Abstract
To investigate the estimated glomerular filtration rates of chronic hepatitis B (CHB) patients with or without liver cirrhosis, and to explore the related risk factors.
A total of 559 CHB patients were enrolled. Liver cirrhosis was diagnosed with ultrasound. The Child-Pugh scoring system was used to stage patients with liver cirrhosis. The Modification of Diet in Renal Disease (MDRD) formula was used to calculate the estimated glomerular filtration rate (eGFR).
A total of 296 patients were involved. The results showed that the incidence of renal impairment in patients with liver cirrhosis was 8.45% (25/296). The incidence of renal impairment in Child-Pugh C patients was significantly higher than that in Child-Pugh B and Child-Pugh Grade A patients (i.e., 17.2% [17/99] vs 6.67% [7/105] vs 1.09% [1/92], respectively, P < .001); age, hyperuricemia, and Child-Pugh score are all risk factors for impaired renal function.
With the deterioration of liver function in patients with cirrhosis, the incidence of impaired renal function has increased significantly, and renal function should be closely monitored to guide patients in clinical medication.
Keywords: Child-Pugh score, cirrhosis, estimated glomerular filtration rate, hepatitis B virus, renal function impairment
1. Introduction
Chronic hepatitis B (CHB) remains a global health burden.[1] It is reported that an estimated 240 million persons worldwide are chronically infected with hepatitis B virus (HBV), placing them at increased risk of developing cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).[1,2] The Asia-Pacific region, including China, is a high-risk area for HBV infection.[3] Studies showed that the HBV surface antigen (HBsAg) carrier rate in the general population in China was 7.18%. There are ∼93 million people with chronic HBV infection in China currently.[4]
In addition to causing liver damage such as chronic hepatitis and cirrhosis, HBV can also affect renal function through immune-mediated mechanisms.[5] HBV-related glomerular diseases occurring in patients with CHB are important causes of end-stage renal disease and renal replacement therapy.[6–8] Renal insufficiency is common in patients with HBV-related liver cirrhosis, and renal function impairment accompanied by hemodynamic changes will further exacerbate the decline in glomerular filtration rate, form hepatorenal syndrome, and increase patient mortality.[5,9–11] Studies found that among patients with CHB, the estimated glomerular filtration rates (eGFR) are significantly decreased.[12–14] However, until now, the difference in eGFR between CHB patients and CHB patients with cirrhosis is still unknown. There is also no study report on eGFR distribution in adult newly treated CHB patients with liver cirrhosis and the related risk factors.
Therefore, this study retrospectively analyzed the clinical data of CHB patients and CHB-related cirrhosis patients. The study aimed to evaluate the difference between CHB patients and CHB-cirrhosis patients, and to analyze the risk factors affecting renal function.
2. Subjects and methods
2.1. Subjects
This study included CHB patients and HBV-related cirrhosis patients from January 1, 2015 to December 31, 2018. The enrollment criteria included: Subjects must
-
1.
be aged over 18 years,
-
2.
have hepatitis B surface antigen (HBsAg) positive for more than 6 months,[15,16]
-
3.
not have received any anti-HBV treatment before.
Diagnosed with CHB related cirrhosis based on medical history and abdominal ultrasound. Conversely, patients were excluded when they met the following criteria:
-
1.
evidence of co-infection with hepatitis C, hepatitis D, or human immunodeficiency virus,
-
2.
have autoimmune liver disease, and
-
3.
heavy alcohol consumption or alcohol abuse, defined as alcohol consumption of >10 g/day.[17]
The Institutional Review Board approved the study. Each enrolled patient provided written informed consent.
2.2. Patient information collection
Patient information was extracted from medical records, including demographic data, diagnosis of type 2 diabetes (T2DM), hyperuricemia, and kidney stones, which may be related to eGFR decline.[18–20] Data on liver function, HBV DNA quantification, and HBV serological marker were also collected. The unit of serum creatine is umoI/L, and the unit of ALT is U/L. Patients were classified according to the Child-Pugh score classification criteria: A score of 5 to 6 points was classified as A, 7 to 9 points was classified as B, and a score ≥10 points was classified as C.[21] HBV DNA was quantified using fluorescent quantitative PCR detection, HBV serological markers were applied using ELISA.
2.3. Renal function assessment
The GFR was estimated according to the MDRD formula recommended by the American Kidney Foundation, which is listed below: eGFR (mL/min/1.73 m2) = 175∗(serum creatinine)−1.154∗(age)−0.203∗(0.742 if female).
