Supplemental Digital Content is available in the text
Keywords: aprepitant, chemotherapy-induced nausea and vomiting, meta-analysis, systematic review
Abstract
Objective:
To systematically evaluate the efficacy and safety of antiemetic regimen with aprepitant in the prevention of chemotherapy-induced nausea and vomiting (CINV) and provide updated information for clinical practice.
Methods:
Pubmed, Embase, the Cochrane Library, and 3 Chinese literature databases were systematically searched. Randomized controlled trials comparing standard regimen (5-hydroxytryptamine-3 receptor antagonist and glucocorticoid) with aprepitant triple regimen (aprepitant plus the standard regimen) for preventing CINV were screened. Literature selection, data extraction, and quality evaluation were performed by 2 reviewers independently. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in the meta-analysis using RevMan 5.3 software.
Results:
A total of 51 randomized controlled trials were finally included in the systematic review. Compared with the standard regimen, the aprepitant triple regimen significantly improved the complete response in the overall (OR 1.88, 95% CI 1.71–2.07), acute (OR 1.96, 95% CI 1.65–2.32) and delayed (OR 1.96, 95% CI 1.70–2.27) phases, regardless of emetogenic risk of chemotherapy. Aprepitant could also significantly enhance the proportions of patients who have no emesis, nausea, or use of rescue medication respectively in the overall, acute and/or delayed phases. Aprepitant was found to be associated with decreased risk of constipation (OR 0.85, 95% CI 0.74–0.97), but increased the incidence of hiccup (OR 1.26, 95% CI 1.05, 1.51). There were no statistically significant differences between the 2 groups on other safety outcomes.
Conclusion:
The aprepitant triple regimen is effective for the prevention of CINV in patients being treated with moderately or highly emetogenic chemotherapy, and has a significant tendency to reduce the risk of constipation and increase the incidence of hiccup.
1. Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a series of common adverse reactions during chemotherapy, happening in 70% to 80% of treated patients.[1–3] CINV can be classified into acute (within the first 24 hours after chemotherapy initiation) and delayed (24 to 120 hours post-chemotherapy) events. Nausea and vomiting can reduce patients’ quality of life and treatment compliance, increase their fear for treatment, and even result in discontinuation of the anti-tumor therapy.
Patients receiving highly emetogenic chemotherapy (HEC, eg, anthracycline and cyclophosphamide [AC] regimen) and moderate emetogenic chemotherapy (MEC, eg, carboplatin or oxaliplatin) are the major populations that suffering CINV,[4] which can be prevented by prophylactic antiemetic agents. Glucocorticoids, most commonly dexamethasone, were first used for treating CINV in the early 1990 s.[5] Thereafter, the addition of 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) showed additional improvement in acute CINV, which acts by blocking the peripheral nervous pathways of gastrointestinal tracts.[6]
Recent studies showed that the combination of the standard regimen (5HT3RA plus glucocorticoid) and neurokinin-1 receptor antagonists (NK-1RAs) could make greater advances in preventing CINV. Currently, the National Comprehensive Cancer Network,[7] the American Society of Clinical Oncology,[8] and the Multinational Association of Supportive Care in Cancer/ European Society of Medical Oncology[9] guidelines endorsed the use of NK-1RAs plus standard regimen in patients receiving HEC for preventing CINV. However, the American Society of Clinical Oncology and Multinational Association of Supportive Care in Cancer/ European Society of Medical Oncology guidelines did not recommend the addition of NK-1RA for MEC patients, while the National Comprehensive Cancer Network guideline recommended that an NK-1RA should be added to the standard regimen for select patients with additional risk factors or previous treatment failure with standard regimen alone. As for the Chinese guideline,[10] NK-1RA was recommended to be selectively used in part of MEC patients.
Aprepitant is the first NK-1RA approved by the U.S. Food and Drug Administration for the prevention and treatment of CINV. In China, it's officially approved only for the prevention of CINV in HEC patients. There are some published systematic reviews[11–13] on the prevention of CINV by aprepitant plus standard regimen. However, they primarily focused on a specific chemotherapy regimen, age group, or emetogenic risk group. Our research group previously performed a comprehensive evaluation of aprepitant on both HEC and MEC patients, regardless of chemotherapy regimen and age.[14] A number of additional clinical trials investigating the correlations between aprepitant use and CINV prevention had been published and therefore a more comprehensive analysis was allowed. Thus we conducted an updated systematic evaluation to evaluate the efficacy and safety of antiemetic regimen with aprepitant in preventing CINV, so as to provide state-of-the-art evidence for clinical decision making.
