Abstract
Coronavirus disease 2019 (COVID-19) is a novel infection of which we still have much to learn. Microvascular and macrovascular complications are increasingly recognised as being among the drivers of morbidity and mortality in patients with this condition. Here we present a case of a woman with COVID-19 who suffered massive and bilateral middle cerebral artery strokes, which presented as reduced consciousness several days into admission. Clinicians need to be aware of possible causes of reduced consciousness in COVID-19 patients, particularly as delirium appears to be a common complication, and revisit working diagnoses if the clinical picture does not fully fit. Studies into both anticoagulation and the management of stroke in the context of COVID-19 are urgently needed to help inform future practice.
Keywords: infections, pneumonia (respiratory medicine), stroke, end of life decisions (geriatric medicine)
Background
Coronavirus disease 2019 (COVID-19) is a new infection of which there is much to learn. Based on literature, particularly from intensive care units (ICU), we have seen that microvascular complications are an emerging hypothesis to explain the multiorgan failure seen in deteriorating patients. It is currently unclear to what extent macrovascular complications contribute to the mortality and morbidity of COVID-19.
Case presentation
A woman in her 70s presented to hospital with a 4-day history of cough and dyspnoea. She had been brought to hospital by her family as she had been feeling increasingly breathless. As she was known to have asthma, her family had tried using her inhalers but this had not helped her breathlessness. She had not had contact with any known COVID-19 cases. On the way to hospital, the ambulance crew noted that she was pyrexial.
She had a medical history of asthma and type 2 diabetes mellitus. She was also severely obese, with a body mass index of 40. In terms of her regular medications, she took metformin 500 mg twice a day, senna 15 mg at night, lansoprazole 30 mg in the morning, paracetamol as required and salbutamol inhalers as required. She had also been started on 30 mg prednisolone by her general practitioner 2 days prior to admission.
She lived with her family, was living downstairs and only mobilised inside the house. She also required help from family with activities of daily living. They were however managing without a formal package of care.
On examination, she was found to have a respiratory rate of 30 breaths/min. She was not in respiratory distress and was not using her accessory muscles. On chest auscultation, there were bilateral crackles but no wheeze. Cardiovascular examination was unremarkable with a regular pulse. Abdominal examination was also unremarkable. She was well perfused and had no peripheral oedema. She was alert and oriented, and neurological examination at this stage was unremarkable.
She was requiring 40% FiO2 supplemental oxygen to maintain saturations of 94%.
An arterial blood gas (ABG) done on 40% FiO2 showed a pH of 7.42, pCO2 of 5.4 kPa, pO2 of 7.5 kPa, a normal bicarbonate and a lactate of 2.4 mM.
A chest radiograph (CXR) showed dense bilateral airspace shadowing consistent with COVID-19 pneumonia (figure 1).
Figure 1.
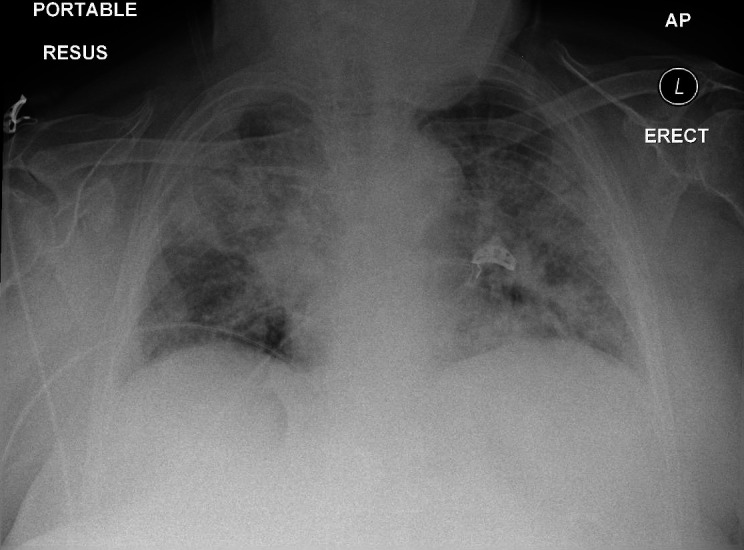
Initial chest radiograph demonstrating dense bilateral airspace shadowing consistent with COVID-19 pneumonia. COVID-19, coronavirus disease 2019.