2.4. Statistical methods
The data were analyzed using the SPSS software (version 19; SPSS Inc, Chicago, IL). The comparison of variables was performed using the chi-square test or Student's t test when appropriate. Multivariate logistic regression analysis was used to analyze the risk factors of renal impairment. Values of P < .05 (two-sides) indicates statistical difference.
3. Results
3.1. Patient demographics
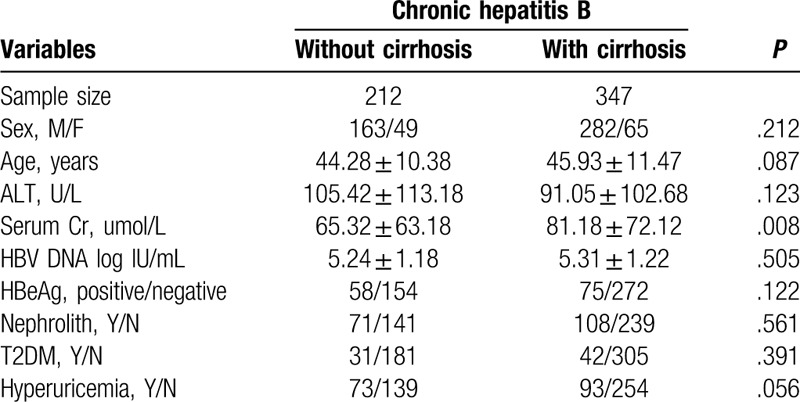
A total of 559 patients were involved. Among them, 212 were CHB patients without cirrhosis and 347 of them were CHB-related cirrhosis patients. The sex, age, ALT level, HBV DNA level, HBeAg status, proportion of nephrolith, T2DM, and hyperuricemia were similar for the two groups. Serum creatinine (Cr) was significantly higher in CHB-related cirrhosis patients (P = .008), as shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics by groups.
3.2. Renal function impairment among CHB patients
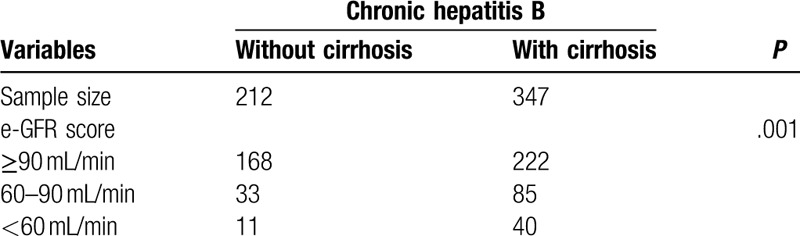
Among the 559 patients involved, we analyzed the eGFR level between these two groups. The results are shown in Table 2. A total of 33 (15.6%) CHB patients had eGFR of 60–90 mL/min while 11 (5.2%) patients had eGFR < 60 mL/min. In CHB-related cirrhosis patients, 85 (24.5%) patients had eGFR of 60 to 90 mL/min and 40 (11.5%) had eGFR < 60 mL/min. CHB-related cirrhosis patients showed a more severe renal function impairment (P = .001).
Table 2.
Proportion of renal function by groups.
3.3. Risk factors related to impaired renal function in all patients involved
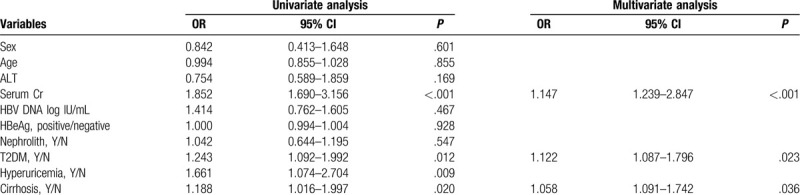
Univariate and multivariate analyses were conducted to explore the independent factors that affected decreased eGFR. The univariate analysis results showed that serum Cr, T2DM, hyperuricemia, and liver cirrhosis were the factors associated with the decrease in eGFR in CHB patients. However, multivariate analysis suggested that serum Cr (P < .001), T2DM (P = .023), and liver cirrhsosis (P = .036) were the independent factors that affected the decreased eGFR in CHB patients (Table 3).
Table 3.
Risk factors associated with impaired renal function in CHB.