2. Methods
This systematic review and meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions[15] and is presented per the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline.[16] It was registered on the International Prospective Register for Systematic Reviews (No. CRD 42019120574). This article was based on previously conducted studies and did not contain any studies with human participants or animals performed by any of the authors.
2.1. Search strategy and selection criteria
We systematically searched PubMed, Embase, the Cochrane Library, and 3 Chinese databases (China National Knowledge Infrastructure, Wangfang, and Chinese Biomedical Literature Database). The brand and generic drug names “aprepitant OR Emend” were used as search terms. Our previous evaluation searched the literature from inception to August 2015, so database search was limited between August 2015 and June 2018 for the current study. A manual search of reference lists of relevant reviews was additionally performed.
Two authors (TQ and PM) carried out the literature screening independently. The research questions and eligibility criteria for the systematic review conformed to the PICOS (participants, interventions, comparators, outcomes, and study design) approach. Studies meeting the following criteria were considered for inclusion:
-
(1)
Participants: malignant tumor patients who received HEC or MEC.
-
(2)
Interventions: aprepitant plus 5HT3RA and glucocorticoid (i.e. the aprepitant triple regimen) for the prevention of CINV.
-
(3)
Comparators: 5HT3RA and glucocorticoid (i.e. the standard regimen) for the prevention of CINV, with or without a placebo.
-
(4)
Outcomes:
Efficacy: complete response (CR, defined as no emesis and no use of rescue medication) in the overall (0 to 120 hours), acute and delayed phases; the proportion of patients who have no emesis, nausea, or use of rescue medication in the phases above; the Functional Living Index-Emesis (FLIE) score;
Safety: incidence of ≥1 adverse event, serious adverse event, discontinuation due to adverse events, febrile neutropenia, asthenia/fatigue, constipation, diarrhea, nausea, headache, hiccup, neutropenia, and anorexia.
-
(5)
Study design: randomized controlled trial (RCT).
Reviews, editorials, guidelines, and case reports were excluded. Considering the basic requirement of the publication quality, Chinese RCTs that not published on Source Journals for Chinese Scientific and Technical Papers and Citations were excluded.
2.2. Data extraction and quality assessment
Data extraction was collected and arranged by researchers using a collection form. Study and patients characteristics, intervention details, as well as outcome measures mentioned earlier, were extracted. Corresponding authors were contacted for data not available within studies, or when outcomes were presented in an unsuitable format for data synthesis. The data was first extracted by 2 authors and cross-checked.
Two authors independently assessed the quality of included studies. Discrepancies were resolved by discussion or through consultation with the third reviewer. The potential risks of bias in RCTs were assessed according to the criteria developed using the Cochrane risk of bias tool.
2.3. Statistical analyses
Outcomes were pooled using Review Manager 5.3 software (RevMan, Cochrane, London). Dichotomous data were presented as odds ratios (ORs). For continuous data, estimates were pooled using the inverse variance methodology to calculate weighted mean differences. All results were estimated from each study with 95% confidence intervals (CIs). Heterogeneity was assessed using the chi-square test and the I2 statistic. If I2 was no more than 50%, a fixed-effect model with the Mantel-Haenszel method was used; otherwise, the random-effect model was adopted. If the extracted data was not sufficient for a quantitative meta-analysis, a narrative approach was conducted to summarize the study-specific results. Potential publication biases of CR in the overall, acute and delayed phases as primary outcomes were assessed by drawing funnel plots.
To explore any potential risk factors that might affect the CR results, 4 prespecified subgroup analyses were conducted, according to patient's age, 5HT3RA treatment time length, race, emetogenic risk of chemotherapy and whether glucocorticoid dose was adjusted in the intervention group.
