Abstract
Selective serotonin reuptake inhibitors (SSRIs), commonly used to treat depression, are associated with loss of motivation, anergy, and lack of curiosity often referred collectively as apathy. However, this association has not been systematically assessed using a specific rating scale for measuring apathy syndrome. Our objective was to study the association between SSRI use and apathy syndrome.
We conducted a retrospective chart review of 125 patients enrolled in an outpatient psychiatry clinic. The prevalence of apathy syndrome and its clinical significance (based on standardized assessment) were compared between patients treated and not treated with SSRIs. Apathy was assessed using the Apathy Evaluation Scale-clinician version with a score ranging 18–72 with higher score for worse apathy. A score of greater than 30 is considered clinically significant apathy.
Among 119 patients, the mean apathy scores were significantly higher in those treated with SSRIs compared to those not treated with SSRIs (42.5 ± 9.2 vs 31.3 ± 6, P < .0001). The SSRI group also had a significantly higher percentage of patients with clinically significant apathy (92% vs 61%, P < .0001). Use of all SSRIs was associated with the presence of apathy. Apathy was seen in all mental health diagnostic categories with highest Apathy evaluation scale-clinician version scores in those with dementia.
SSRI use may be associated with higher rates of apathy syndrome. Clinicians should specifically inquire about iatrogenic apathy syndrome when evaluating patients on an SSRI if there is suspicion of loss of motivation. Limitations of this study included retrospective nature of this study, and that majority of the sample was males. Prospective studies are needed to elucidate information regarding the prevalence, etiology, and treatment response for SSRI-associated apathy syndrome.
Keywords: sertraline, antidepressants, apathy, citalopram, selective serotonin reuptake inhibitors
1. Introduction
It is being increasingly realized that apathy and depression are distinct and separable entities which may respond differently to different medications.[1,2] Apathy syndrome is a primary loss of motivation independent of any intellectual impairment, emotional distress, or decreased consciousness.[3] Oftentimes apathy goes unnoticed because of the patient's indifference toward his or her situation. The presence of apathy has been well described in patients with major neurocognitive disorders, schizophrenia and major depressive disorder. Extensive literature exists on negative symptoms of schizophrenia and a recent study (N = 1208) with patients from the European Schizophrenia Cohort adds to the evidence that depression may not mediate the negative symptoms.[4] Apathy symptoms, such as loss of interest, psychomotor retardation, anergy, and lack of insight may mistakenly be attributed to depressive disorders.[5] Apathy has a direct impact on the functional status of patients even after controlling for the effect of depression. In 1 study, patients with apathy were 3.2 times more likely to have deficits in activities of daily living when compared to those without apathy.[6] Apathy may also hinder compliance with treatment recommendations. Our group found that the presence of apathy syndrome even in the absence of depression, was linked with poor compliance with self-care for diabetes and poor glycemic control.[7] Several teams including ours have found that the treatment of apathy is distinct from the treatment of depression.[8–10] Furthermore, presence of apathy increases caregiver burden.[11] Thus, it is important to screen for apathy in clinical settings.
