Abstract
Flecainide is a class IC antiarrhythmic indicated for ventricular and supraventricular arrhythmias in pediatric patients without structural heart disease. Flecainide has a narrow therapeutic window and proarrhythmic effect even in therapeutic doses and could lead to a life-threatening intoxication. Dosage errors, accidental intakes, and drug or food interactions, especially with dairy products, can be the cause of the intoxication. We report three consecutive cases of flecainide intoxication in children with supraventricular tachycardia (SVT) in our hospital from 2017 to 2019. Two cases had complete and spontaneous normalization of electrocardiogram (ECG) after flecainide removal. However, admission to the intensive care was required due to a sustained ventricular tachycardia in one case. Flecainide intoxication can be a life-threatening complication in patients with SVT. We believe all children should have close monitoring with serial ECG and plasma levels of flecainide during the 48–72 h after initiation of treatment, and consider hospitalization for patients <1 year of age.
Keywords: Arrhythmia, children, flecainide, intoxication, supraventricular tachycardia
INTRODUCTION
Flecainide is a class IC antiarrhythmic indicated for ventricular and supraventricular tachycardia (SVT) in pediatric patients without structural heart disease.[1] Flecainide antagonizes NaV1.5 sodium channels, responsible for generating the fast sodium input current (INa), prolonging depolarization and repolarization of the myocardial cell. It has been shown to be effective in the control of recurrences of SVT in children (73% to 100%, depending on mechanism).[1] Flecainide toxicity is rare in pediatrics, but due to its narrow therapeutic window and its interaction with dairy products, it is potentially lethal, leading to hypotension and arrhythmias.[2] Flecainide intoxication has an estimated mortality rate of <1%.[3]
We present a series of three consecutive cases of flecainide intoxication in our hospital in children with a diagnosis of SVT from March 2017 to June 2019. We describe their clinical presentation, electrocardiogram (ECG) changes, and possible causes.
CASE REPORTS
Case 1
A 3-day-old girl with the diagnosis of SVT was initially started on propranolol, and subsequently, flecainide was added for further recurrences. She was discharged home on day 10 of life on propranolol 2.8 mg/kg/day and flecainide 3.3 mg/kg/day. Flecainide was given in a liquid solution of 20 mg/mL and was prepared in the hospital local pharmacy. On day 25th of life, she was brought to the emergency department with irritability and dyspnea after her last flecainide dose. The initial vital signs were normal. A 12-lead ECG revealed sinus rhythm with a prolonged PR interval, a complete right bundle branch, and a prolonged QTc interval. The serum flecainide concentration at 18 h postingestion was 0.72 mcg/mL (therapeutic range: 0.30–1 mcg/mL). Follow-up during hospitalization showed progressive ECG normalization after flecainide suppression over the next 12 h, and she was discharged home with only oral propranolol with no further SVT recurrences. No alterations in the concentration of the master formula were noted, and the family reported no errors in the administration schedule, always 30 min after the breastfeeding.
Case 2
A 6-month-old boy treated with propranolol 2.7 mg/kg/day and flecainide 3.6 mg/kg/day given as a liquid preparation of 20 mg/mL, for recurrent SVT diagnosed after the birth was seen in a routine cardiology follow-up. He had a current febrile episode, but no vomiting or diarrhea was present. His ECG showed sinus bradycardia with prolonged PR interval, a complete right bundle branch block, and a prolonged QTc interval [Figure 1a]. A serum flecainide concentration of 1.1 mcg/mL was reported at 8 h after the last dose of flecainide. Control ECGs after flecainide removal demonstrated a progressive normalization of PR interval and QRS complex [Figure 1b]. The boy was discharged home without flecainide after 48 h. No alterations in the concentration of the master formula were noted, and the family reported no errors in the administration schedule.
Figure 1.
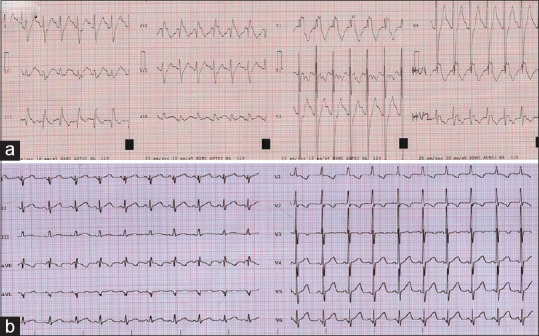
Electrocardiogram on admission and after normalization of alterations for case 2. (a) Electrocardiogram showed sinus rhythm with prolonged PR interval (174 ms), complete right bundle branch block (QRS of 174 ms), and prolonged QTc interval (518 ms). (b) Electrocardiogram after flecainide removal
Case 3
A 5-months-old boy presented to the emergency department with decay and respiratory distress. He was diagnosed with SVT after birth and treated with propranolol 3 mg/kg/day, and flecainide 4 mg/kg/day had been added after further SVT recurrence. Flecainide was administered using a liquid preparation of 20 mg/mL, administered by the hospital pharmacy. During his outpatient clinic controls, his doses were not adjusted due to no further SVT recurrences. On the day of his emergency consultation, his treatment doses were propranolol 2 mg/kg/day and flecainide 1.33 mg/kg/day orally. His vital signs showed a heart rate of 50–100 beats/min and blood pressure of 56/33 mmHg. ECG revealed sinus rhythm with episodes of the variable atrioventricular block (first, second and 2,1) and a right bundle branch block [Figure 2a]. He was transferred to the pediatric intensive care unit, where he presented a sustained monomorphic ventricular tachycardia [Figure 2b] that spontaneously terminated after 1 mEq/kg of sodium bicarbonate intravenously. Inotropic support with 5 mcg/kg/min of dobutamine was given for low cardiac output during 2 h. The plasma flecainide concentration was 2.06 mcg/mL at 12-h postingestion. He presented no further arrhythmias, and his ECG progressively normalized after 36 h of his admission [Figure 2c]. The patient was with an untrained family relative during flecainide dose administration.
