Age-related macular degeneration (AMD) is a progressive neurodegenerative disease with a global prevalence of 8.7% in people over the age of 45. It is one of the leading causes of central retinal blindness in the industrialized world leading to loss of an individual’s ability to read, drive and see fine details, such as recognition of faces. By 2040, it is estimated that 288 million individuals worldwide will be diagnosed with AMD due to improved life expectancy (Wong et al., 2014).
AMD can present in two forms, namely ‘dry’ and ‘wet’ AMD that have different end stages. Geographic atrophy (GA) is the end stage of dry AMD which has a slower progression. In GA, the retinal pigment epithelium (RPE) cell layer that underlies the retina begins to degenerate and becomes atrophic, subsequently leading to cone photoreceptor cell death and eventual central vision loss (Handa et al., 2019). The end stage of wet AMD is termed choroidal neovascularization (CNV) and is characterized by the growth of abnormal new blood vessels that originate from the choroid. Therapeutic intervention for the treatment of CNV secondary to wet AMD involves monthly intraocular injections of anti-vascular endothelial growth factor biologics which can inhibit blood vessel growth. In contrast, there are no therapies currently available for GA treatment apart from recommended lifestyle changes such as dietary modification and cessation of smoking. Great advancements have been made in AMD research, however the underlying molecular aetiology of GA is still unknown and poorly understood.
The majority of research studies investigating GA pathobioalogy have focused on the RPE or pathways leading to RPE degeneration due to this being the most obvious pathology observed in human subjects (Handa et al., 2019). Many of these studies have implicated roles for inflammation, and in particular the complement cascade, accumulation of lipids, mitochondrial dysfunction or oxidative stress in dry AMD pathology. The retina, due to its high energy and metabolic daemand, is extremely sensitive to perturbations in numerous intracellular pathways that can lead to chronic oxidative stress and this can also be affected by ageing. One of the key diagnostic hallmarks for both wet and dry AMD is the presence of drusen between the RPE and Bruch’s membrane. Drusen contains many of the components implicated in AMaD pathology including lipids and complement cascade components and the presence of drusen is also sufficient in activating inflammatory processes. Drusen accumulation within an individual diagnosed with AMD has high symmetry in location and distribution between each eye, which intriguingly correlates with a high degree of symmetry in retinal blood vessel patterning of an individual’s right and left eyes (Mann et al., 2011). Numerous studies have also shown a genetic contribution to AMD risk and burden with variants associated with genes including ApoE, ARMS2/HTRA1 and complement components such as complement factors H and B (Handa et al., 2019).
The macula although only accounting for 3% of the retinal surface area has the highest oxygen consumption and can be classified into four regions, the foveal avascular zone, fovea, parafoveal, and perifoveal regions. Although the region of the foveal avascular zone is devoid of retinal blood vessels, the other regions are extensively vascularized with a dense capillary network appearing from the slope of the foveal pit. The parafoveal region of the macula is the thickest due to the dense inner nuclear layer. The accumulation of drusen and RPE atrophy has typically been found to occur in the parafoveal and perifoveal region of the macula before expanding to the fovea.
Until recently, the involvement of the inner retinal microvasculature has been neglected in studies, as AMD has always been considered a disease of the RPE and outer retina with limited inner retinal involvement. Recently, however, we have discovered that the inner blood-retina barrier (iBRB) may be an early initiating factor in GA secondary to dry AMD pathology (Hudson et al., 2019). The iBRB is formed by the endothelial cells that line the inner retinal blood vessels which are essential in maintaining retinal homeostasis. The presence of highly specialised tight junctions between adjacent endothelial cells helps to regulate the movement of molecules and ions between the blood and neural retina. The barrier is also essential in restricting entry of potential blood-borne damaging agents such as immune cells, pathogens and anaphylatoxins (Klaassen et al., 2013). Our research has shown that the iBRB is highly dynamic and expression of the most highly enriched tight junction component, claudin-5 (Daneman et al., 2010), is regulated in a circadian-mediated manner (Hudson et al., 2019). Critically, claudin-5 expression is dose-dependent as mice lacking all or most of their claudin-5 die early (Nitta et al., 2003; Greene et al., 2018).
