Medicine has long sought to match diagnostic and treatment approaches to the particular needs and risks of individual patients. The decreasing cost and increasing ease of genetic sequencing have propelled the rise of precision medicine. Precision medicine aims to use genetic and other information to provide care tailored to the individual patient, with the goal of improving clinical outcomes and minimizing unnecessary diagnostic and therapeutic interventions. Although developments in genetic sequencing have the potential to transform clinical care, there are important limitations, including uncertainty in the clinical interpretation of many genetic variants and concerns about privacy, discrimination, and cost.
To help clinicians understand the basics of genetic sequencing and how to apply it in clinical practice, Annals of Internal Medicine is launching a new “Precision Medicine” series. This introduction provides a general overview of clinical sequencing, with a focus on germline variation. Subsequent articles will use a case-based format to provide concise summaries of specific clinical precision medicine scenarios that are relevant to the practice of internal medicine. These cases will highlight specific clinical indications; interpretation of genetic test results; and ethical, legal, cost, and privacy issues related to genetic testing. The goal is to provide practical information on the appropriate application and interpretation of genomics in routine clinical practice.
In general, clinicians might consider genetic testing in 2 situations: to establish a diagnosis in symptomatic persons (diagnostic testing), or to assess predisposition for disease in asymptomatic persons who have increased risk due to family history or personal characteristics (predisposition or predictive testing). In some circumstances, population-wide genetic testing may be used for newborn screening or universal carrier screening for reproductive purposes.
Diagnostic genetic testing in symptomatic persons can clarify the diagnosis and prognosis, suggest the most appropriate management strategies, and indicate other associated features for which medical surveillance or intervention may be helpful. Identifying an underlying molecular genetic cause may also help in family planning and counseling of blood relatives.
To determine disease risk for unaffected relatives in a family with a medical condition (such as colon cancer), it is best to start genetic testing in an affected family member to determine whether there is an identifiable hereditary factor. If the affected person has a familial mutation, targeted mutation analysis in unaffected family members allows for the most cost-effective and informative risk stratification. When a familial mutation is identified, a normal genetic test result in asymptomatic family members is “informative” and reduces their disease risk to the level in the general population. However, when the affected family member is unavailable for or unwilling to undergo testing, normal results in asymptomatic family members are uninformative. Clinicians and patients must recognize that for common diseases with substantial risk in the general population, such as breast cancer, no one—not even those with an informative genetic test result—is risk-free.
What Genetic Sequencing Strategies Are Available in Clinical Practice?
The most common genetic testing strategies that are available in clinical practice are targeted gene sequencing, gene panel sequencing, and clinical exome sequencing (ES). Targeted gene sequencing using the Sanger method is useful for diagnostic testing when the clinician suspects a mutation in a specific gene. It is not easily scalable, so it is limited to sequencing of a small number of genes. Panel sequencing and ES use next-generation sequencing, which can sequence many genes simultaneously and provides reliable, rapid, and cost-effective detection of genetic variants.
Panel sequencing interrogates a preselected set of genes known to be involved in a particular condition, such as cancer or cardiomyopathy, for which mutations in any 1 of several genes can cause similar phenotypes. It enables coverage of all relevant regions of the genes and is usually optimized to also capture a range of variants that are not easily detectable by Sanger sequencing, such as insertions, deletions, and other rearrangements. One major disadvantage of this strategy is that the panels require frequent updating with the discovery of new relevant genes.
Exome sequencing involves sequencing of the coding regions (exomes) of all genes, and genome sequencing (GS) involves sequencing of both coding and noncoding regions. The exomes represent about 1% of the genome. Exome sequencing is available for clinical diagnostics for some indications, and GS is used predominantly in the research setting.
Because ES involves sequence analysis of all genes in the genome, it can identify mutations in genes that are not suspected on the basis of clinical presentation or are not yet known to cause disease. When initial analysis is unable to establish a diagnosis, ES data can be reinterrogated as new genes for a given condition are discovered and new exome analysis methods are developed.
