Abstract
This study aimed to assess the prevalence and occult rates of uterine leiomyosarcoma (ULMS) in women with smooth-muscle tumors undergoing gynecological surgery. A retrospective study was performed at an academic cancer center from 2008 to 2015. Patients undergoing either hysterectomy or myomectomy via laparoscopic, abdominal, vaginal, and hysteroscopic approaches were identified with the validated pathology diagnosis of either ULMS or leiomyomas. All patients initially operated at our institute were included and reviewed. The prevalence and occult rates of ULMS were calculated and compared between different age groups.
Twenty-eight patients with original ULMS were identified in 9556 gynecological surgeries. The prevalence of overall and occult ULMS in our study was 0.25% (1 in 345 patients) and 0.07% (1 in 1429 patients). The proportion of occult in all ULMSs was 25%. The prevalence rates of overall ULMS were 0.21%, 0.13%, 0.52%, 2.12%, and 6.67% in the 30 to 39, 40 to 49, 50 to 59, 60 to 69, and ≥70-year age groups, respectively. There was a significantly increased risk of ULMS after 50 years of age. The prevalence rates of occult ULMS were 0.05%, 0.08%, and 0.12% for the 30 to 39, 40 to 49, and 50 to 59 year age groups, respectively. There was no statistically significant difference among age the groups. The prevalence of ULMS was 0.41% and 0.16% for solitary and multiple tumor masses, respectively. Patients with solitary uterine tumors were at a significantly increased risk of ULMS (OR = 2.601, 95% CI = 1.108–6.141).
Our retrospective data in part reflects the clinical characteristics of overall and occult ULMS and forms the basis for further prevention of occult ULMS.
Keywords: leiomyosarcoma, myomas, prevalence, uterine neoplasms
1. Introduction
Uterine leiomyosarcoma (ULMS) is a rare smooth-muscle tumor with an annual incidence of only 0.35–0.64 per 100,000 women in the US.[1,2] An epidemiologic study on soft tissue sarcoma (STS) in China reported that 10.9% of all STS cases were located in female genital organs and 5.97% of all STS cases were leiomyosarcoma.[3] The incidence of ULMS was very low, but the proportion of unexpected ULMS was as high as 46% of all ULMS cases.[4] One of the greatest challenges is the differential diagnosis between ULMS and leiomyoma preoperatively because symptoms and signs of both these tumors are similar.[5] Studies have demonstrated that preoperative endometrial sampling, magnetic resonance imaging, and measurements of serum lactate dehydrogenase level may be helpful but still have limitations in making a differential diagnosis.[5,6]
The risk of occult ULMS is between 0.12% and 2.3% for women undergoing minimally invasive hysterectomy or myomectomy for benign indications.[4,7,8] All women with uterine smooth muscle tumors carry the risk of developing ULMS; however, the distribution of occult ULMS throughout overall ULMS, as well as all uterine smooth muscle tumors, is not clearly understood due to limited literature. In this context, the objective of this study was to obtain additional data by calculating the risk of overall and occult ULMS during gynecological procedures, which would improve the accuracy of such models and better inform patients.
2. Patients and methods
This study was approved by the institutional review board of the Fourth Hospital of Hebei Medical University, and the requirement for informed consent was waived due to the retrospective nature of the study. We identified patients diagnosed with smooth-muscle tumors of the uterus, including leiomyosarcoma and leiomyoma (epithelioid, myxoid, cellular, bizarre, angioleiomyoma, and angiomyoleiomyoma) who underwent gynecological surgery between January 2008 and December 2017, using the medical record system. The medical records of all patients were retrieved and reviewed. Patients with a histopathologically confirmed diagnosis of ULMS and leiomyoma, who underwent intimal surgical treatment at our institute, were included. This naturally implies that referral patients after primary tumor resection at other hospitals were excluded.
Cases were classified as occult ULMS if any type of malignancy was neither considered preoperatively nor stated as an indication for surgery. The study was divided into two 5-year periods: 2008 to 2012 and 2013 to 2017. Patients were categorized into 6 10-year age groups: ≤29, 30 to 39, 40 to 49, 50 to 59, 60 to 69, and ≥70 years.
Characteristics of all samples and ULMS were compared between periods and age groups, and the number of tumors using chi-squared or Fisher exact tests for categorical variables and Mann–Whitney U tests for continuous variables. All P-values were two-tailed. All statistical analyses were performed using the SPSS version 25.0 program (SSPS, Inc., Chicago, IL). The level of significance was set at 0.05.
3. Results
From January 2008 to December 2017, 9556 women with ULMS and leiomyoma, aged 44 years (median range 14–92 years) were included in this study. Of these patients, 28 had ULMS with a median age of 52 years (range 34–76 years), and 9528 cases of leiomyoma with a median age of 44 years (range 14–92 years). The age difference between ULMS and leiomyoma patients was statistically significant by the Mann–Whitney U test (Z = -4.630, P < .001).