Interestingly, our study has discovered that the integrity of retinal blood vessels changes during the course of the day, with vessels being ‘leakier’ in the evening compared to the morning when fundus fluorescein angiograms were carried out in mice (Hudson et al., 2019). This phenomenon also occurred in a non-human primate model as well as healthy 18–30 year-old human adults. These observed changes arose due to reduced expression of claudin-5 in the evening compared to morning which was dependent on BMAL-1, a core clock transcription factor (McMahon et al., 2014). These changes in claudin-5 expression across a 24-hour period were not specific to the retina but were also observed in all other tissues studied including different brain regions, lung, heart and kidney.
Regulation of claudin-5 expression levels at the inner retina can very rapidly lead to a GA-like phenotype in mouse and non-human primate models using adeno-associated virus vectors expressing claudin-5 shRNA (Hudson et al., 2019). In a similar manner to other AMD studies, the supplementation of a high cholesterol rich diet to our mouse model along with claudin-5 suppression induced an RPE-like atrophy phenotype which could be observed by optical coherence tomography and by immunohistochemical analysis of RPE flatmounts. A similar model used in the macula of a non-human primate also led to an RPE atrophy-like phenotype when optical coherence tomography images were collected and post-mortem analysis also showed distinct RPE degeneration un-distinguishable from GA lesions observed in post-mortem human donor eye tissues. This is the first ever study that showed a persistent and size-selective dysregulation of the iBRB can lead to a GA-like pathology (Hudson et al., 2019). Additionally, a recent study also observed, for the first time, moderate BRB leakage and increased plasma protein accumulation when assessing post-mortem dry AMD donor eyes (Schultz et al., 2019).
Previously, retinal translocation studies were undertaken in an effort to restore visual acuity in patients with CNV secondary to wet AMD and GA secondary to dry AMD. Ophthalmologists surgically translocated healthy neural retina from the atrophic RPE region to an area of intact RPE cells. Although successful for a sub-group of patients with sub-foveal CNV, in those diagnosed with GA, recurrence of RPE atrophy was detected in the translocated region (Cahill et al., 2005), suggesting the inner retina could promote RPE cell stress and death. Breakdown of the iBRB has also been implicated in other ocular conditions, such as the rare juvenile retinal condition Coats’ disease and diabetic retinopathy. In Coats’ disease there is acute vascular leakage of blood derived cholesterol crystals and lipid-laden macrophages infiltrate the sub-retinal space (Ghorbanian et al., 2012). Once again, this lends weight to the idea that the dysregulated inner retinal vasculature can deposit material from the blood to the RPE as in the case with our novel model of dry AMD. In contrast, diabetic retinopathy arises due to chronic iBRB breakdown that subsequently leads to greatly enhanced vascular leakage, independent of any RPE atrophy.
It is well established that as we age, our circadian rhythms can become dysfunctional resulting in disturbed sleep patterns. Loss of circadian clock genes can contribute to aberrant visual function during development and ageing and also have been implicated in many neurodegenerative diseases including diabetic retinopathy (McMahon et al., 2014). There is mounting evidence that other cell types associated with the iBRB, such as pericytes and glial cells, can also be affected by clock gene disruption resulting in iBRB instability and astrogliosis. We postulate that circadian entrained cycling of the inner retinal vascular permeability is key in allowing daily replenishment of substrates to the photoreceptors and clearing of waste material from the neural retina via osmotic gradients. In the ageing eye, with dysregulation of the circadian clock, this cycling in the inner retinal vasculature ceases to be tightly regulated, resulting in the RPE becoming overloaded with dietary components and metabolites. This material will eventually mediate drusen deposition and ultimately atrophy of the overworked RPE.
Collectively, our study is the first to implicate a novel role of the iBRB and in particular claudin-5 as a key mediator in initiating dry AMD pathology and subsequent RPE pathology. The generation of a more relevant animal model for dry AMD, and (Figure 1) ultimately GA, for research purposes may open up avenues of research to investigate in detail, the mechanisms in GA progression and potential novel therapeutic targets to slow down or prevent GA development. On-going studies to elucidate the retinal permeability changes in preclinical species and AMD patients will expand on our observations to date so far. Overall, our findings suggest that restoration of the dynamic expression of claudin-5 in the ageing eye may prevent or indeed halt the progression of AMD.
Figure 1.