Genome sequencing provides information on non–protein coding variation; gives more complete coverage of the coding regions; and enables more accurate detection of structural variants, such as translocations, deletions, and duplications. However, despite its comprehensiveness, GS is infrequently used in clinical settings because of its higher cost and greater computational requirements compared with ES (1–3).
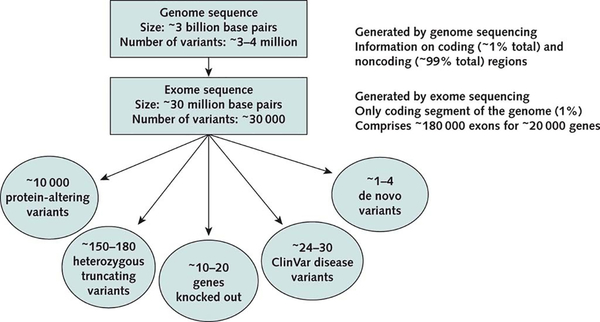
Figure 1 summarizes the approximate numbers of genetic variants found in a typical human exome. On average, ES detects approximately 10 000 protein-altering variants, including 150 to 180 protein-truncating variants, 20 to 30 known disease-causing (mainly recessive) variants, and 1 to 4 de novo variants that are not present in parents. The key challenge relates to interpretation of genetic variants. As of 1 February 2019, the Online Mendelian Inheritance in Man database indicated that out of 20 000 protein-coding genes, only 3652 (approximately 18%) had been found to cause known single-gene disorders. The process of establishing associations of the other genes with diseases remains laborious and continues to evolve (6). Moreover, because some genes have been studied more extensively than others, the level of evidence is highly variable for different Mendelian disorders.
Figure 1.
Expected findings from genome and exome sequencing for an individual patient. The numbers shown are approximate and depend on specific sequencing platforms and populations being studied. Protein-altering variants are variants that alter amino acid sequence in any way. Truncating variants are a subset of protein-altering variants that lead to a premature stop codon or truncation of a protein. “Knocked-out genes” are genes carrying homozygous loss-of-function variants. ClinVar disease variants are known pathogenic variants that cause human diseases according to ClinVar (Table 2). De novo variants are new mutational events that, by definition, are not inherited from parents. (Adapted from references 4 and 5.)
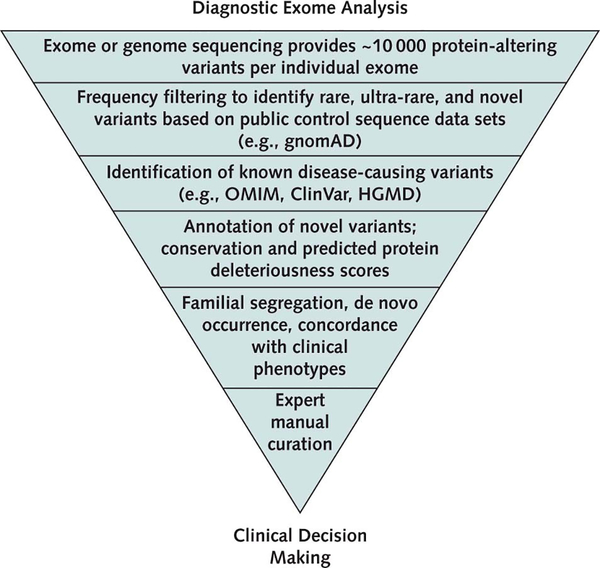
In practice, routine application of bioinformatic methods narrows the search for a diagnostic variant in the clinical analysis of ES data (Figure 2). Expert review is usually required to interpret variant pathogenicity and to assess the concordance of molecular findings with clinical features. The final determination of the molecular diagnosis may require genetic testing of other family members to assess whether the clinical phenotype travels with the genetic variant within the family (segregation analysis).
Figure 2.
Steps in the diagnostic sequence analysis of an individual exome. gnomAD = Genome Aggregation Database; HGMD = Human Gene Mutation Database; OMIM = Online Mendelian Inheritance in Man.
How Should Clinicians Interpret Genetic Test Results?