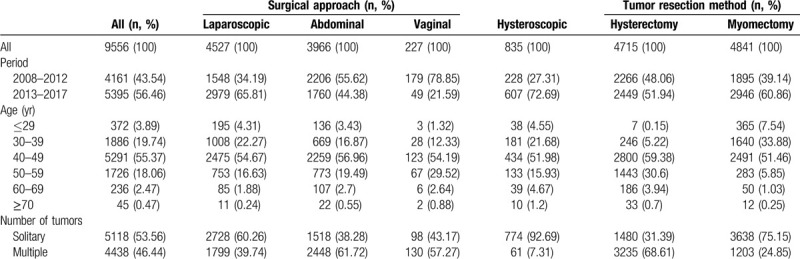
Table 1 shows the differences in patient characteristics among the surgical approaches and tumor resection methods. Minimal invasive surgeries, including laparoscopic and hysteroscopic surgeries, increased from 42.68% during 2008 to 2012 to 66.47% during 2013 to 2018. There was a statistically significant upward trend in the number of women who underwent laparoscopic and hysteroscopic surgeries in the last 5 years (χ2 = 305.81, P < .001, and χ2 = 98.134, P < .001, respectively). In contrast, myomectomy cases showed a significant increase in the last 5 years (χ2 = 77.216, P < .001).
Table 1.
Sample characteristics.
The overall prevalence of ULMS was 0.29% or 1:345 patients and that of occult ULMS was 0.07% (7 patients) or 1:1429 patients. Occult ULMS was observed in 25.00% of all ULMS cases. Table 2 provides details on the characteristics and prevalence rates of ULMS. The prevalence of overall and occult ULMS was 0.34% and 0.12%, respectively, during 2008 to 2012, and was slightly decreased to 0.26% and 0.04%, respectively, during 2013 to 2018. The difference was not statistically significant (χ2 = 0.476, P = .490, and P = .251). The prevalence rates of overall ULMS were 0.21%, 0.13%, 0.52%, 2.12%, and 6.67% for the 30 to 39, 40 to 49, 50 to 59, 60 to 69, and ≥70 age groups, respectively. There was a significant difference among age groups (χ2 = 93.845, P < .001). The prevalence rates of occult ULMS were 0.05%, 0.08%, and 0.12% for the 30 to 39, 40 to 49, and 50 to 59 age groups, respectively. There was no statistically significant difference among age groups.
Table 2.
Characteristics and prevalence of ULMS.
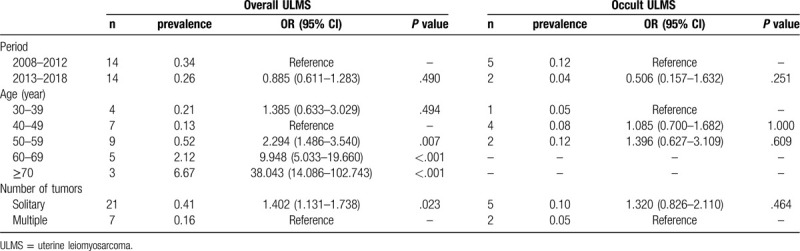
Figure 1 represents the age distribution and prevalence of ULMS. The number of overall ULMS was distributed in a normal pattern between 30 and over 70 years of age with a peak at 50 to 59 years of age. However, the prevalence rates were similar in the age groups of 30 to 39 years and 40 to 49 years, and consistently increased after 50 years of age. The number of occult cases was distributed in a normal pattern between 30 and 59 years of age with a peak at 40 to 49 years of age. The prevalence rates were similar among the three groups. Interestingly, occult ULMS skewed to the left side, which corresponds to younger age of patients and lower prevalence of ULMS. There was no proportional relationship in terms of number or prevalence between overall and occult ULMS. Another interesting finding was that the prevalence of ULMS was 0.41% and 0.16% for solitary and multiple tumor masses, respectively. Patients with solitary masses were associated with a statistically significant increased risk of ULMS (OR = 1.402, 95% CI = 1.131–1.738).
Figure 1.