3. Results
3.1. Baseline characteristics and risk of bias
The selection process for articles included in the systematic review is shown in Supplemental Digital Content (Figure S1). From the 1625 citations identified by the literature searching and from other sources, 25 trials[17–41] met the inclusion criteria for inclusion. A total of 51 RCTs[17–67] were finally included in the systematic review.
A total of 11217 patients were enrolled. According to the emetogenic risk of chemotherapy, patients in 30 trials received HEC, 17 received MEC, 3 received HEC and MEC, while 1 trial did not report. Most of the patients in the intervention groups were treated with aprepitant for 3 days. See Supplemental Digital Content (Table S1) for detailed information.
The risk of bias summary is shown in Supplemental Digital Content (Figure S2), and an assessment of the risk of bias for each of the studies selected is shown in Supplemental Digital Content (Figure S3). Random sequence generation was adequate in 35 trials, and allocation concealment was adequately described in 11 trials. 4 trials were considered to be at high risk of performance and detection bias. All studies were judged to be at low risk of attrition, reporting, and other bias.
3.2. Prevention of CINV
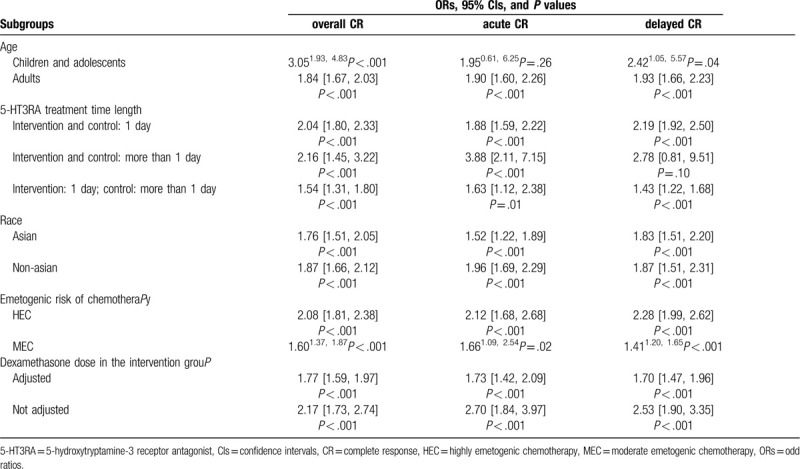
Compared with the standard regimen, the aprepitant triple regimen significantly improved CR in the overall (OR 1.88, 95% CI 1.71 –2.07, P < .001), acute (OR 1.96, 95% CI 1.65 –2.32, P < .001) and delayed (OR 1.96, 95% CI 1.70 –2.27, P < .001) phases [Supplemental Digital Content (Figure S4)]. The results of the subgroup analyses were shown in Table 1. Aprepitant triple regimen still retained the advantages against the standard regimen on CR in most subgroups. Compared with the standard regimen, the aprepitant triple regimen significantly improved the proportion of patients who have no emesis event during the overall (OR 2.50, 95% CI 2.16 –2.91, P < .001), acute (OR 2.09, 95% CI 1.76 – 2.49, P < .001), and delayed phases (OR 2.49, 95% CI 2.14–2.89, P < .001). It significantly improved the proportion of patients who have no nausea event during the overall (OR 1.53, 95% CI 1.13 c1.75, P < .001) and delayed phases (OR 1.50, 95% CI 1.30 – 1.74, P < .001), but not in the acute phase (OR 1.18, 95% CI 0.97–1.43, P = .09). The aprepitant triple regimen significantly improved the proportion of patients who have no use of rescue medication in the overall phase (OR 1.45, 95% CI 1.26∼1.68, P < .001), while data for acute and delayed phases was not reported. Significant publication biases of primary outcomes (CR in the overall, acute or delayed phases) were not found by drawing funnel plots [see Supplemental Digital Content (Figure S5)].
Table 1.
Subgroup analyses for complete response.
3.3. FLIE score
A total of 8 studies reported the results of the FLIE score.[23,33,34,49,50,57,59,65] Meta-analysis was not performed due to the inconsistency among reporting types of the results. The FLIE scores of the aprepitant triple regimen were higher than those of the standard regimen, and the FLIE questionnaire results showed that significantly more patients in the aprepitant group reported minimal or no impact of CINV on daily life, compared with patients in the control groups.