Guidelines from the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology classify genetic variants for Mendelian disorders into 1 of 5 categories: pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, or benign (Table 1) (7). Pathogenic variants are considered to be disease-causing and should be acted on as such. Likely pathogenic variants have a 90% estimated probability of being pathogenic, and clinical geneticists typically act on them as if they are pathogenic. Variants of uncertain significance are common and should not trigger clinical action unless they are reclassified as likely pathogenic or pathogenic. However, they are more likely to eventually be reclassified as likely benign or benign. Variants that are likely benign or benign are often not included on genetic test reports because of their lack of clinical significance.
Table 1.
American College of Medical Genetics and Genomics Variant Classifications
| Classification | Meaning |
|---|---|
| Pathogenic | Disease-causing |
| Likely pathogenic | >90% chance variant is disease-causing |
| Variant of uncertain significance | Uncertain whether variant is disease-causing |
| Likely benign | >90% chance variant is not disease-causing |
| Benign | Not disease-causing |
ClinVar (Table 2) is a free “open resource” that provides classifications of all clinically relevant variants. Each entry includes a 4-star scale about the level of confidence in the classification. Clinicians and patients can check ClinVar to see the current classification of a variant if they are considering action in light of that variant. Over time, ClinVar will reclassify variants using data from the general population (especially allele frequency in unaffected persons), information within families that demonstrates that the variants are de novo or segregate with disease, and additional research. To assist with correct reclassification, some laboratories offer free genetic testing to family members for segregation analysis when the index patient has a VUS. This enables determination of whether the same variant co-occurs with disease in all affected family members and thus may help to reclassify variants.
Table 2.
Useful Resources
| Resource (URL) | Description |
|---|---|
| American College of Medical Genetics and Genomics practice guidelines (www.acmg.net/ACMG/Medical-Genetics-Practice-Resources/Practice-Guidelines.aspx) | Provides up-to-date clinical guidelines on genetic testing for specific pediatric and adult conditions, as well as guidelines on predisposition testing; carrier screening; and preconception, prenatal, and newborn genetic screening. |
| ClinVar (www.ncbi.nlm.nih.gov/clinvar) | Provides expert-graded evidence for strength of association between a gene and a disease based on available literature, including the number of reported cases with variants in a disease-causing gene and supporting experimental data. Each variant is accompanied by assertions about its pathogenicity (or lack thereof) for the associated disease phenotype. The number of stars accompanying an entry reflects the strength of the evidence supporting the classification. |
| Online Mendelian Inheritance in Man (https://omim.org) | A complete database of Mendelian disorders derived from curation of the primary literature; includes phenotypic features, associated genes, and reported causal variants with references. |
| Genome Aggregation Database (http://gnomad.broadinstitute.org) | A sequence repository that includes whole-exome sequencing data from 123 136 unrelated persons and whole-genome sequencing data from 15 496 unrelated persons; provides estimates of population frequencies across 7 major global populations; excludes related persons and those with severe early-onset disorders but includes persons affected by various adult-onset diseases. |
Why Is Diagnostic Interpretation of Sequence Data Challenging?
Diagnostic interpretation of genetic sequence data is difficult for several reasons. First, persons have many ultra-rare and private (unique) variants in known disease genes. Second, the functional effect of new missense variants is difficult to assess. Third, variant population frequencies are not available for many ethnicities. Fourth, clinical databases are not well curated, and many benign variants are erroneously classified as pathogenic. Finally, parents of adult patients are frequently unavailable for testing of segregation or de novo occurrence. Our knowledge of variant pathogenicity is in a state of flux, especially for persons of non-European ancestry, for whom less reference sequence is available to categorize variants as rare. As a result, current sequencing tests report many VUSs, which make the testing less informative and can lead to confusion and unnecessary clinical follow-up (7). Because the genetic knowledge base is rapidly evolving, periodic reinterpretation of sequence data may be prudent, but it is unclear who is responsible for this and who will pay for it.