Selective serotonin reuptake inhibitors (SSRIs) are usually considered first-line treatment for depression. Their use is preferred over other agents due to their relatively favorable safety profile and ease of administration. However, decreased emotional range, loss of motivation, loss of intellectual curiosity, all components of the apathy syndrome, have been reported as potential adverse effects of these medications.[12–16] The association of apathy with SSRI use is increasingly being recognized to be a class effect, to be quick in onset, reversible with discontinuation of SSRIs in some cases and to be irrespective of age of the patients or the indication for the use of SSRIs. In a sample of 181 patients with Parkinson's disease, use of SSRIs but no other anti-depressants were associated with apathy indicating the class effect of these medications. The association was seen even after controlling for depressive symptoms, age, education, gender, and use of other psychotropic agents.[17] The onset of apathy with SSRI use may be very quick. In study of healthy volunteers, 1 week of citalopram use has been linked with reduction in activity in the reward networks of ventral striatum and ventral medial/orbitofrontal cortex.[18] SSRI-associated apathy is seen in patients with various diagnoses including depression, panic disorder, and obsessive-compulsive disorder which suggests that the association is due to the medication rather than the diagnosis.[13] Bolling et al conducted semi-structured telephone interviews of patients who completed a trial of antidepressants and found that nearly 20% of the 161 subjects complained of “apathy” and “loss of creativity” though no specific assessment of apathy was performed.[19] Although most of these reports are in young adults, Wongpakaran et al reported such association in geriatric patients. In a post-hoc analysis of a hospital database apathy sub-scales were derived from the Geriatric Depression Scale and Hamilton Depression Scales by adding items pertinent to apathy.[20] They separated 384 subjects into 2 groups based on SSRI use and found that apathy was more prevalent for patients taking SSRIs than not taking SSRIs.[20]
None of these studies to date have used validated rating scales to specifically assess apathy associated with SSRI use. The Apathy Evaluation Scale-Clinician version (AES-C) is an 18-item scale that is able to capture apathy as a syndrome and differentiate it from depression.[21] The purpose of the current retrospective chart review was to use a validated apathy scale to investigate an association, if any, between apathy and the use of SSRI medications. We also wanted to test if the association of SSRI use with apathy differed by the specific SSRI. We hypothesized that sertraline may be least likely to be associated with apathy due to its dopaminergic actions.
2. Methods
Approval from the Institutional Review Board was obtained to query a clinical database incorporating validated evidence-based outcome measures for this chart review. Charts from 125 consecutive patients from the Mental Health Clinic, Geriatric Psychiatry Clinic, and the Recovery Center at the University affiliated Veterans Affairs Medical center were reviewed. Demographic data including the age, mental health diagnoses, and medications were recorded. When there were more than 1 mental health diagnoses, 1 primary diagnosis was picked for the purposes of this paper, based on the presenting symptoms in the index visit and the overall burden caused by the diagnosis. Although a rating scale for severity of depression, was not obtained in this sample, among those with MDD, severity of current depression episode was captured from medical records. Patients were grouped into those being treated with SSRIs and those not being treated with these agents.
Apathy syndrome was ascertained from the scores on the AES-C.[20] The AES-C is an 18-item scale that assesses a patient's initiative, motivation, persistence and other aspects of apathy, all of which are rated on a 4-point Likert scale. AES-C captures the behavioral, cognitive and emotional aspects of apathy. Total scores range from 18 to 72 with higher scores indicating worse apathy. Three items have negative syntax to ensure validity of responses. The AES-C has been tested in various neurodegenerative disorders and has good internal consistency (coefficient alpha = .86), and test-retest reliability (r = .76).[21] The clinician-administered version is known to reliably differentiate apathy from depression.[21] A score greater than 30 on the AES-C is generally considered to indicate clinically significant apathy.[21]
Analysis of variance was performed to compare the AES-C scores in both groups. Chi-Square analysis was used to compare the percentage of patients in each group with clinically significant apathy (AES-C > 30). The Chi-Square test was also performed on the number of patients using specific SSRIs. The Fisher Exact test was used when N ≤ 5 in any cell.
3. Results
Of the 125 patient records reviewed, 6 were excluded for missing information about their diagnoses and/or medications. The mean age of the 119 patients included in the analysis was 55.8 ± 13.6 (Standard Deviation) years and the mean AES-C score was 37.13 ± 9.5. Major depressive disorder was the most common diagnosis in the sample followed by bipolar disorder and schizophrenia (Table 1). Sixty-two patients (52%) were being treated with SSRIs. Patients not being treated with SSRIs were younger than those being treated with SSRIs, but the difference was not statistically significant [(54.2 ± 11.5) vs (57.2 ± 15.4)] (Table 1). The SSRI group had a trend towards higher proportion of major depressive disorder than the non-SSRI group (42% vs 26%, P < .07), as well as having a significantly lower proportion of patients with schizophrenia (11% vs 30%, P < .01) (Table 1). The SSRI group had significantly higher scores on the AES-C (42.5 ± 9.2 vs 31.3 ± 6, P < .0001) as well as a significantly higher percentage of patients with clinically significant apathy (AES-C scores > 30) (92% vs 61%, P < .0001) than the non-SSRI group. The AES-C scores were analyzed by individual SSRIs. Number of patients receiving different types of SSRIs are listed in Table 2. All 5 agents were associated with high scores on the AES-C ranging from 35 ± 6.4 for escitalopram group to 45.6 ± 9.4 with fluoxetine group (Table 2). Most of the patients being treated with these agents had clinically significant apathy ranging from 75% in the escitalopram group to 100% with paroxetine and fluoxetine (Fig. 1). Clinically significant apathy was noted across all psychiatric diagnoses with highest scores noted in patients with dementia, followed by anxiety disorders, and major depressive disorder (Table 2). The mean AES-C score was lower in the MDD-severe (N = 29) (38.4 ± 9.8) compared to group with MDD-moderate and mild combined (N = 12) (41.3 ± 9.3) (p = 0.39). The percentage of clinically significant apathy was 100% in those with dementia, followed by anxiety (93%) and MDD (80%). The mean apathy score was the least in patients with chronic paranoid schizophrenia (35.6 ± 8.3). In this subgroup, there was no statistically significant difference in apathy scores between those on SSRIs compared to those not on SSRIs (P = .54).