3.4. Safety outcomes
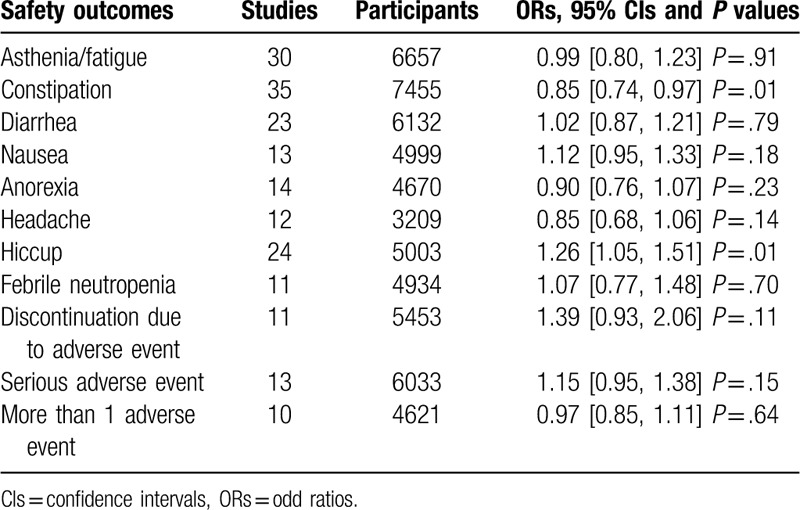
There were no significant differences between the aprepitant triple regimen and standard regimen for most outcomes (Table 2), except for constipation and hiccup. The aprepitant group had a significant tendency to reduce the risk of constipation (OR 0.85, 95% CI 0.74–0.97, P = .01) and increase the incidence of hiccup (OR 1.26, 95% CI 1.05–1.51, P = .01).
Table 2.
Meta-analysis results of safety outcomes.
4. Discussion and conclusion
The primary efficacy results of our systematic review and meta-analysis were consistent with previous studies. Compared to the standard regimen, the aprepitant triple regimen significantly improved the CR in the overall, acute, and delayed phases. Regardless of the emetogenic risk of chemotherapy, the aprepitant triple regimen consistently improved the prevention of CINV in the overall, acute, and delayed phases. This provides evidence for the recommended aprepitant for cancer patients receiving MEC.
The results of subgroup analyses enriched the knowledge of aprepitant treatment. The OR estimates of CR in the children and adolescents subgroups were larger than those in the adult group. A previous study has shown that younger patients had a higher risk of CINV.[68] The results suggested that the benefit of aprepitant combination in children and adolescents may be more remarkable; however, the result in the acute phase was not statistically significant. Also, when 5HT3RA was used for more than 1 day in both the aprepitant and standard regimen groups, aprepitant significantly improved CR in the overall and the acute phases, but not in the delayed phase. These results may be partially explained by the relatively small population size of the available trials. Meanwhile, the mechanisms of CINV in acute or delayed phases are quite different. Serotonin mediates the early CINV process that occurs within 8 to 12 hours following chemotherapy, after which time substance P acting at NK receptors becomes the dominant mediator of vomiting.[69]
The first and second generations 5HT3RAs are proved to be associated with a small number of patients experiencing mild headache, diarrhea, or constipation.[70] Our result showed that the addition of aprepitant was able to lower the risk of constipation. Hiccups are often observed in patients treated with cisplatin-based chemotherapy. A previous cluster analysis[71] indicated that aprepitant was not a major risk factor for the onset of hiccups. However, the meta-analysis showed an opposite result. More large-scale trials are still required to further investigate the issue.
However, there are still some limitations in this study. Firstly, a few trials included in the meta-analysis were found to have a possible unclear or high risk of bias in some domains. This potential bias may reduce the credibility of the corresponding results. Interpretations of these findings must be made with caution. Secondly, the adverse events were generally not reported as primary endpoints in the included RCTs. Definitions of adverse events used in the included RCTs were not specifically stated, which could mean some inconsistency across studies. Also, our analyses were based on trial-level, rather than patient-level, data. Detailed information, such as the exact time of constipation occurrence, or any dechallenges or rechallenges, was not reported.