When clinical factors strongly suggest a specific genetic cause, interpretation of negative genetic test results presents an additional challenge for clinicians. It is important to recognize that certain types of genetic variation, such as copy number variants, are often missed by conventional ES and may require additional analyses or alternative tests, such as GS or chromosomal microarray. Moreover, ES can yield false-negative results when disease-causing variants reside in genomic regions that are not well captured by ES, such as highly repetitive regions that are refractory to short-read sequencing. For example, ES does not readily detect triplet repeat disorders, such as Huntington disease. Consequently, when clinical suspicion is high and ES results are negative, additional testing may be required using targeted sequencing methods that are optimized for the genomic regions of interest or using long-range sequencing.
What Is the Clinical Role of Genetic Tests in Internal Medicine Practice?
In current practice, most genetic testing in adults involves panel-based tests, such as hereditary cancer panels. Many professional groups have developed practice guidelines that discuss the role of genetic testing for specific disorders, such as thrombophilia (8), cancer predisposition (9, 10), or hyperlipidemia (11). For many of these disorders, genetic testing can pinpoint the molecular cause, enabling surveillance, early detection of complications, and sometimes indications for specific therapies. For example, a genetic diagnosis of Lynch syndrome, which accounts for 3% to 5% of colorectal cancer cases, could trigger more frequent colonoscopy, screening for endometrial and ovarian cancer in female carriers, and consideration of colectomy for tumors that cannot be removed via colonoscopy (12). Genetic testing is recommended for suspected familial hypercholesterolemia because low-density lipoprotein cholesterol levels frequently do not distinguish heterozygotes from homozygotes, who require much more aggressive lipid-lowering therapy (11, 13). Identification of PCSK9 mutations provides a rationale for therapy with proprotein convertase subtilisin/kexin type 9 inhibitors, whereas low-density lipoprotein receptor–null homozygotes do not respond to such agents (14).
In addition to these classic examples, recent studies have discovered genetic forms of adult diseases without a previously suspected inherited component, such as chronic kidney disease, pulmonary hypertension, and amyotrophic lateral sclerosis. About 10% of chronic kidney disease cases have a monogenic cause (15). For some chronic kidney disease subcategories, such as nephrotic syndrome, immunosuppression is contraindicated in genetic forms but is indicated for idiopathic disease (15, 16). Although genetic testing for germline cancer genes has been available for more than 15 years, it has become evident that many mutation carriers do not have a positive family history. Thus, expanded genetic testing in patients with cancer, particularly young patients, may be warranted. For example, a recent study detected germline mutations, mostly clustered in DNA repair pathways, in 12.2% of patients with metastatic cancer from 30 primary sites, providing a rationale for genetic counseling and clinical genetic testing for all patients with cancer (17). Although keeping up with these rapid developments is difficult, internists should become familiar with clinical scenarios that may warrant genetic evaluation (Table 3).
Table 3.
Indications for Genetic Work-up or Referral to a Genetic Specialist
| Patients with clinical findings indicative of a specific monogenic syndrome or disorder (e.g., polycystic kidneys in a patient with renal dysfunction, multiple polyps on routine colonoscopy, low ceruloplasmin levels in a patient with neurologic deterioration) |
| Patients with a rare condition that has an established genetic predisposition (e.g., cancer syndromes, severe hyperlipidemia, long QT syndrome, cardiomyopathies, nephrotic syndrome, Huntington disease) |
| Patients with early disease onset and a strongly positive family history |
| Patients with rare, unexplained disorders and unrevealing standard diagnostic work-ups |
| Healthy persons with a family history of a disease for which early diagnosis allows preventive intervention (e.g., sudden cardiac death, ovarian cancer) |
| Couples preparing to conceive whose ethnicities have a high carrier frequency for specific disorders (e.g., Ashkenazi Jewish) or couples who are related by bloodline (as commonly occurs in the Middle East) |
Carrier screening is another important sequencing application. Approximately 1 in 280 births results in a child with a monogenic disorder (18). Thus, although they are individually rare, known monogenic disorders cumulatively represent a significant disease burden. Until recently, recommendations for carrier screening were limited to such disorders as cystic fibrosis and spinal muscular atrophy, and screening for disorders such as Tay–Sachs disease or hemoglobinopathies was based on ethnicity. Currently, it is recommended that all pregnant women be offered screening to determine their carrier status for specific autosomal recessive disorders (19, 20). However, increasing rates of migration and intermarriage make an ethnicity-based approach inefficient. Expanded pan-ethnic carrier screening allows detection of carrier status for more than 200 disorders at a lower cost per patient than previous ethnicity-based testing (21). Targeted genotyping approaches with a limited number of mutations for any specific disorder are still common in practice but are being replaced by targeted sequencing. It should also be noted that although most carrier screening is performed during pregnancy, preconception screening is preferable because it allows carrier couples more reproductive choices, including preimplantation genetic diagnosis, in which embryos created by in vitro fertilization are genetically evaluated before implantation, resulting in only unaffected pregnancies.