An ECG showed sinus tachycardia with occasional ventricular ectopics, but no acute ischaemic changes.
The initial impression was of severe COVID-19 pneumonia, together with type 1 respiratory failure.
She was reviewed by the ICU team who felt that she was not a suitable candidate for ICU due to her poor baseline physiological reserve and instead recommended ward-based care.
She was started on intravenous amoxicillin and levofloxacin to cover bacterial pneumonia as per local guidelines, and COVID-19 nasopharyngeal swabs were sent, together with a COVID-19 blood panel. (In addition to full blood count (FBC), urea and electrolyes, C-reactive protein (CRP), it also included fibrinogen, troponin, lactate dehydrogenase, D-dimer, ferritin and a clotting screen.) She was transferred to the COVID-19 ward once swab results confirmed COVID-19 infection.
Five days into admission, she was found to be extremely drowsy during the morning ward round. As her first language was not English, nursing staff tried speaking in other languages familiar to the patient but got no response. At this point, she was still requiring 4 L/min of supplemental oxygen via a nasal cannula, and she remained tachycardic. An ABG showed type 1 respiratory failure. Given that she was at day 9–10 of symptoms, and that she appeared agitated and was also pulling off her nasal cannula and face mask, the impression was of hypoactive delirium secondary to COVID-19 pneumonia.
Her family were informed of her deterioration. She initially remained stable, but was then reviewed by the ward consultant later in the day. A neurological examination at the time showed that she was responding to pain, had a Glasgow Coma Scale (GCS) of 7 (M5V1E1), with equal tone, equal and reactive pupils and an equivocal right plantar reflex with an extensor left plantar reflex. The consultant considered worsening COVID-19 as a possibility but noted that the deterioration in conscious level was reasonably abrupt. Her current medications were continued and repeat blood tests were sent.
She was again reviewed the following morning; overnight her supplemental oxygen requirements had increased from 4 L/min to 8 L/min. Her antibiotics were switched to piperacillin/tazobactam, and treatment continued. The main feature, as earlier, was the drop in GCS—now M2V1E1. She was still maintaining her own airway.
Her case was discussed with the neurology team on the third day of the deterioration, as there was a concern that we were missing a diagnosis such as COVID-19 encephalitis or ‘Neuro COVID-19’. A computed tomograph of the head (CT Head) was requested. The CT Head showed a large left middle cerebral artery (MCA) territory infarction with foci of hyperdensity representing acute haemorrhage (figure 2), and a 9 mm midline shift to the right. It also showed a right MCA branch territory infarction (figure 3).
Figure 2.
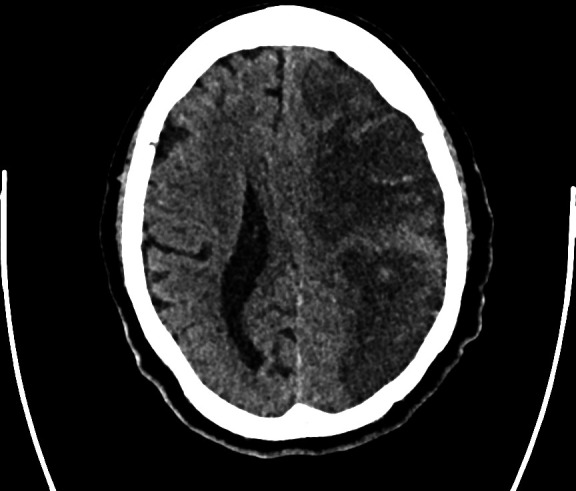
A slice of the CT images of the head demonstrating massive left middle cerebral artery infarction with small areas of haemorrhagic transformation.
Figure 3.