In conclusion, aprepitant triple regimen not only effectively improves the prevention of CINV in both highly and MEC patients, but also reduces the incidence of constipation. However, more attention to the increased risk of hiccup should be paid.
Author contributions
Conceived and designed the study: XC SZ TQ PM XX. Performed the study: TQ PM. Analyzed the data: TQ PM. Wrote the paper: TQ PM. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 5-HT3RA = 5-hydroxytryptamine-3 receptor antagonist, CINV = chemotherapy-induced nausea and vomiting, CIs = confidence intervals, CR = complete response, FLIE = functional living index-emesis, HEC = highly emetogenic chemotherapy, MEC = moderate emetogenic chemotherapy, NK-1RA = neurokinin-1 receptor antagonists, ORs = odd ratios, RCT = randomized controlled trial.
How to cite this article: Qiu T, Men P, Xu X, Zhai S, Cui X. Antiemetic regimen with aprepitant in the prevention of chemotherapy-induced nausea and vomiting: an updated systematic review and meta-analysis. Medicine. 2020;99:33(e21559).
TQ and PM contributed equally to this work.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- [1].Hesketh PJ. Treatment of chemotherapy-induced emesis in the 1990 s: impact of the 5-HT3 receptor antagonists. Support Care Cancer 1994;2:286–92. [DOI] [PubMed] [Google Scholar]
- [2].De Boer-Dennert M, de Wit R, Schmitz PI, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 1997;76:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Passik SD, Kirsh KL, Rosenfeld B, et al. The changeable nature of patients’ fears regarding chemotherapy: implications for palliative care. J Pain Symptom Manage 2001;21:113–20. [DOI] [PubMed] [Google Scholar]
- [4].Navari RM. Management of chemotherapy-induced nausea and vomiting:Focus on newer agents and new uses for older agents. Drugs 2013;73:249–62. [DOI] [PubMed] [Google Scholar]
- [5].Grunberg SM, Slusher B, Rugo HS. Emerging treatments in chemotherapy-induced nausea and vomiting. Clin Adv Hematol Oncol 2013;11: 2 suppl 1: 1–8. [PubMed] [Google Scholar]
- [6].Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT(3) and tachykinin NK(1) receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2012;684:1–7. [DOI] [PubMed] [Google Scholar]
- [7].National Comprehensive Cancer Network. Antiemesis 2019;1:2019. [Google Scholar]
- [8].Hesketh PJ, Bohlke K, Lyman GH, et al. Antiemetics: American Society of Clinical Oncology Focused Guideline Update. J Clin Oncol 2016;34:381–6. [DOI] [PubMed] [Google Scholar]
- [9].Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27: suppl 5: v119–33. [DOI] [PubMed] [Google Scholar]
- [10].Yu SY, Yin JL, Qin SK, et al. Tumor treatment related vomiting prevention guide (2014 edition). Chin Clin Oncol 2014;19:263–73. [Google Scholar]
- [11].Di Maio M, Baratelli C, Bironzo P, et al. Efficacy of neurokinin-1 receptor antagonists in the prevention of chemotherapy-induced nausea and vomiting in patients receiving carboplatin-based chemotherapy: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;124:21–8. [DOI] [PubMed] [Google Scholar]
- [12].Chapell R, Aapro MS. Efficacy of aprepitant among patients aged 65 and over receiving moderately to highly emetogenic chemotherapy: a meta-analysis of unpublished data from previously published studies. J Geriatr Oncol 2013;4:78–83. [DOI] [PubMed] [Google Scholar]
- [13].Abdel-Rahman O. Neurokinin-1 inhibitors in the prevention of nausea and vomiting from highly emetogenic chemotherapy: a network meta-analysis. Ther Adv Med Oncol 2016;8:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang MM, Xu XH, Zhai SD. Health technology assessment on aprepitant to control chemotherapy induced nausea and vomiting. China Health Insurance 2016;11:50–6. [Google Scholar]
- [15].Higgins J, Green SE (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at: http://handbook-5-1.cochrane.org/ Accessed 22 November 2017. [Google Scholar]