An important emerging indication for more comprehensive ES and GS is for disorders with unknown causes. There are currently no universal guidelines on ES or GS in adults because most published studies have focused on pediatric populations (22, 23). However, the yield is likely to be greatest for diseases with early age at onset, positive family history, or atypical presentation. In adults, studies indicate a high diagnostic yield for patients with chronic kidney disease of unknown cause, particularly in the setting of a positive family history (15, 24). Moreover, studies have shown that 1% to 2% of patients referred for ES or GS actually carry 2 distinct genetic disorders, often leading to a convergence of symptoms that cannot be disaggregated in other ways (22, 23, 25). With wider availability, decreasing costs, and broader public awareness, clinical use of ES and GS will increase. Large systematic studies are needed to guide appropriate, evidence-based indications for testing.
What Are Incidental or Secondary Genetic Findings?
When an entire exome or genome is sequenced, pathogenic variants may be identified that are unrelated to the reason for the testing. Such results have been termed “incidental” or “secondary” findings (26–28). The ACMG lists 59 genes that are recommended for secondary analysis for patients undergoing ES or GS. Most of these involve monogenic disorders causing cancer or cardiovascular diseases that are associated with significant morbidity or mortality if they are not recognized and acted on. The ACMG recommends offering patients the opportunity to opt out of receipt of secondary findings, but more than 90% of patients elect to receive them. Within ACMG’s list of 59 genes, only pathogenic variant results are reported, maximizing the specificity but not the sensitivity of secondary findings. Therefore, if the report includes no pathogenic variants in these genes, clinicians should not assume that the patient has no mutation in them, especially if there is a suggestive family history. Approximately 1% to 4% of the population carries a pathogenic variant in 1 of these genes (29–32).
What Are the Potential Harms of Genetic Testing?
The key problem with widespread genetic testing is uncertainty in interpretation of results, particularly for predictive testing. Despite accelerated gene discovery and improved efforts in sharing genetic data, even targeted sequencing continues to return a large number of VUSs. This problem grows as more genes are sequenced because of the high prevalence of ultra-rare and novel variants in any human genome, leading to the potential for ambiguous findings. The shortage of reference data for diverse ancestries contributes to uncertainty in variant interpretation for non-Europeans and can lead to health care disparities, adverse effects, and increased costs if genetic findings of uncertain significance result in unnecessary follow-up testing, surveillance, or interventions.
Limited genetic literacy among most patients and physicians amplifies these limitations and makes it difficult to communicate nuanced genetic findings or uncertainties related to a variant classification. Smart electronic clinical decision support systems could mitigate this problem and assist with iterative reinterpretation of genetic data, but most electronic health records (EHRs) are not equipped to handle sequence information, leaving the genetic laboratory and the provider to bear the burden of reinterpreting genetic variants.
Another critical problem is that even after an unambiguous genetic diagnosis, no effective targeted treatments are currently available for most genetic diseases. Although our ability to make accurate genetic diagnoses continues to improve, the vision of wider application of precision medicine will ultimately depend on the development of targeted treatments for a much larger proportion of human disease-causing genes.