Table 1.
Demographic and diagnostic data for mental health clinic patients.
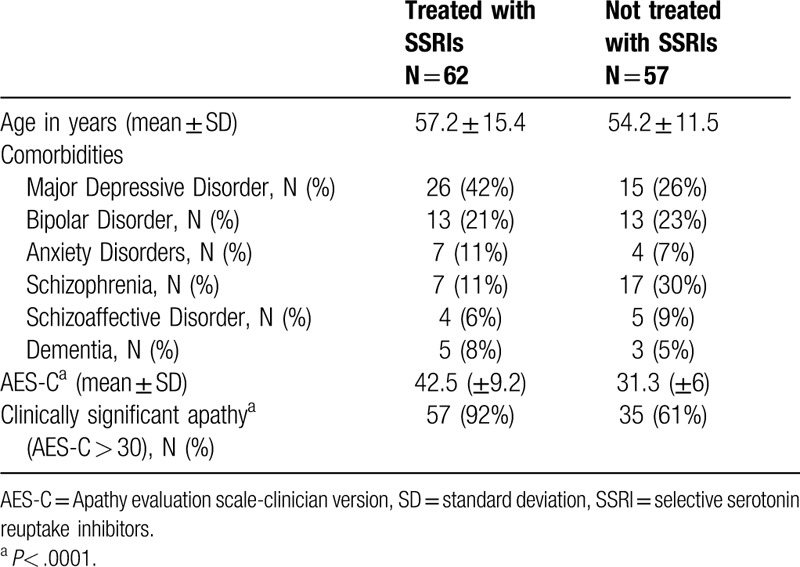
Table 2.
Apathy scores per individual SSRI agent and comorbidities.
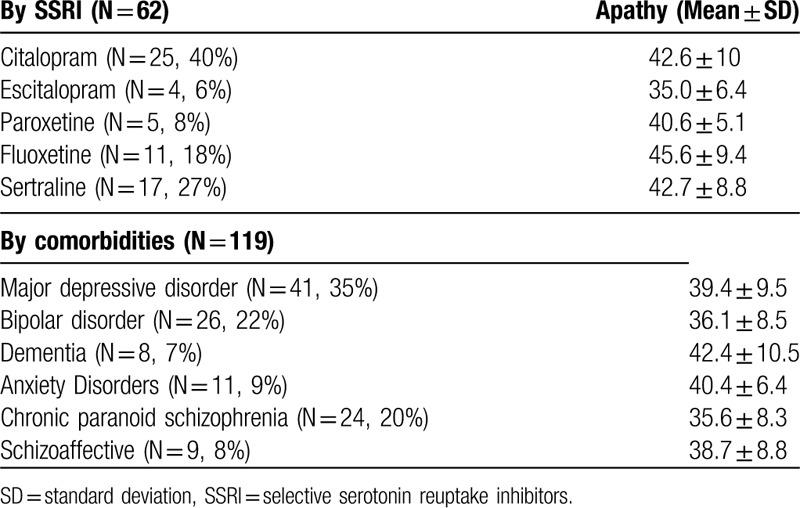
Figure 1.