- [16].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].An N, Lan H, Zhang M, et al. Clinical research on aprepitant foe the prevention of nausea and vomiting due to EP regimen chemotherapy in small-cell lung cancer patients. Med J West china 2018;30:242–5. [Google Scholar]
- [18].Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 2014;25:1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kusagaya H, Inui N, Karayama M, et al. Evaluation of palonosetron and dexamethasone with or without aprepitant to prevent carboplatin-induced nausea and vomiting in patients with advanced non-small-cell lung cancer. Lung Cancer 2015;90:410–6. [DOI] [PubMed] [Google Scholar]
- [20].Yahata H, Kobayashi H, Sonoda K, et al. Efficacy of aprepitant for the prevention of chemotherapy-induced nausea and vomiting with a moderately emetogenic chemotherapy regimen: a multicenter, placebo-controlled, double-blind, randomized study in patients with gynecologic cancer receiving paclitaxel and carboplatin. Int J Clin Oncol 2016;21:491–7. [DOI] [PubMed] [Google Scholar]
- [21].Kim JE, Jang JS, Kim JW, et al. Efficacy and safety of aprepitant for the prevention of chemotherapy-induced nausea and vomiting during the first cycle of moderately emetogenic chemotherapy in Korean patients with a broad range of tumor types. Support Care Cancer 2016;25:1–9. [DOI] [PubMed] [Google Scholar]
- [22].Bubalo J, Mulverhill K, Meyers G, et al. A randomized, placebo-controlled pilot trial of aprepitant combined with standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting in patients undergoing cyclophosphamide-based conditioning regimens prior to hematopoietic stem cell transplant (HSCT). Bone Marrow Transplant 2018;53:1010–8. [DOI] [PubMed] [Google Scholar]
- [23].Ishido K, Higuchi K, Azuma M, et al. Aprepitant, granisetron, and dexamethasone versus palonosetron and dexamethasone for prophylaxis of cisplatin-induced nausea and vomiting in patients with upper gastrointestinal cancer. Anticancer Drugs 2016;27:884–90. [DOI] [PubMed] [Google Scholar]
- [24].Aridome K, Mori SI, Baba K, et al. A phase II, randomized study of aprepitant in the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapies in colorectal cancer patients. Mol Clin Oncol 2016;4:393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaushal P, Atri R, Soni A, et al. Comparative evaluation of triplet antiemetic schedule versus doublet antiemetic schedule in chemotherapy-induced emesis in head and neck cancer patients. Ecancermedicalscience 2015;9:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sugimori Y, Ota T, Ujihira T, et al. A phase II randomised study to evaluate the efficacy of aprepitant plus palonosetron for preventing delayed-phase CINV associated with TC therapy in gynaecological cancer. J Obstet Gynaecol Res 2017;43:1454–9. [DOI] [PubMed] [Google Scholar]
- [27].Song Z, Wang H, Zhang H, et al. Efficacy and safety of triple therapy with aprepitant, ondansetron, and prednisone for preventing nausea and vomiting induced by R-CEOP or CEOP chemotherapy regimen for non-Hodgkin lymphoma: a phase 2 open-label, randomized comparative trial. Leuk Lymphoma 2017;58:816–21. [DOI] [PubMed] [Google Scholar]
- [28].Guo XY, Zheng RJ, Li JY, et al. Effect analysis of Aprepitant for chemotherapy patients with lung cancer. Clin Pulm Med 2015;20:2153–6. [Google Scholar]
- [29].Jiang M, Chi F, Zeng Y. Efficacy of aprepitant in treatment of chemotherapy-induced nausea and vomiting in patients with advance breast cancer. Clinical Misdiagnosis and Mistherapy 2017;30:95–8. [Google Scholar]
- [30].Jiang M, Chi F, Wu R. Efficacy of aprepitant in preventing chemotherapy-induced nausea and vomiting in patients with breast cancer. Practical Pharmacy And Clinical Remedies 2017;20:293–7. [Google Scholar]
- [31].Kang YX, Min J, Zhang DW, et al. Clinical observation of Aprepitantcombined with Tropisetron in the prevention of nausea and vomiting caused by high vomiting chemotherapy regimen. Modern Oncol 2016;24:1926–48. [Google Scholar]