Related to this critical issue are concerns about the willingness of insurers to cover genetic tests. Currently, most plans pay for specific diagnostic testing and counseling for patients when they are “clinically indicated.” This usually requires a relevant family or personal history to suggest increased risk and an available clinical action that will depend on the results. The most common type of genetic test covered by insurers is a panel of genes for hereditary cancer and targeted gene panel testing for some adult-onset conditions. Coverage of ES varies by indication because of concerns that the diagnostic yield is low and the results do not inform care for most adult conditions. The latter point is important because patients and physicians could seek genetic testing to identify variants that allow risk stratification but for which there is no specific medical intervention. For example, the ApoE4 allele is associated with increased risk for Alzheimer disease, but there is currently no clinical intervention that knowledge of the patient’s ApoE4 status would direct. Consequently, insurers generally do not cover ApoE4 testing. The limited evidence for clinical utility of ES or GS for most adult conditions significantly hampers reimbursement, but this is likely to change as effective treatments emerge for more genetic disorders. Rigorously designed clinical studies that assess costs and benefits of genomic testing by specific clinical indication are needed.
Beyond issues with regard to the cost of genetic testing, insurers and policymakers are concerned that widespread implementation of ES and GS will increase downstream health care costs without delivering the promised benefits. Costs may include expenditures on unnecessary testing, procedures, and specialist referrals related to clinical evaluation of VUSs. Additional costs relate to the information technology infrastructure needed to handle next-generation sequencing data and training of practicing clinicians. Whether the potential benefits of genomic testing will offset these costs is unclear.
The potential for discriminatory use of genetic information is another concern. The Genetic Information Nondiscrimination Act prohibits increasing health insurance rates or denying coverage or employment on the basis of genetic predisposition. However, the law does not extend to life insurance, long-term care insurance, or disability insurance.
What Can We Expect With Regard to the Clinical Application of Genetic Sequencing Over the Next Decade?
As the cost of sequencing continues to decrease, the technology and related bioinformatics are becoming more robust and standardized. With approximately 200 new Mendelian disorders being discovered annually and tens of thousands of new genomes being added continuously to public databases, our ability to interpret genetic variation is improving rapidly. The number of clinical indications for genetic testing is increasing, as is the availability of direct-to-consumer genetic testing, fostering public demand. Thus, in the future, genetic testing for many indications could be widely available to patients, enabling rapid clinical interrogation and reinterpretation based on a specific clinical context.
As computational methods evolve, we anticipate major improvements in the diagnostic algorithms that automate the analysis and interpretation of sequence variation. For example, electronic phenotyping based on EHR data may provide additional context for variant interpretation (33, 34). We also foresee major improvements in predictive models of disease that incorporate sequence data in addition to EHR-derived information and exposure data captured by mobile technology. Automated analysis of relatedness and linkage of individual patients into expanded pedigrees across EHR systems may further enhance assessment of familial risk (35). Sequence-derived pharmacogenomic information could be incorporated into drug prescribing algorithms to tailor dosing and reduce adverse effects. In the future, clinicians will probably receive actionable information about inherited risk along with real-time clinical decision support for management of specific disease predispositions. This vision of precision medicine is likely to become a reality over the next decade as evidence of clinical validity and utility of genetic testing accumulates across various subspecialties.
Key Summary Points.
Genetic testing can be helpful in 2 clinical situations: diagnostic testing in persons who are symptomatic, and predisposition testing in those who are asymptomatic but at risk on the basis of family history and personal factors.
Diagnostic testing is currently the most common type of genetic testing in internal medicine practice and includes targeted Sanger sequencing for suspected monogenic disorders and focused panel sequencing of genes for hereditary cancer and cardiac diseases.
Exome sequencing (ES) targets all protein-coding segments (exons) of the genome and can reliably establish a molecular diagnosis for known genetic disorders. Unlike targeted testing, ES can identify a previously unsuspected molecular diagnosis.
Genome sequencing (GS) involves sequencing of the entire human genome, providing information on noncoding regions and copy number variants and enabling derivation of polygenic risk scores for complex traits. It is not routinely used in clinical practice.
In addition to diagnostic information for monogenic disorders, both ES and GS provide information on actionable secondary findings.
Interpretation of genetic variation detected by sequencing is challenging but continues to improve with data sharing and accelerated gene discovery for monogenic diseases.