3.4. Patient demographics among CHB-related cirrhosis patients
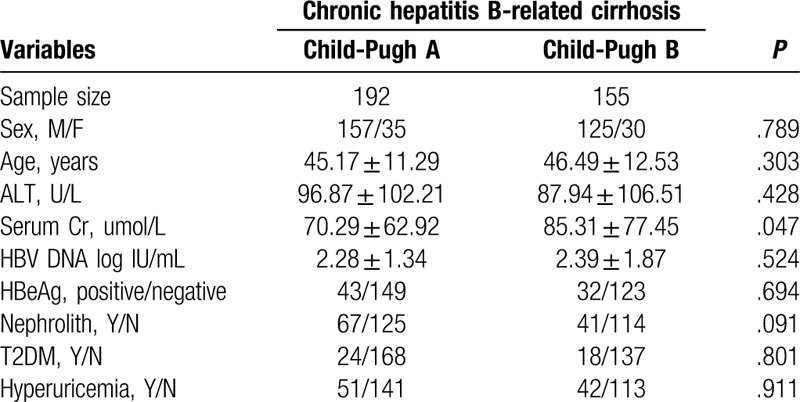
To further evaluate the association between liver cirrhosis and renal function in CHB patients, we compared the differences in patients’ demographics in CHB-related cirrhosis patients. Among the 347 patients with cirrhosis, 192 staged with Child-Pugh A and 155 staged with Child-Pugh B. Serum creatinine (Cr) was significantly higher in Child-Pugh B patients (P = .047) as shown in Table 4.
Table 4.
Baseline demographic and clinical characteristics by groups.
3.5. Renal function in patients with different Child-Pugh stage
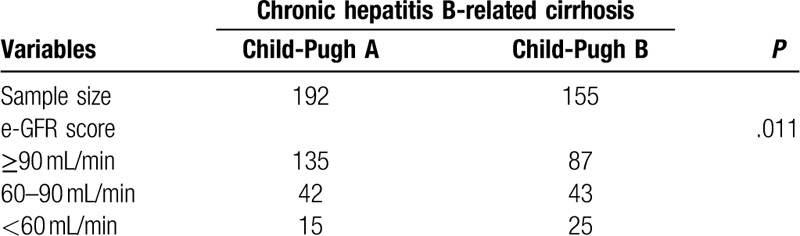
The incidence of impaired renal function was different in patients with different Child-Pugh stage. In patients with Child-Pugh A, 42 had eGFR 60 to 90 mL/min and 15 had eGFR < 60 mL/min, while in Child-Pugh B, 43 patients who had eGFR 60 to 90 mL/min and 25 had eGFR < 60 mL/min (P = .011) as shown in Table 5.
Table 5.
Proportion of renal function by groups.
3.6. Risk factors related to impaired renal function in liver cirrhosis patients
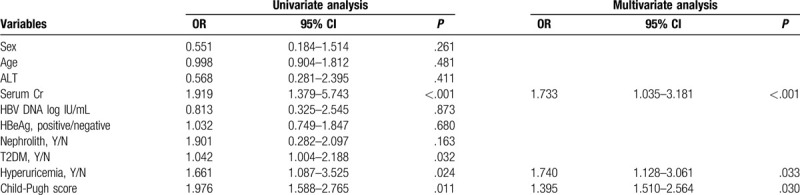
Univariate and multivariate analyses were conducted to explore the independent factors that affected decreased eGFR in CHB-related cirrhosis patients. The multivariate analysis suggested that serum Cr (P < .001), hyperuricemia (P = .033) and Child-Pugh score (P = .030) were the independent factors that affected the decreased eGFR in CHB-related cirrhosis patients (Table 6).
Table 6.
Risk factors associated with impaired renal function in CHB-related cirrhosis.
4. Discussion
In HBV infected patients, kidney damage can be caused by circulating immune complexes and in situ immune complexes.[22] Studies from Hong Kong found that 29% of HBV-associated nephritis developed into renal failure.[23,24] HBV-related liver cirrhosis is the end stage of liver disease.[25] Liver cirrhosis and the accompanying portal hypertension can lead to changes in systemic hemodynamics and damage to renal function.[25,26] Early renal damage is a functional change rather than an organic change, and timely treatment can reverse renal damage. Hence, the early identification of patients with renal impairment using risk factors could be an effective way for timely treatment. However, changes in renal function and the risk factors associated with decreased eGFR have not been reported yet. In our study, we found significantly more patients with impaired renal function in CHB-related cirrhosis compared with CHB patients. Moreover, among CHB-related cirrhosis patients, renal impairment was more severe in Child-Pugh B patients compared with Child-Pugh A patients. Multivariate analysis suggested that serum Cr, T2DM, and liver cirrhosis were the independent factors that affected decreased eGFR in CHB patients, while for patients with cirrhosis, serum Cr, hyperuricemia, and Child-Pugh score were the independent factors that affected decreased eGFR in cirrhosis patients.