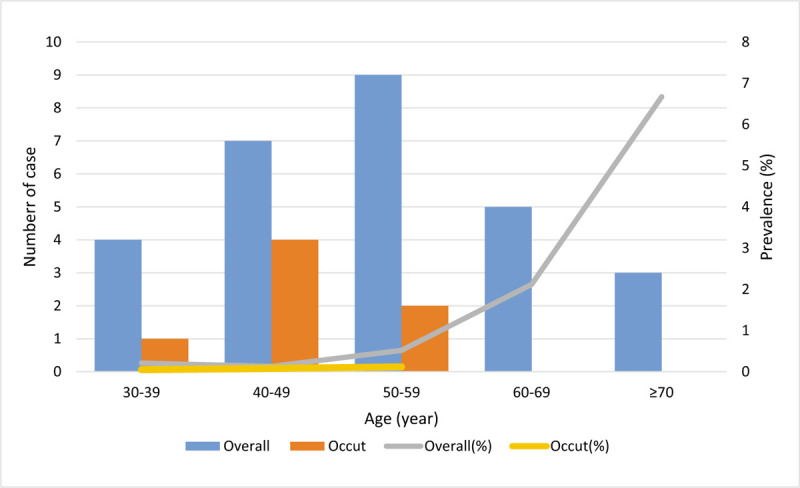
The age distribution and prevalence of ULMS. The occult ULMS distributes in low age groups and there was no overlap between the overall and occult number of ULMS. The prevalence of occult ULMS does not increase with overall prevalence. ULMS = uterine leiomyosarcoma.
4. Discussion
This cohort study evaluated all pathologically validated ULMS cases in our institute from January 2008 to December 2017. In contrast to previous studies that only included laparoscopic surgery or morcellation patients for presumed leiomyoma, our study included all ULMS patients and all types of surgery. The broad inclusion of tumor and surgery types provides a true picture of the relationship between occult and overall ULMS as well as all uterine smooth muscle tumors.
The prevalence rates of overall and occult ULMS were 0.29% or 1:345 patients and 0.07% or 1:1429 patients in our study population. Our result is in agreement with a previous study conducted by Van Den Haak et al[4] which reported that the prevalence rates of overall and occult ULMS were 0.25% or 1:400 patients and 0.12% or 1 in 865 patients undergoing all types of surgeries. Occult ULMS, especially after morcellation, is a serious clinical problem that warrants investigation. The U.S. Food and Drug Administration (FDA) published its initial safety communication, which reported that the risk of occult ULMS after morcellation was 0.2% or 1 in 498 patients.[9] Rodriguez et al. recently reported that the prevalence of occult ULMS in presumed morcellation cases was 0.14% or 1 in 714 patients.[10] Cao et al reported that the prevalence of occulted ULMS was 0.11% (29/26643) and that of morcellation was 0.02%.[11]
Few studies have reported the proportion of occulted cases in all ULMS. Van Den Haak et al reported that the proportion of occult ULMS was 46%.[4] The proportion of occult ULMS in the present study was 25%, which is lower than that reported in a previous study.[4] This may be explained by the fact that the ULMS cases were retrieved from different types and levels of the hospitals. Study conducted in the Netherlands included different types and levels of hospitals nationwide, while the present study included only one cancer center in our province.
There was no proportional relationship between the number and prevalence of overall and occult ULMS. We calculated the prevalence of overall ULMS based on age groups and found that it constantly increased with age after 50 years. Moreover, we found that the prevalence of occult ULMS was stable between 30 and 59 years of age and even decreased to zero after 60 years of age. A population-based study emphasized the same trend in overall uterine sarcoma prevalence among patients with leiomyoma, undergoing surgery.[12] However, a study published by Rodriguez et al[10] reported that the incidence of occult ULMS increased with age following laparoscopic hysterectomy or myomectomy. They also stated that no occult leiomyosarcoma was identified in patients aged 40 years and younger, who underwent laparoscopic myomectomy. Given that young women, especially those aged 40 years and younger, are at the lowest risk, several gynecological associations take into consideration possible consequences following an increased number of abdominal hysterectomies in them. We cannot share this view because it will increase the occurrence of occult ULMS that is presumably excluded for malignancy in low-risk populations. As shown in Figure 1, occult ULMS was skewed towards younger age with a stable prevalence, while no case was found in the elderly, who were at the highest risk of ULMS. The possible explanation for this by Van Den Haak et al[4] is that the highest risk for preoperatively unrecognized ULMS was at age 40 to 50 years, in which malignancy was suspected in only 15% of women, as opposed to 53%, 63%, and 80% in women aged 50 to 60, 60 to 70, and over 70 years. A study at Johns Hopkins University demonstrated that only one woman underwent morcellation of an occult uterine sarcoma during 10 years.[13] Therefore, patient safety may improve, and the risk of occult ULMS would reduce if we suspect malignancy in all patients with uterine smooth muscle tumors and perform comprehensive preoperative assessments.