- [32].Meng N, Zhu Q, Li YM, et al. Clinical effect of aprepitant on chemotherapy-induced nausea and vomiting patients with lung cancer. Guangxi Medical Journal 2018;40:112–5. [Google Scholar]
- [33].Meng WJ, Wang X, Jia YS, et al. Efficacy and safety evaluation on aprepitant in prevention of nausea and vomiting induced by highly emetogenic chemotherapy. J Jilin Univ (Med Edit) 2016;42:331–5. [Google Scholar]
- [34].Pan GH, Xu Y, Hu SS, et al. Clinical observation of triple therapy with aprepitant, ondansetron and dexamethasone on prevention of adjuvant chemotherapy induced nausea and vomiting for post-operative patients with rectal carcinoma. Chin J Pharmacoepidemiol 2017;11:725–8. [Google Scholar]
- [35].Qi J. Aprepitant as secondary prevention in moderate-severe vomiting caused by AC chemotherapy for breast cancer. J Pharm Pract 2017;35:158–60. [Google Scholar]
- [36].Su J, Luo Y, Zhu YH, et al. Clinical observation of aprepitant combined with tropisetron and dexamethasone in the prevention of vomiting induced by cisplatin chemotherapy. J Hunan Normal Univ (Med Sci) 2016;13:60–3. [Google Scholar]
- [37].Tian X, Wu LN, Hu TY, et al. Clinical research on aprepitant for the prevention of nausea and vomiting due to paclitaxeland cisplatin chemotherapy in lung adenocarcinoma patients. Practical Pharmacy And Clinical Remedies 2016;19:1152–5. [Google Scholar]
- [38].Tian X, Xuan Y, Hu TY, et al. Clinical observation of NKl receptor antagonist aprepitant for the prevention of nausea and vomiting induced by pf chemotherapy regimen with head and neck cancer. J Med Res 2016;45:49–53. [Google Scholar]
- [39].Yan Y, Ren Y. The observation of multipathway blocking the effect of nausea and vomiting induced by chemotherapy in breast cancer. Mondrn Oncol 2017;25:68–71. [Google Scholar]
- [40].Yu HQ, Chen HY, Ren RM, et al. Therapeutic effect of aprepitant triple regimen on patients receiving high emetogenic chemotherapy. Chin J Integr Med 2016;25:274–6. [Google Scholar]
- [41].Zhao GF, Xiong J, Ye T, et al. Clinical observation of aprepitant in the antiemetic treatment of doxorubicin and ifosfamide regimen induced vomiting. J Clin Oncol 2017;23:802–6. [Google Scholar]
- [42].Albany C, Brames MJ, Fausel C, et al. Randomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a Hoosier Oncology Group study. J Clin Oncol 2012;30:3998–4003. [DOI] [PubMed] [Google Scholar]
- [43].Bakhshi S, Batra A, Biswas B, et al. Aprepitant as an add-on therapy in children receiving highly emetogenic chemotherapy: a randomized, double-blind, placebo-controlled trial. Support Care Cancer 2015;23:3229–37. [DOI] [PubMed] [Google Scholar]
- [44].Campos D, Pereira JR, Reinhardt RR, et al. Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol 2001;19:1759–67. [DOI] [PubMed] [Google Scholar]
- [45].Chawla SP, Grunberg SM, Gralla RJ, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer 2003;97:2290–300. [DOI] [PubMed] [Google Scholar]
- [46].De Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol 2003;21:4105–11. [DOI] [PubMed] [Google Scholar]
- [47].Gore L, Chawla S, Petrilli A, et al. Aprepitant in adolescent patients for prevention of chemotherapy-induced nausea and vomiting: a randomized, double-blind, placebo-controlled study of efficacy and tolerability. Pediatr Blood Cancer 2009;52:242–7. [DOI] [PubMed] [Google Scholar]
- [48].Herrstedt J, Muss HB, Warr DG, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer 2005;104:1548–55. [DOI] [PubMed] [Google Scholar]
- [49].Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin - The Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112–9. [DOI] [PubMed] [Google Scholar]
- [50].Hu Z, Cheng Y, Zhang H, et al. Aprepitant triple therapy for the prevention of chemotherapyinduced nausea and vomiting following highdose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trial. Support Care Cancer 2014;22:979–87. [DOI] [PubMed] [Google Scholar]