Barriers to widespread implementation of diagnostic ES include uncertainty related to variant interpretation, insufficient data on persons of diverse ancestries, unwillingness of some insurers to cover testing, a small workforce of genetic professionals, limited genetic literacy among patients and physicians, concerns about privacy and genetic discrimination, and a lack of standards for reinterpretation of genomic data over time.
Acknowledgments
Grant Support: The authors were supported by the Columbia Precision Medicine Initiative, Columbia University, New York, New York, as well as the following grants from the National Institutes of Health: Columbia Clinical and Translational Science Award number UL1TR001873 from the National Center for Advancing Translational Sciences; the Electronic Medical Records and Genomics Network grant number U01HG8680 from the National Human Genome Research Institute; the Columbia Kidney Precision Medicine Project grant number UG3DK114926 from the National Institute of Diabetes and Digestive and Kidney Diseases; and the All of Us Network grant number UG3OD023183 from the Office of the Director, National Institutes of Health.
Disclosures: Dr. Gharavi reports grants from the Renal Research Institute and other support from AstraZeneca during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-0425.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Krzysztof Kiryluk, College of Physicians and Surgeons, Columbia University, New York, New York.
David B. Goldstein, College of Physicians and Surgeons, Columbia University, New York, New York.
John W. Rowe, Mailman School of Public Health, Columbia University, New York, New York.
Ali G. Gharavi, College of Physicians and Surgeons, Columbia University, New York, New York.
Ronald Wapner, College of Physicians and Surgeons, Columbia University, New York, New York.
Wendy K. Chung, College of Physicians and Surgeons, Columbia University, New York, New York.
References
- 1.Cooper DN, Chen JM, Ball EV, Howells K, Mort M, Phillips AD, et al. Genes, mutations, and human inherited disease at the dawn of the age of personalized genomics. Hum Mutat. 2010;31:631–55. doi: 10.1002/humu.21260 [DOI] [PubMed] [Google Scholar]
- 2.Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet. 2013;14:681–91. doi: 10.1038/nrg3555 [DOI] [PubMed] [Google Scholar]
- 3.Ku CS, Naidoo N, Pawitan Y. Revisiting Mendelian disorders through exome sequencing. Hum Genet. 2011;129:351–70. doi: 10.1007/s00439-011-0964-2 [DOI] [PubMed] [Google Scholar]
- 4.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. ; 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. ; Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–76. doi: 10.1038/nature13127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasouly HM, Groopman EE, Heyman-Kantor R, Fasel DA, Mitrotti A, Westland R, et al. The burden of candidate pathogenic variants for kidney and genitourinary disorders emerging from exome sequencing. Ann Intern Med. 2018. doi: 10.7326/M18-1241 [DOI] [PubMed] [Google Scholar]
- 8.Baglin T, Gray E, Greaves M, Hunt BJ, Keeling D, Machin S, et al. ; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149:209–20. doi: 10.1111/j.1365-2141.2009.08022.x [DOI] [PubMed] [Google Scholar]
- 9.Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–7. doi: 10.1200/JCO.2015.63.0996 [DOI] [PubMed] [Google Scholar]
- 10.Hampel H, Bennett RL, Buchanan A, Pearlman R, Wiesner GL; Guideline Development Group, American College of Medical Genetics and Genomics Professional Practice and Guidelines Committee and National Society of Genetic Counselors Practice Guidelines Committee. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70–87. doi: 10.1038/gim.2014.147 [DOI] [PubMed] [Google Scholar]
- 11.Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. ; European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–57. doi: 10.1093/eurheartj/ehu274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. ; US Multi-Society Task Force on Colorectal Cancer. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2014;147:502–26. doi: 10.1053/j.gastro.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 13.Green RF, Dotson WD, Bowen S, Kolor K, Khoury MJ. Genomics in public health: perspective from the Office of Public Health Genomics at the Centers for Disease Control and Prevention (CDC). Healthcare (Basel). 