An epidemiological survey showed that eGFR ≤60 mL/min accounted for 1.70% in Chinese population,[19] and there is no report on the decline of glomerular filtration rate in special populations (such as patients with HBV-related cirrhosis). Our study showed that among the 296 patients with newly diagnosed liver cirrhosis, the proportion of patients with impaired renal function was higher than that of the general population. The treatment guideline recommends that once a cirrhosis patient is diagnosed, the use of antiviral drugs should be determined.[27] However, oral anti-HBV drugs are excreted through the kidneys, so patients should be tested for eGFR before oral anti-HBV treatment.[5]
Currently, the antiviral drugs widely used in China's antiviral therapy include lamivudine (LAM), adefovir dipivoxil (ADV), telbivudine (LDT), entecavir (ETV), and tenofovir disoproxil (TDF). Previous study on the effects of long-term treatment with nucleoside drugs on renal function have shown differences between different drugs.[5] A cohort study reported a significantly more eGFR <50 mL/min in the ADV treatment group than in the non-ADV group.[26] Other reports on LDT showed that it was possible to improve renal function in patients, but the specific mechanism had not been elucidated.[5] Therefore, for patients with CHB-related cirrhosis, even those with asymptomatic compensatory cirrhosis, the renal safety of antiviral drugs should be considered when selecting antiviral drugs. CHB patients may develop renal impairment through three mechanisms. First, HBV infection may cause kidney function changes through the deposition of immune complexes, such as membranous nephropathy and vascular membrane capillary nephritis. Secondly, patients with end-stage CHB disease and liver cirrhosis often have renal impairment, which can be due to many reasons, including insufficient renal perfusion due to lack of effective circulating blood volume. Third, since all currently available nucleoside drugs are eliminated in an unaltered form mainly by renal, these drugs are believed to be related to dose-dependent nephrotoxicity, especially nucleotide analogs.
A number of studies analyzing chronic kidney disease (CKD)-related risk factors have suggested that age, gender, hypertension, diabetes, hyperuricemia, renal cysts, and kidney stones are CKD-related risk factors.[18,20] Our study suggested serum Cr, T2DM, and liver cirrhosis were the independent factors that affected decreased eGFR in CHB patients, while for patients with cirrhosis, serum Cr, hyperuricemia, and Child-Pugh score were the independent factors that affected the decreased eGFR in cirrhosis patients. Hyperuricemia was a risk factor for impaired renal function, which was consistent with previous reports. In addition, this study suggests that Child-Pugh classification is also a risk factor for impaired renal function in patients with liver cirrhosis, suggesting that as liver disease progresses, renal function damage also gradually increases.
This study had some limitations. All the patients were from one medical center, a prospective multicenter study is thus necessary. In our study, we failed to explore further the reasons why hyperuricemia and liver cirrhosis contributed to the decreased eGFR. We believe that a well-designed controlled study is needed to answer these questions. Another potential limitation is that only HBV-related patients were included in our study. The value of our work will be higher if included results from non-HBV related cirrhosis as well. In order to explore the effect of HBV on renal function, a study of cirrhosis with different etiology is needed.
In summary, this study evaluated the eGFR levels in CHB and CHB-related cirrhosis patients. Based on the results, it is recommended that renal function should be reviewed regularly in this group of people to avoid the use of drugs with potential nephrotoxicity, and to adjust antiviral drugs according to renal function in a timely manner. This is especially important for hyperuricemia patients and high Child-Pugh score patients.
Author contributions
XXXX.
Footnotes
Abbreviations: ADV = adefovir dipivoxil, CHB = chronic hepatitis B, CKD = chronic kidney disease, Cr = creatinine, eGFR = estimated glomerular filtration rate, ETV = entecavir, HBsAg = HBV surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, LAM = lamivudine, LDT = telbivudine, MDR = modification of diet in renal disease, T2DM = type 2 diabetes, TDF = tenofovir disoproxil.