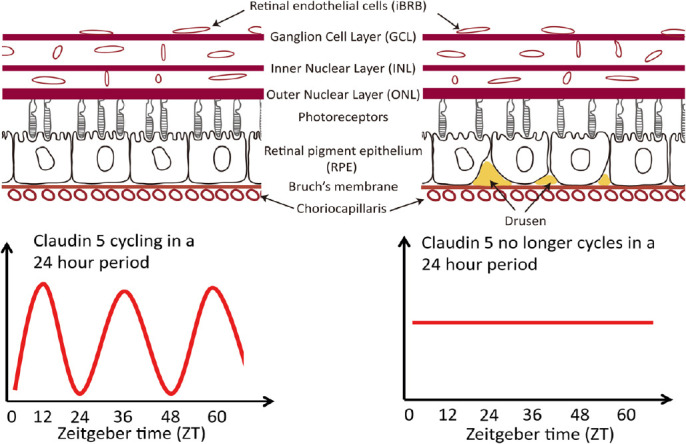
Proposed model for inner blood-retina barrier involvement in dry age-related macular degeneration (AMD) pathology and in particular the end-stage, geographic atrophy.
Cessation of claudin-5 cyclical expression at the iBRB can lead to the accumulation of drusen and the subsequent degeneration and atrophy of the retinal pigment epithelium (RPE).
This work was supported by grants from Science Foundation Ireland (SFI) (12/YI/B2614 and 11/PI/1080), The Health Research Board of Ireland (HRB), the BrightFocus Foundation, the Irish Research Council (IRC) and Enterprise Ireland (EI). The Campbell lab at Trinity College Dublin is also supported by an SFI Centres grant supported in part by a research grant from SFI under grant number 16/RC/3948 and co-funded under the European Regional Development fund by FutureNeuro industry partners.
Additional file: Open peer review reports 1 (87.5KB, pdf) and 2 (87.8KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Pio Conti, University of Chieti-Pescara, Italy; Yuntao Hu, Beijing Tsinghua Changgung Hospital, Beijing, China.
P-Reviewers: Conti P, Hu Y; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Cahill MT, Mruthyunjaya P, Bowes Rickman C, Toth CA. Recurrence of retinal pigment epithelial changes after macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2005;123:935–938. doi: 10.1001/archopht.123.7.935. [DOI] [PubMed] [Google Scholar]
- 2.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghorbanian S, Jaulim A, Chatziralli IP. Diagnosis and treatment of coats’ disease: a review of the literature. Ophthalmologica. 2012;227:175–182. doi: 10.1159/000336906. [DOI] [PubMed] [Google Scholar]
- 4.Greene C, Kealy J, Humphries MM, Gong Y, Hou J, Hudson N, Cassidy LM, Martiniano R, Shashi V, Hooper SR, Grant GA, Kenna PF, Norris K, Callaghan CK, Islam MD, O’Mara SM, Najda Z, Campbell SG, Pachter JS, Thomas J, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23:2156–2166. doi: 10.1038/mp.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handa JT, Bowes Rickman C, Dick AD, Gorin MB, Miller JW, Toth CA, Ueffing M, Zarbin M, Farrer LA. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun. 2019;10:3347. doi: 10.1038/s41467-019-11262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson N, Celkova L, Hopkins A, Greene C, Storti F, Ozaki E, Fahey E, Theodoropoulou S, Kenna PF, Humphries MM, Curtis A, Demmons E, Browne A, Liddie S, Lawrence MS, Grimm C, Cahill M, Humphries P, Doyle SL, Campbell M. Dysregulated claudin-5 cycling in the inner retina causes retinal pigment epithelial cell atrophy. JCI Insight. 2019;4:e130273. doi: 10.1172/jci.insight.130273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Mann SS, Rutishauser-Arnold Y, Peto T, Jenkins SA, Leung I, Xing W, Bird AC, Bunce C, Webster AR. The symmetry of phenotype between eyes of patients with early and late bilateral age-related macular degeneration (AMD) Graefes Arch Clin Exp Ophthalmol. 2011;249:209–214. doi: 10.1007/s00417-010-1483-x. [DOI] [PubMed] [Google Scholar]
- 9.McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz H, Song Y, Baumann BH, Kapphahn RJ, Montezuma SR, Ferrington DA, Dunaief JL. Increased serum proteins in non-exudative AMD retinas. Exp Eye Res. 2019;186:107686. doi: 10.1016/j.exer.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