Figure 2.
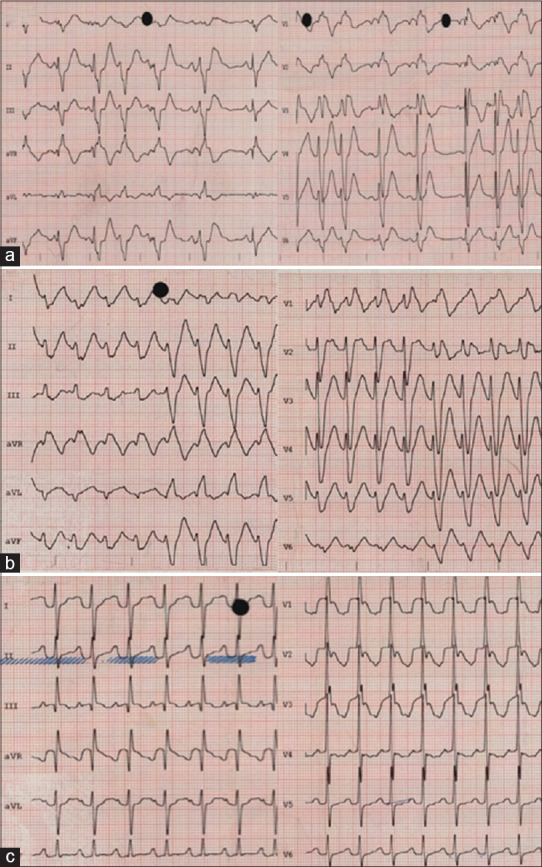
Electrocardiogram on admission (a) during a ventricular tachycardia episode (b) and after 36 h of admission (c) in case 3. (a) Sinus bradycardia with a second-degree atrioventricular block and right bundle branch block. (b) Sudden change of morphology noted after forth beat with a sustained monomorphic ventricular tachycardia with a right bundle branch block morphology and superior axis
DISCUSSION
Flecainide has a narrow therapeutic window and proarrhythmic effect, even in therapeutic doses. Dosage errors, accidental intakes, and drug or food interactions, especially with dairy products, can be the cause of the intoxication.[4,5,6] However, sometimes, it is not possible to find the explanation why the intoxication happened, like in our first case, where no alterations in the concentration of the master formula were noted, nor errors in the administration schedule, and the dose was administered 30 min after the breastfeeding.
At a pediatric age, the exclusive feeding with dairy products during the first 6 months makes the interaction with flecainide inevitable, so it is advisable to monitor plasma levels, especially after the first 2 days of treatment with serial ECGs. It is important to be aware of possible intoxications when the complementary feeding starts since the decrease in milk intake with the same dose of flecainide may lead to greater absorption of flecainide and cause intoxication. We believe this was the most probable cause of our second case, as the master formula was tested, and no reported administration mistakes were noted. In the third patient, an administration mistake was suspected, given that the patient was with an untrained family member. This reason can be very frequent in cases where the child is not with his parents or when patients begin kindergarten.
When using drugs with narrow therapeutic window that could lead to a life-threatening intoxication, it is very important to give written instructions to parents on how to administer the medication and its possible interactions, to ensure the correct administration. This is even more important in infants and young children, where drugs must be administered with master formulations that can lead to errors in dosing. Therefore, we believe that all children should have close monitoring with serial ECG and plasma levels of flecainide during the 48–72 h after the initiation of treatment. Furthermore, in patients <1 year of age in whom the risk of intoxication is higher, initiation of flecainide as an inpatient with continuous ECG monitoring for 48–72 h should be considered.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Brugada J, Blom N, Sarquella-Brugada G, Blomstrom-Lundqvist C, Deanfield J, Janousek J, et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace. 2013;15:1337–82. doi: 10.1093/europace/eut082. [DOI] [PubMed] [Google Scholar]
- 2.Köppel C, Oberdisse U, Heinemeyer G. Clinical course and outcome in class IC antiarrhythmic overdose. J Toxicol Clin Toxicol. 1990;28:433–44. doi: 10.3109/15563659009038586. [DOI] [PubMed] [Google Scholar]
- 3.Perry JC, Garson A., Jr Flecainide acetate for treatment of tachyarrhythmias in children: Review of world literature on efficacy, safety, and dosing. Am Heart J. 1992;124:1614–21. doi: 10.1016/0002-8703(92)90081-6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson B, Mangat J, Barton C, et al. Decreased milk drinking causing flecainide toxicity in an older child. Case Reports. 2012:2012. doi: 10.1136/bcr.02.2012.5810. bcr0220125810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Close BR, Banks CJ. Pediatric flecainide toxicity from a double dose. Am J Emerg Med. 2012;30:2095e1–2. doi: 10.1016/j.ajem.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 6.D'Alessandro LC, Rieder MJ, Gloor J, Freeman D, Buffo-Sequiera I. Life-threatening flecainide intoxication in a young child secondary to medication error. Ann Pharmacother. 2009;43:1522–7. doi: 10.1345/aph.1L549. [DOI] [PubMed] [Google Scholar]