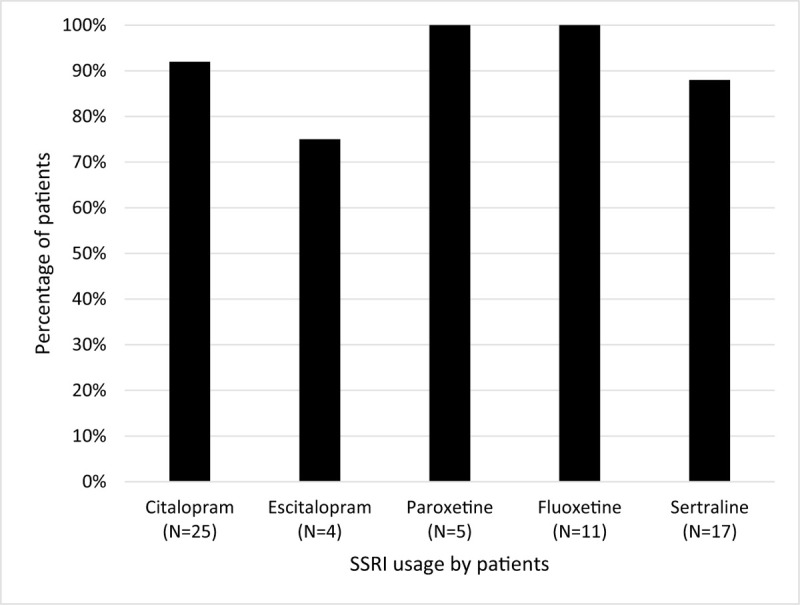
Percentage of patients with clinically significant Apathy by each SSRI. SSRI = selective serotonin reuptake inhibitors.
4. Discussion
In the present study, patients being treated with SSRIs had higher apathy scores than those who were not taking SSRIs. Most of the patients being treated with SSRIs (92%) had clinically significant apathy suggesting that the high scores on the AES-C were not influenced by few outliers. This supports the previous reports of apathy syndrome being associated with the use of SSRIs.[12–19] Our study is differentiated from the previous studies on apathy as we assessed apathy as a syndrome rather than a symptom. We also used a validated rating scale specifically designed to differentiate apathy syndrome from depression. The fact that high scores on AES-C were seen with all SSRIs in this report suggests an association of apathy syndrome with the SSRIs as a class. It is possible that the higher proportion of MDD in the SSRI group could in part explain the association with apathy. While some of the association is explained by the overlap of symptomatology between depression and apathy, the difference in apathy scores between the groups may not be explained completely by the phenomenological overlap. For instance, a difference of 3.3 points on AES-C is typically considered a clinically meaningful difference and, in this sample, the SSRI group had scored more than 10 points greater than the non-SSRI group.[8] The current literature of SSRI induced apathy is dominated mainly by case reports, and our study confirms the findings of SSRI induced apathy with a large sample size relative to the existing research.
We hypothesized that sertraline with some mild dopamine reuptake inhibition properties in addition to SSRI activity may be less likely to be associated with high apathy scores; however, we did not find this to be true in our population. Apathy may result from a number of different etiologies. Studies of patients following brain injury demonstrate that apathy results from damage to medial prefrontal, anterior cingulate, and anterior temporal paralimbic areas such as the amygdale.[22] Strokes involving the posterior limb of the internal capsule can also cause apathy.[23] Apathy is a frequent behavioral disorder in Alzheimer's dementia (AD). Single photon emission computed tomography studies of AD patients revealed decreased perfusion in anterior regions in apathetic patients, and in posterior regions of patients without apathy.[24] These findings support the suggestion that the neural mechanisms of apathy involve the brainstem and forebrain circuits that mediate goal-directed behavior.
Dopaminergic abnormality is also known to cause apathy. Apathy syndrome has been reported after damage to the anterior cingulate cortex which receives extensive dopaminergic input from the ventral tegmental area. Hoehn-Saric et al offer a plausible model suggesting dual impact of SSRIs on the frontal cortex, 1 via serotonergic system and other by the indirect modulation of the midbrain dopaminergic systems projecting to the prefrontal cortex.[15] This SSRI-induced hypodopaminergic state leads to symptoms of apathy. Furthermore, dopaminergic agents, such as methylphenidate, have been reported to treat apathy independent of depression.[9,10,25,26] A similar mechanism could explain the pathophysiology of SSRI-associated extrapyramidal symptoms.