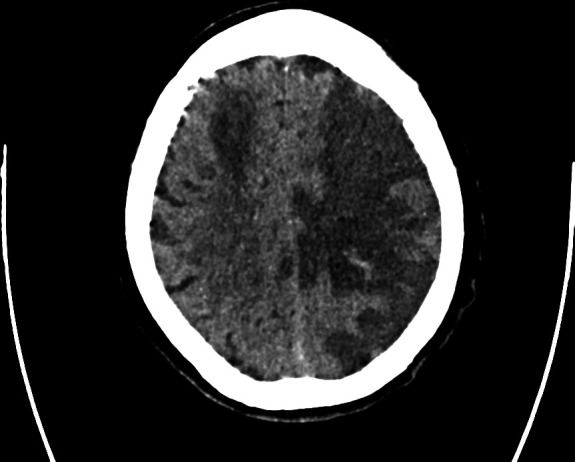
A further slice demonstrating right-sided wedge-shaped infarction in the right middle cerebral artery territory.
Investigations
Initial blood tests were consistent with COVID-19 infection and showed a raised CRP of 87, normal haemoglobin and white cell count but with mild neutrophilia (8.9), lymphopaenia (0.46) and eosinopaenia (0.00). Her D-dimer and fibrinogen were both elevated at 855 and 4.55, respectively, as was her high sensitivity troponin-I (43). She had a slightly low serum albumin (30), but with unremarkable renal function and liver function tests.
Her admission CXR (figure 1) showed bilateral infiltrates consistent with COVID-19 infection. This was confirmed with a positive nasopharyngeal Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) reverse transcriptase PCR result.
Repeat blood tests after her deterioration in consciousness showed a rise in CRP (136), slightly lower albumin (26) and mildly deranged Alanine Transaminase (ALT) (77). However, renal function remained within normal limits, and her FBC was unremarkable. Similarly, an ABG done after her deterioration showed only a low pO2 of 8.9 kPa, with a normal lactate, pH and bicarbonate. These results strengthened the case for searching for neurological causes of drowsiness.
A CT Head was performed later in the admission to rule out structural causes of reduced consciousness (eg, space occupying lesion, infarcts, oedema) and showed bilateral infarcts, with a 9 mm midline shift and small areas of haemorrhagic transformation (figures 2 and 3).
Differential diagnosis
Our initial thoughts were of a hypoactive delirium or a delirium secondary to metabolic decompensation in the context of severe COVID-19 infection. Factors that made us consider this diagnosis were widespread reports of delirium with the aforementioned severe infection, the fact that she was in an unfamiliar environment, that she was being spoken to in a language that she was not familiar with and that she had no family members accompanying her due to infection control measures. As patients with COVID-19 can develop a bacterial pneumonia, there was also a concern that the deterioration was due to an undertreated bacterial pneumonia—if the worsening presentation was due to progression of the COVID-19 pneumonia, then effectively this patient was at her ceiling of treatment as she was not a candidate for intensive care. However, the relatively stable markers of infection and stable renal function (with normal lactate on a blood gas) undermined this diagnosis.
Other diagnoses that were then considered included non-convulsive status epilepticus, COVID-related encephalitis or a massive intracerebral event. These diagnoses were considered to be less likely than the previous differentials because neurological examination following the patient’s deterioration was inconclusive. In addition to that, there was no seizure activity noted during the period before or after her reduced consciousness. She was not known to suffer from epilepsy, and she had no history or imaging to suggest a structurally abnormal brain which could predispose her to seizures. Having said that, she was on amoxicillin and levofloxacin, both of which can reduce a person’s seizure threshold. Ultimately the final diagnosis was clinched via imaging.
Treatment
Given her poor baseline function and the extent of the infarction, which was more than two-thirds of the left hemisphere, it was the opinion of both the stroke team and the treating team that if she did survive there was no potential for rehabilitation. Based on this, they did not recommend investigations into the aetiology of the stroke as it would not be of benefit to the patient. Her parent team then offered supportive and palliative care.
Outcome and follow-up
The patient was closely monitored on the ward over a period of 10 days; at the time of writing she is comfortable, but has not regained consciousness.