Our data demonstrates that the risk of occult ULMS in women undergoing uterine surgery is not necessarily increased by the preference of adopting minimally invasive surgery. Although the proportion of laparoscopic and hysteroscopic lesions increased from 42.68% during 2008 to 2012 to 66.47% during 2013 to 2018, the prevalence of overall ULMS remains stable and that of occult ULMS was almost the same. A study at Johns Hopkins Hospital reported low risk of occult uterine malignancy when the utilization of minimally invasive surgeries increased significantly from 39.9% to 82.9% during 2005 to 2014.[13] After the FDA released its initial safety communication, which discourages the use of power morcellation for the treatment of leiomyoma, there has been a new reliance on abdominal myomectomy to avoid tissue fragmentation in sarcoma cases.[14,15] Several options, such as scalpel-based or in-bag, contained morcellation, may be a safer alternative for tissue extraction in minimally invasive gynecological surgeries. Another way to control the risk of occult ULMS at a low level is to adopt a more nuanced approach using careful preoperative patient selection, with peer-review and rigorous informed consent processes. However, the best way to eliminate occult ULMS is to develop reliable, novel, and early detection strategies for differential diagnosis of ULMS and leiomyoma.
The prevalence of overall and occult ULMS was 0.29% and 0.07% in all surgeries for the treatment of uterine smooth muscle tumors. Although there is no method to accurately diagnose ULMS preoperatively, there is still hope for reducing occult ULMS because the prevalence of occult and overall ULMS is not proportional to age. Maintaining a potential malignant diagnosis in mind is critical to reducing the prevalence of occult ULMS.
Author contributions
LW, SL, ZZ, JJ, and BS participated in the design, collection and reviewing the data, the statistical analysis, interpretation the data and drafting the manuscript.
All authors read and approved the final manuscript.
Footnotes
Abbreviations: STS = soft tissue sarcoma, ULMS = uterine leiomyosarcoma.
How to cite this article: Wang L, Li S, Zhang Z, Jia J, Shan B. Prevalence and occult rates of uterine leiomyosarcoma. Medicine. 2020;99:33(e21766).
This study was approved by the Institutional Review Board of the Fourth Hospital of Hebei Medical University.
The patients’ informed consent was waived due to the retrospective nature of the study.
The study was supported by the Medical Scientific Research Foundation of Hebei Province, China (20150313).
The data was confidential.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26,758 cases. Int J Cancer 2010;119:2922–30. [DOI] [PubMed] [Google Scholar]
- [2].Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma: a gynecologic oncology group study. Cancer 2015;71(S4):1702–9. [DOI] [PubMed] [Google Scholar]
- [3].Gockley AA, Rauh-Hain JA, del Carmen MG. Uterine leiomyosarcoma: a review article. Int J Gynecol Cancer 2014;24:1538–42. [DOI] [PubMed] [Google Scholar]
- [4].Van Den Haak L, de Kroon CD, Warmerdam MI, et al. Incidence and groups at risk for unexpected uterine leiomyosarcoma: a Dutch nationwide cohort study. Arch Gynecol Obstet 2019;299:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goto A, Takeuchi S, Sugimura K, et al. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer 2002;12:354–61. [DOI] [PubMed] [Google Scholar]
- [6].D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol 2010;116:131–9. [DOI] [PubMed] [Google Scholar]
- [7].Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol 1994;83:414–8. [PubMed] [Google Scholar]
- [8].Paul P, Rengaraj V, Das T, et al. Uterine sarcomas in patients undergoing surgery for presumed leiomyomas: 10 years’ experience. J Minim Invasive Gynecol 2016;23:384–9. [DOI] [PubMed] [Google Scholar]
- [9].Stentz NC, Cooney LG, Sammel M, et al. Changes in myomectomy practice after the US Food and Drug Administration safety communication on power morcellation. Obstetrics & Gynecology 2017;129:1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodriguez AM, Asoglu MR, Sak ME, et al. Incidence of occult leiomyosarcoma in presumed morcellation cases: a database study. Eur J Obstet Gynecol Reprod Biol 2016;197:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cao H, Li L, Yang B, et al. Unexpected uterine sarcomas after hysterectomy and myomectomy for presumed leiomyoma: a retrospective study of 26,643 patients. Cancer Manag Res 2019;11:7007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mao J, Pfeifer S, Zheng XE, et al. Population-based estimates of the prevalence of uterine sarcoma among patients with leiomyomata undergoing surgical treatment. JAMA Surg 2015;150:368–70. [DOI] [PubMed] [Google Scholar]
- [13].Ricci S, Angarita A, Cholakian D, et al. Preoperative patient stratification results in low rates of occult uterine malignancy in women undergoing uterine surgery and morcellation. Gynecol Oncol 2015;137:11–2. [Google Scholar]
- [14].Food, Administration D. UPDATED laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. In: WHO Geneva; 2016. [Google Scholar]
- [15].Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol 2016;214:98. [DOI] [PubMed] [Google Scholar]