In 1 case report of SSRI (fluoxetine) induced apathy, a decrease in blood flow in the frontal lobes was noted along with worsening of apathy. These findings were reversed with discontinuation of fluoxetine.[15] SSRI-induced apathy thus appears to be dose and duration dependent, as well as reversible.[12–16] Our team published a report supporting the reversibility of the SSRI associated apathy syndrome with either discontinuation or lowering the dosage of the agent.[27]
The limitations of this study include but are not limited to, the retrospective nature of this study, lack of blinding of the reviewers to SSRI status, lack of an assessment of confounding psychosocial factors, substance use factors or an assessment of ongoing and worsening medical illness. The differences in the diagnostic categories, such as more affective disorders in the SSRI group, may have had an impact on the apathy scores. However, in the non-SSRI group, apathy scores were low despite having higher proportion of patients with schizophrenia known for negative symptoms. Interestingly, in the sub-analysis of patients with schizophrenia, no statistically significant association was found between apathy and SSRI use perhaps owing to the small number of patients on SSRIs in this sub-sample. We could not analyze gender effects in our sample as only 8 participants were women owing to the setting of the Department of Veterans Affairs. Prevalence of apathy in aging men is an important finding as it may be associated with declining testosterone.[28] Despite these limitations, our data suggests that the SSRIs may be related to the presence of apathy. Other studies suggest apathy improves after the discontinuation of SSRIs or augmentation with dopaminergic agents such as bupropion. Prospective studies are needed to identify the effects of SSRIs in the onset and worsening of apathy in patients with depression and other psychiatric diagnoses.
5. Conclusions
This study indicates that there is an association between use of SSRIs and co-occurring apathy syndrome. It further validates that the association may be true for all SSRIs and may be seen in various mental health conditions. Prospective studies are needed to elucidate information regarding whether development of Apathy Syndrome is causally related to starting therapy with an SSRI, as well as the prevalence, etiology, and treatment response for SSRI-associated apathy syndrome.
Author contributions
Conceptualization: Prasad R. Padala.
Data analysis: Prasad R. Padala.
Interpretation of the analyses: Kalpana P. Padala, Dennis H. Sullivan.
Investigation: Kalpana P. Padala.
Patient interviews: Prasad R. Padala.
Regulatory approvals: Prasad R. Padala.
Validation: Anusha S. Majagi.
Writing – original draft: Prasad R. Padala, Kalpana P. Padala, Anusha S. Majagi, Kimberly K. Garner, Richard A. Dennis, Dennis H. Sullivan.
Footnotes
Abbreviations: AES-C = Apathy evaluation scale-clinician version, SSRI = selective serotonin reuptake inhibitors.