Discussion
SARS-CoV-2 is a novel coronavirus, first reported in Wuhan in the Hubei Province of China, which has now spread to almost every country on earth. It has infected more than 2 million people worldwide and has caused hundreds of thousands to become critically ill with COVID-19.1 We consider this case to be of interest in the current climate of continually emerging evidence on the pathophysiology of this new disease, of which we still have much to learn.
An area of growing interest is the effect this coronavirus has on vasculature. Microvascular complications identified at autopsy in patients with COVID-19 are beginning to be documented in the international literature and are postulated to play a significant role in severe forms of the disease.2 3 At present, macrovascular complications are also being recognised, particularly in COVID-19 patients who become critically ill and who are admitted to ICUs. It is theorised that the incidence of thromboembolic disease is increased in this cohort as a result of a systemic inflammatory response, as well as relative or complete immobility, tissue hypoxia and in some cases disseminated intravascular coagulation.4 This can result in both arterial and venous thromboembolism, causing increased morbidity in an already vulnerable patient group.
In a Dutch study of 184 ICU patients with COVID-19, the cumulative incidence of pulmonary embolism, deep vein thrombosis, ischaemic stroke, myocardial infarction and systemic arterial embolism was 31%, despite chemical thromboprophylaxis being given to all patients.4 The authors of this study feel that this percentage is likely an underestimate of the true incidence of thrombotic events, and that vigilance for thrombotic complications in patients with COVID-19 is paramount.
In a retrospective observational study from Wuhan, China, acute ischaemic stroke complicated 5% (11 of 221) of hospital admissions with COVID-19 during the study period.5 As a comparator, data from a 2009 study in Seoul prior to COVID-19 reported ischaemic stroke complicating 0.04%–0.06% of hospital admissions for all clinical presentations.6 While the geography and temporality of these studies are not directly comparable, we can still deduce that this likely represents a significant increase from a baseline hospital inpatient population.
In the Wuhan study, all patients with infarcts were noted to have pre-existing risk factors for stroke. These data suggest significant independent relationships between incidence of stroke and age >50 years, severity of COVID-19 and risk factors for both stroke and COVID-19. However, it is notable that there is significant overlap between risk factors for both COVID-19 and ischaemic stroke, and that no further analysis of confounding factors was undertaken in this cohort.5 Although the number of patients with stroke is small here, a significant proportion (36%) did not survive, suggesting that ischaemic stroke in COVID-19 patients may be a poor prognostic marker. This theory is echoed by a larger retrospective case series from Wuhan in which 5 out of 214 (2.3%) patients had an acute ischaemic stroke, 4 of whom were classed as having severe COVID-19 infection. Of note, 13 patients in the study (6%) had ‘impaired consciousness’, which again correlated with severity of disease.7
Intimately linked to vascular complications is the derangement of coagulation pathways caused by SARS-CoV-2 in acutely ill patients. A relationship has been repeatedly noted between raised D-dimer levels and increased mortality in COVID-19,7–9 as well as a similar trend with increased ferritin, CRP and ESR.8 D-dimer, a fibrin degradation product, is considered to be a largely non-specific serological marker present in many inflammatory conditions, however when raised it indicates fibrinolysis and activation of coagulation pathways. In COVID-19, a raised D-dimer likely reflects the systemic activation of pro-thrombotic pathways due to a combination of previously described contributing factors. It is feasible that activation of these pathways further increases the risk of thrombosis, and therefore macrovascular complications, in combination with other risk factors previously described.
Data such as the above have prompted the question of whether treatment dose anticoagulation should be considered for all critically ill COVID-19 patients, particularly if they have pre-existing risk factors for thrombosis. Anecdotally, some UK hospital trusts are giving increased doses of prophylactic low-molecular-weight heparin for patients with COVID-19 who require intensive care admission, or even treatment dose anticoagulation. However, there are no national or international guidelines at the time of writing.
Bilateral ischaemic stroke is a relatively uncommon presentation in the general population and is usually the result of a cardio-embolic cause such as atrial fibrillation, recent myocardial infarction or coronary valve disease.10 While this patient had pre-existing risk factors for ischaemic stroke (type 2 diabetes mellitus, advanced age), her admission ECG showed sinus rhythm and she had no known history of cardiac disease with no cardiac murmurs on auscultation.