How to cite this article: Wang D, Yan X, Zhang M, Ren C, Wang L, Ma J, Guo L. Association between liver cirrhosis and estimated glomerular filtration rates in patients with chronic HBV infection. Medicine. 2020;99:33(e21387).
DW and XY contributed equally to this work.
The authors have no funding information to disclose.
The authors have no financial or personal relationships with other people or organizations that could inappropriately influence this work.
Compliance with Ethical standards.
Ethical approval: The Institutional Review Board of Sixth People's Hospital had approved this study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent: Informed consent was obtained from all patients for inclusion in the study.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chinese Society Of Hepatology CMA, Chinese Society Of Infectious Diseases CM. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Chin J Hepatol 2015;23:888–905. [DOI] [PubMed] [Google Scholar]
- [5].Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J 2016;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amarapurkar DN, Patel N. Increased eGFR with telbivudine in combination therapy of chronic hepatitis B infection. Indian J Gastroenterol 2014;33:89–91. [DOI] [PubMed] [Google Scholar]
- [7].Gupta A, Quigg RJ. Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis 2015;22:343–51. [DOI] [PubMed] [Google Scholar]
- [8].Wang Y, Liu C, Hong S, et al. Effect of recombinant hepatitis B virus on human glomerular mesangial cell apoptosis. Biotechnol Biotechnol Equip 2014;28:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parlati L, Laurain A, Pol S. Epidemiology update for HCV and HBV in ESRD in France: still a lot to do. Liver Int 2017;37:815–6. [DOI] [PubMed] [Google Scholar]
- [10].Shi X, Zhu P, Yan G, et al. Clinical characteristics and long-term outcome of acute kidney injury in patients with HBV-related acute-on-chronic liver failure. J Viral Hepat 2016;23:920–9. [DOI] [PubMed] [Google Scholar]
- [11].Trinh S, Le AK, Chang ET, et al. Changes in renal function in patients with chronic HBV infection treated with Tenofovir Disoproxil Fumarate vs Entecavir. Clin Gastroenterol Hepatol 2019;17:948–56. [DOI] [PubMed] [Google Scholar]
- [12].Mauss S, Berger F, Filmann N, et al. Effect of HBV polymerase inhibitors on renal function in patients with chronic hepatitis B. J Hepatol 2011;55:1235–40. [DOI] [PubMed] [Google Scholar]
- [13].Gane EJ, Deray G, Liaw YF, et al. Telbivudine improves renal function in patients with chronic hepatitis B. Gastroenterology 2014;146:138–46. [DOI] [PubMed] [Google Scholar]
- [14].Yapali S, Lok AS. Potential benefit of telbivudine on renal function does not outweigh its high rate of antiviral drug resistance and other adverse effects. Gastroenterology 2014;146:15–9. [DOI] [PubMed] [Google Scholar]
- [15].Cai S, Yu T, Jiang Y, et al. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med 2016;16:429–36. [DOI] [PubMed] [Google Scholar]
- [16].Zheng Z, Liao W, Liu L, et al. Effect of nucleos(t)ide analogue on serum HBsAg level in chronic hepatitis B patients: a 3-years study. Biomed Pharmacother 2020;122:109698. [DOI] [PubMed] [Google Scholar]
- [17].Cai S, Ou Z, Liu D, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United Eur Gastroenterol J 2018;6:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weiner DE, Tighiouart H, Elsayed EF, et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol 2008;19:1204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–22. [DOI] [PubMed] [Google Scholar]
- [20].Chen N, Wang W, Huang Y, et al. Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant 2009;24:2117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- [22].Appel G. Viral infections and the kidney: HIV, hepatitis B, and hepatitis C. Cleve Clin J Med 2007;74:353–60. [DOI] [PubMed] [Google Scholar]
- [23].Tang S, Lai FM, Lui YH, et al. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int 2005;68:1750–8. [DOI] [PubMed] [Google Scholar]
- [24].Tang S, Lai KN. Hepatitis B-related membranous nephropathy should be treated with a specific anti-viral agent. Kidney Int 2006;70:818. [DOI] [PubMed] [Google Scholar]
- [25].Zheng C, Yan H, Zeng J, et al. Comparison of pegylated interferon monotherapy and de novo pegylated interferon plus tenofovir combination therapy in patients with chronic hepatitis B. Infect Drug Resist 2019;12:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ha NB, Ha NB, Garcia RT, et al. Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology 2009;50:727–34. [DOI] [PubMed] [Google Scholar]
- [27].European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]