2015;3:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.France M, Rees A, Datta D, Thompson G, Capps N, Ferns G, et al. ; for HEART UK Medical Scientific and Research Committee. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis. 2016;255:128–39. doi: 10.1016/j.atherosclerosis.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 15.Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380:142–51. doi: 10.1056/NEJMoa1806891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groopman EE, Rasouly HM, Gharavi AG. Genomic medicine for kidney disease. Nat Rev Nephrol. 2018;14:83–104. doi: 10.1038/nrneph.2017.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, et al. Integrative clinical genomics of metastatic cancer. Nature. 2017;548:297–303. doi: 10.1038/nature23306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734–42. doi: 10.1001/jama.2016.11139 [DOI] [PubMed] [Google Scholar]
- 19.Committee on Genetics. Committee opinion no. 691: carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41–e55. doi: 10.1097/AOG.0000000000001952 [DOI] [PubMed] [Google Scholar]
- 20.Grody WW, Thompson BH, Gregg AR, Bean LH, Monaghan KG, Schneider A, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482–3. doi: 10.1038/gim.2013.47 [DOI] [PubMed] [Google Scholar]
- 21.Nazareth SB, Lazarin GA, Goldberg JD. Changing trends in carrier screening for genetic disease in the United States. Prenat Diagn. 2015;35:931–5. doi: 10.1002/pd.4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013;369:1502–11. doi: 10.1056/NEJMoa1306555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Deignan JL, Dorrani N, Strom SP, Kantarci S, Quintero-Rivera F, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. 2014;312:1880–7. doi: 10.1001/jama.2014.14604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lata S, Marasa M, Li Y, Fasel DA, Groopman E, Jobanputra V, et al. Whole-exome sequencing in adults with chronic kidney disease: a pilot study. Ann Intern Med. 2018;168:100–9. doi: 10.7326/M17-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posey JE, Harel T, Liu P, Rosenfeld JA, James RA, Coban Akdemir ZH, et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med. 2017;376:21–31. doi: 10.1056/NEJMoa1516767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. ; American College of Medical Genetics and Genomics. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565–74. doi: 10.1038/gim.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. CORRIGENDUM: ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2017;19:606. doi: 10.1038/gim.2017.18 [DOI] [PubMed] [Google Scholar]
- 28.Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017;19:249–55. doi: 10.1038/gim.2016.190 [DOI] [PubMed] [Google Scholar]
- 29.Retterer K, Juusola J, Cho MT, Vitazka P, Millan F, Gibellini F, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2016;18:696–704. doi: 10.1038/gim.2015.148 [DOI] [PubMed] [Google Scholar]
- 30.Johnston JJ, Rubinstein WS, Facio FM, Ng D, Singh LN, Teer JK, et al. Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. Am J Hum Genet. 2012;91:97–108. doi: 10.1016/j.ajhg.2012.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gambin T, Jhangiani SN, Below JE, Campbell IM, Wiszniewski W, Muzny DM, et al. Secondary findings and carrier test frequencies in a large multiethnic sample. Genome Med. 2015;7:54. doi: 10.1186/s13073-015-0171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart MR, Biesecker BB, Blout CL, Christensen KD, Amendola LM, Bergstrom KL, et al. Secondary findings from clinical genomic sequencing: prevalence, patient perspectives, family history assessment, and health-care costs from a multisite study. Genet Med. 2018. doi: 10.1038/s41436-018-0308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son JH, Xie G, Yuan C, Ena L, Li Z, Goldstein A, et al. Deep phenotyping on electronic health records facilitates genetic diagnosis by clinical exomes. Am J Hum Genet. 2018;103:58–73. doi: 10.1016/j.ajhg.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastarache L, Hughey JJ, Hebbring S, Marlo J, Zhao W, Ho WT, et al. Phenotype risk scores identify patients with unrecognized Mendelian disease patterns. Science. 2018;359:1233–9. doi: 10.1126/science.aal4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polubriaginof FCG, Vanguri R, Quinnies K, Belbin GM, Yahi A, Salmasian H, et al. Disease heritability inferred from familial relationships reported in medical records. Cell. 2018;173:1692–1704.e11. doi: 10.1016/j.cell.2018.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]