The standard of care for patients who are diagnosed with an ischaemic stroke would usually involve initiation of secondary prevention such as statins, in addition to further investigation of the underlying cause of the stroke, for example with carotid artery Doppler ultrasonography, transthoracic echocardiography and 24-hour ECG recordings. Additionally, they would be continuously monitored for any change in neurology in the initial period in case neurosurgical intervention was required. These measures did not happen in this case due to the extensive nature of the infarcts and the patient’s poor functional status, as this would not have changed the clinical outcome. Therefore, although it is not possible to know the precise aetiology of this patient’s strokes, the severity of the infarcts and their bilateral nature could in this case point towards an alternative precipitating factor such as a hypercoagulable state.
This was a difficult diagnosis to make based on her clinical features alone. Timely diagnosis of a new vascular event such as we have described may be even more difficult in patients with COVID-19 who require intubation and ventilation. This requires sedation, which prohibits elements of a complete neurological examination including GCS, power and sensation. In patients with COVID-19 where new neurological abnormalities are noted, it is vital to have a high index of suspicion for intracranial events and to arrange urgent imaging of the brain to investigate this. An even lower threshold for intracranial imaging may be prudent in patients who are more difficult to assess, such as those under sedation or with other causes of impaired consciousness, to ensure that a cerebrovascular event is not missed.
To date we have found one other paper documenting cases of bilateral infarcts in three patients with COVID-19,11 in whom D-dimer was significantly elevated and who all had pre-existing risk factors for stroke. It is important to note that there is a paucity of high-level evidence regarding macrovascular complications in COVID-19, and that further work is required in this area.
Learning points.
Although the exact pathophysiological mechanism is not known, patients with coronavirus disease 2019 (COVID-19) are at increased risk for macrovascular complications including deep vein thromboses, pulmonary emboli and arterial pathology including myocardial infarction and stroke, the latter of which has been demonstrated by this case.
While delirium is common in the critically ill, it is important to maintain a high index of suspicion for other causes of reduced consciousness in those patients with COVID-19, including cerebrovascular complications.
Imaging of the central nervous system should be considered promptly in the evaluation of new neurological abnormalities in patients with COVID-19, particularly in critically ill patients on mechanical ventilation who are more difficult to assess comprehensively. Focal neurological signs may be more difficult to detect in these patients due to the effects of sedation.
Although there is a paucity of high-level evidence, national consensus regarding thromboprophylaxis in COVID-19 patients based on emerging data and expert opinion would still be beneficial. This would standardise care and could potentially reduce the incidence of COVID-19-related vascular complications.
Acknowledgments
We are grateful to Dr M Ziaullah Khan for helping us to communicate with the patient’s next of kin in the process of obtaining consent and explaining the article and its figures.
Footnotes
IZBM and LB contributed equally.
Contributors: IZBM was involved in the care of the patient, and drafted the initial case report. LB drafted the discussion section. SM was the consultant in charge of her care at the time of the events and provided oversight and input into discussion and flow of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.World Health Organisation Coronavirus disease (COVID-19) pandemic. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Accessed Apr 2020].
- 2.Magro C, Mulvey JJ, Berlin D, et al. . Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020;220:1–13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciceri F, Beretta L, Scandroglio AM, et al. . Microvascular COVID-19 blood vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip M, van der Meer NJM, et al. . Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Li M, Wang M, et al. . Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 2020. 10.1136/svn-2020-000431. [Epub ahead of print: 02 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HJ, Cho HJ, Kim Y-D, et al. . Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol 2009;16:582–8. 10.1111/j.1468-1331.2009.02538.x [DOI] [PubMed] [Google Scholar]
- 7.Mao L, Wang M, Chen S, et al. . Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. SSRN Journal 2020. 10.2139/ssrn.3544840 [DOI] [Google Scholar]
- 8.Chen T, Wu D, Chen H, et al. . Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, Li D, Wang X, et al. . Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev 2010;6:150–61. 10.2174/157340310791658730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Xiao M, Zhang S, et al. . Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]