How to cite this article: Padala PR, Padala KP, Majagi AS, Garner KK, Dennis RA, Sullivan DH. Selective serotonin reuptake inhibitors-associated apathy syndrome: a cross sectional study. Medicine. 2020;99:33(e21497).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998;10:314–9. [DOI] [PubMed] [Google Scholar]
- [2].Padala PR, Petty F, Bhatia SC. Methylphenidate may treat apathy independent of depression. Ann Pharmacother 2005;39:1947–9. [DOI] [PubMed] [Google Scholar]
- [3].Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry 1990;147:22–30. [DOI] [PubMed] [Google Scholar]
- [4].Carrà G, Crocamo C, Bartoli F, et al. The mediating role of depression in pathways linking positive and negative symptoms in schizophrenia. A longitudinal analysis using latent variable structural equation modelling. Psychol Med 2020;50:566–74. [DOI] [PubMed] [Google Scholar]
- [5].Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. J Affect Disord 1993;28:117–24. [DOI] [PubMed] [Google Scholar]
- [6].Freels S, Cohen D, Eisdorfer C, et al. Functional status and clinical findings in patients with Alzheimer's disease. J Gerontol 1992;47:M177–82. [DOI] [PubMed] [Google Scholar]
- [7].Padala PR, Desouza CV, Almeida S, et al. The impact of apathy on glycemic control in diabetes: a cross-sectional study. Diabetes Res Clin Pract 2008;79:37–41. [DOI] [PubMed] [Google Scholar]
- [8].Lanctot KL, Chau SA, Herrmann N, et al. Effect of methylphenidate on attention in apathetic AD patients in a randomized, placebo-controlled trial. Int Psychogeriatr 2014;26:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Padala PR, Padala KP, Lensing SY, et al. Methylphenidate for Apathy in Community-Dwelling Older Veterans With Mild Alzheimer's Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Am J Psychiatry 2017;175:159–68. [DOI] [PubMed] [Google Scholar]
- [10].Rosenberg PB, Lanctot KL, Drye LT, et al. Safety and efficacy of methylphenidate for apathy in Alzheimer's disease: a randomized, placebo-controlled trial. J Clin Psychiatry 2013;74:810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thomas P, Clement JP, Hazif-Thomas C, et al. Family, Alzheimer's disease and negative symptoms. Int J Geriatr Psychiatry 2001;16:192–202. [DOI] [PubMed] [Google Scholar]
- [12].Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract 2004;10:196–9. [DOI] [PubMed] [Google Scholar]
- [13].Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child. Adolesc Psychopharmacol 2001;11:181–6. [DOI] [PubMed] [Google Scholar]
- [14].George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette's syndrome and obsessive compulsive disorder. J Clin Psychiatry 1992;53:379–80. [PubMed] [Google Scholar]
- [15].Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry 1991;52:131–3. [PubMed] [Google Scholar]
- [16].Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol 1990;10:343–5. [PubMed] [Google Scholar]
- [17].Zahodne LB, Bernal-Pacheco O, Bowers D, et al. Are selective serotonin reuptake inhibitors associated with greater apathy in Parkinson's disease? J Neuropsychiatry Clin Neurosci 2012;24:326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McCabe C, Mishor Z, Cowen PJ, et al. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 2010;67:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bolling MY, Kohlenberg RJ. Reasons for quitting serotonin reuptake inhibitor therapy: paradoxical psychological side effects and patient satisfaction. Psychother Psychosom 2004;73:380–5. [DOI] [PubMed] [Google Scholar]
- [20].Wongpakaran N, van RR, Wongpakaran T, et al. Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: a case-control study. Ann Gen Psychiatry 2007;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991;38:143–62. [DOI] [PubMed] [Google Scholar]
- [22].Marin RS. Apathy: concept, syndrome, neural mechanisms, and treatment. Semin Clin Neuropsychiatry 1996;1:304–14. [DOI] [PubMed] [Google Scholar]
- [23].Starkstein SE, Fedoroff JP, Price TR, et al. Apathy following cerebrovascular lesions. Stroke 1993;24:1625–30. [DOI] [PubMed] [Google Scholar]
- [24].Benoit M, Koulibaly PM, Migneco O, et al. Brain perfusion in Alzheimer's disease with and without apathy: a SPECT study with statistical parametric mapping analysis. Psychiatry Res 2002;114:103–11. [DOI] [PubMed] [Google Scholar]
- [25].Lanctot KL, Herrmann N, Black SE, et al. Apathy associated with Alzheimer disease: use of dextroamphetamine challenge. Am J Geriatr Psychiatry 2008;16:551–7. [DOI] [PubMed] [Google Scholar]
- [26].Padala PR, Burke WJ, Shostrom VK, et al. Methylphenidate for apathy and functional status in dementia of the Alzheimer type. Am J Geriatr Psychiatry 2010;18:371–4. [DOI] [PubMed] [Google Scholar]
- [27].Padala PR, Padala KP, Monga V, et al. Reversal of SSRI-associated apathy syndrome by discontinuation of therapy. Ann Pharmacother 2012;46:e8. [DOI] [PubMed] [Google Scholar]
- [28].Brodaty H, Altendorf A, Withall A, et al. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. Int Psychogeriatr 2010;22:426–36. [DOI] [PubMed] [Google Scholar]


