical trials. Yonsei Med J. 2012;53:1059–1067. doi: 10.3349/ymj.2012.53.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simunovic MP, Shen W, Lin JY, Protti DA, Lisowski L, Gillies MC. Optogenetic approaches to vision restoration. Exp Eye Res. 2019;178:15–26. doi: 10.1016/j.exer.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Siqueira RC1, Messias A, Gurgel VP, Simões BP, Scott IU, Jorge R. Improvement of ischaemic macular oedema after intravitreal injection of autologous bone marrow-derived haematopoietic stem cells. Acta Ophthalmol. 2015a;93:e174–e176. doi: 10.1111/aos.12473. [DOI] [PubMed] [Google Scholar]
- 49.Siqueira RC, Messias A, Messias K, Arcieri RS, Ruiz MA, Souza NF, Martins LC, Jorge R. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial) Stem Cell Res Ther. 2015b;6:29. doi: 10.1186/s13287-015-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahata K, Katsuki H, Kume T, Nakata D, Ito K, Muraoka S, Yoneda F, Kashii S, Honda Y, Akaike A. Retinal neuronal death induced by intraocular administration of a nitric oxide donor and its rescue by neurotrophic factors in rats. Invest Ophthalmol Vis Sci. 2003;44:1760–1766. doi: 10.1167/iovs.02-0471. [DOI] [PubMed] [Google Scholar]
- 51.Terrell D, Comander J. Current stem-cell approaches for the treatment of inherited retinal degenerations. Semin Ophthalmol. 2019;34:287–292. doi: 10.1080/08820538.2019.1620808. [DOI] [PubMed] [Google Scholar]
- 52.Trapani I, Auricchio A. Seeing the light after 25 years of retinal gene therapy. Trends Mol Med. 2018;24:669–681. doi: 10.1016/j.molmed.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Vidal-Sanz M, Lafuente MP, Mayor-Torroglosa S, Aguilera ME, Miralles de Imperial J, Villegas-Pérez MP. Brimonidine’s neuroprotective effects against transient ischaemia-induced retinal ganglion cell death. Eur J Ophthalmol. 2001;11:S36–40. doi: 10.1177/112067210101102S04. [DOI] [PubMed] [Google Scholar]
- 54.Vugler AA. Progress toward the maintenance and repair of degenerating retinal circuitry. Retina. 2010;30:983–1001. doi: 10.1097/IAE.0b013e3181e2a680. [DOI] [PubMed] [Google Scholar]
- 55.Waldron PV, Di Marco F, Kruczek K, Ribeiro J, Graca AB, Hippert C, Aghaizu ND, Kalargyrou AA, Barber AC, Grimaldi G, Duran Y, Blackford SJI, Kloc M, Goh D, Zabala Aldunate E, Sampson RD, Bainbridge JWB, Smith AJ, Gonzalez-Cordero A, Sowden JC, et al. Transplanted donor- or stem cell-derived cone photoreceptors can both integrate and undergo material transfer in an environment-dependent manner. Stem Cell Reports. 2018;10:406–421. doi: 10.1016/j.stemcr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang HC, Brown J, Alayon H, Stuck BE. Transplantation of quantum dot-labelled bone marrow-derived stem cells into the vitreous of mice with laser-induced retinal injury: Survival, integration and differentiation. Vision Res. 2010;50:665–673. doi: 10.1016/j.visres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Weiss JN, Benes SC, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): Improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment. Neural Regen Res. 2016a;11:1512–1516. doi: 10.4103/1673-5374.191229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss JN, Levy S. Dynamic light scattering spectroscopy of the retina—a non-invasive quantitative technique to objectively document visual improvement following ocular stem cell treatment. Stem Cell Investig. 2019a;6:8. doi: 10.21037/sci.2019.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow derived stem cells in the treatment of Usher syndrome. Stem Cell Investig. 2019b;6:31. doi: 10.21037/sci.2019.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem Cell Investig. 2018;5:18. doi: 10.21037/sci.2018.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION) Stem Cell Investig. 2017;4:94. doi: 10.21037/sci.2017.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss JN, Levy S, Benes SC. Stem cell ophthalmology treatment study (SCOTS): bone marrow-derived stem cells in the treatment of Leber’s hereditary optic neuropathy. Neural Regen Res. 2016b;11:1685–1694. doi: 10.4103/1673-5374.193251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss JN, Levy S, Benes SC. Stem cell ophthalmology treatment study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen Res. 2015a;10:1507–1515. doi: 10.4103/1673-5374.165525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss JN, Levy S, Malkin A. Stem cell ophthalmology treatment study (SCOTS) for retinal and optic nerve diseases: a preliminary report. Neural Regen Res. 2015b;10:982–988. doi: 10.4103/1673-5374.158365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu W, Xu GX. Mesenchymal stem cells for retinal diseases. Int J Ophthalmol. 2011;4:413–421. doi: 10.3980/j.issn.2222-3959.2011.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J Neurosci. 2007;27:4499–4506. doi: 10.1523/JNEUROSCI.0200-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