- [51].Ito Y, Karayama M, Inui N, et al. Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer 2014;84:259–64. [DOI] [PubMed] [Google Scholar]
- [52].Kang HJ, Loftus S, Taylor A, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting in children: a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:385–94. [DOI] [PubMed] [Google Scholar]
- [53].Maehara M, Ueda T, Miyahara D, et al. Clinical efficacy of aprepitant in patients with gynecological cancer after chemotherapy using paclitaxel and carboplatin. Anticancer Res 2015;35:4527–34. [PubMed] [Google Scholar]
- [54].Nasu R, Nann ya Y, Kurokawa M. A randomized controlled study evaluating the efficacy of aprepitant for highly/moderately emetogenic chemotherapies in hematological malignancies. Int J Hematol 2015;101:376–85. [DOI] [PubMed] [Google Scholar]
- [55].Navari RM, Reinhardt RR, Gralla RJ, et al. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist.L-754,030 Antiemetic Trials Group. N Engl J Med 1999;340:190–5. [DOI] [PubMed] [Google Scholar]
- [56].Nishimura J, Satoh T, Fukunaga M, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled phase 3 trial. Eur J Cancer 2015;51:1274–82. [DOI] [PubMed] [Google Scholar]
- [57].Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 2003;97:3090–8. [DOI] [PubMed] [Google Scholar]
- [58].Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer 2010;18:423–31. [DOI] [PubMed] [Google Scholar]
- [59].Schmitt T, Goldschmidt H, Neben K, et al. Aprepitant, granisetron, and dexamethasone for prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: results of a randomized, placebo-controlled phase III trial. J Clin Oncol 2014;32:3413–20. [DOI] [PubMed] [Google Scholar]
- [60].Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in highdose cisplatin treatment. Ann Oncol 2006;17:1000–6. [DOI] [PubMed] [Google Scholar]
- [61].Stiff PJ, Fox-Geiman MP, Kiley K, et al. Prevention of nausea and vomiting associated with stem cell transplant: results of a prospective, randomized trial of aprepitant used with highly emetogenic preparative regimens. Biol Blood Marrow Transplant 2013;19:49–55. [DOI] [PubMed] [Google Scholar]
- [62].Svanberg A, Birgegård G. Addition of aprepitant (Emend) to standard antiemetic regimen continued for 7 days after chemotherapy for stem cell transplantation provides significant reduction of vomiting. Oncology 2015;89:31–6. [DOI] [PubMed] [Google Scholar]
- [63].Takahashi T, Hoshi E, Takagi M, et al. Multicenter, phase II, placebo-controlled, doubleblind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci 2010;101:2455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tanioka M, Kitao A, Matsumoto K, et al. A randomised, placebo-controlled, double-blind study of aprepitant in nondrinking women younger than 70 years receiving moderately emetogenic chemotherapy. Br J Cancer 2013;109:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005;23:2822–30. [DOI] [PubMed] [Google Scholar]
- [66].Yeo W, Mo FK, Suen JJ, et al. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Res Treat 2009;113:529–35. [DOI] [PubMed] [Google Scholar]
- [67].Ding RM, Wang P, Tian Y, et al. Clinical observation of aprepitant on nausea and vomiting caused by FAC chemotherapy regimen for breast cancer. Chin J Diffic and Compl Cas 2015;14:45–8. [Google Scholar]
- [68].Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Supportive Care 2011;19:807–13. [DOI] [PubMed] [Google Scholar]
- [69].Hesketh PJ, Van Belle S, Aapro M, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer 2003;39:1074–80. [DOI] [PubMed] [Google Scholar]
- [70].Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf 2016;15:343–56. [DOI] [PubMed] [Google Scholar]
- [71].Asano H, Watanabe M, Kawaguchi A, et al. A search for the risk factors for hiccups and evaluation of antiemetic therapy in CDDP-based chemotherapy, using cluster analysis. Gan To Kagaku Ryoho 2013;40:1031–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


