Abstract
Background
Symptoms of advanced hepatocellular carcinoma (HCC) represent a substantial burden for the patient and are important endpoints to assess when evaluating treatment. Patient-reported outcomes were evaluated in subjects with advanced HCC and baseline alpha-fetoprotein (AFP) ≥400 ng/mL treated with second-line ramucirumab.
Patients and methods
Patients with AFP≥400 ng/mL enrolled in the REACH or REACH-2 phase 3 studies were used in this analysis. Eligible patients had advanced HCC, Child-Pugh A, Eastern Cooperative Oncology Group performance status 0/1 and prior sorafenib. Patients received ramucirumab 8 mg/kg or placebo once every 2 weeks. Disease-related symptoms and health-related quality of life (HRQoL) were assessed with the Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index (FHSI)-8 and EuroQoL-5-Dimensions (EQ-5D) instruments, respectively. Time to deterioration (TTD) (≥3-point decrease in FHSI-8 total score;≥0.06-point decrease in EQ-5D score, from randomisation to first date of deterioration) was determined using Kaplan-Meier estimation and the Cox proportional hazards model. Both separate and pooled analyses for REACH AFP≥400 ng/mL and REACH-2 patients were conducted.
Results
In the pooled population with AFP ≥400 ng/mL (n=542; ramucirumab, n=316; placebo, n=226), median TTD in FHSI-8 total score was prolonged with ramucirumab relative to placebo (3.3 vs 1.9 months; HR 0.725; (95% CI 0.559 to 0.941); p=0.0152), including significant differences in back pain (0.668; (0.497 to 0.899); p=0.0044), weight loss (0.699; (0.505 to 0.969); p=0.0231) and pain (0.769; (0.588 to 1.005); p=0.0248) symptoms. TTD in EQ-5D score was not significantly different between ramucirumab and placebo groups (median 2.9 vs 1.9 months). Results in the individual trials were consistent with these findings.
Conclusions
Ramucirumab in second-line treatment of advanced HCC demonstrates consistent benefit in the delay of deterioration in disease-related symptoms with no worsening of HRQoL. Taken with previously demonstrated ramucirumab-driven survival benefits in this setting, these data may inform patient–clinician discussions about the benefit–risk profile of this therapy.
Trial registration number
Keywords: hepatocellular carcinoma, ramucirumab, patient-reported outcomes, quality of life
Key questions.
What is already known about this subject?
Ramucirumab has previously demonstrated both efficacy and tolerability as a second-line systemic treatment in patients with advanced HCC in two phase 3 studies (REACH and REACH-2).
Data on patient-reported quality-of-life outcomes in the second-line setting among patients with advanced HCC is limited.
What does this study add?
Time to deterioration in disease symptoms (measured by the Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index) was significantly delayed in patients treated with ramucirumab versus placebo.
Significant differences in time to deterioration for back pain, weight loss and pain were observed.
Delay in deterioration of these cancer-related symptoms extends the previously demonstrated benefit of ramucirumab to survival.
How might this impact on clinical practice?
By demonstrating the potential for second-line ramucirumab to address both the symptoms and HRQoL of patients with HCC, our results will help to guide patient–clinician discussions about the risks and benefits of this therapy.
Introduction
Hepatocellular cancer (HCC) represents the fourth most common cause of cancer-related mortality globally.1 2 Patients with HCC frequently present at an advanced stage, when prognosis is poor and treatment options are limited. Notably, patients with advanced HCC often report a complex variety of symptoms, related to the cancer itself, underlying cirrhosis and other comorbid conditions and side effects of treatment.3 HCC symptoms represent a substantial physiological and psychological burden for patients and can significantly affect their health-related quality of life (HRQoL).3 Primary endpoints in clinical trials of new therapies for advanced HCC primarily focus on improvements in overall survival (OS) and progression-free survival (PFS), but many of the gains observed to date have been relatively small.3 In the non-curative setting, the symptoms and HRQoL experienced by patients with advanced HCC represent important endpoints to assess when evaluating the benefits of treatment.3 In the first-line HCC setting, two recent studies have examined patient-reported HRQoL outcomes,4 5 however, data in the second-line setting are limited.
Ramucirumab, a human IgG1 monoclonal antibody that inhibits ligand activation of vascular endothelial growth factor receptor-2, has demonstrated both efficacy and tolerability as a second-line systemic treatment in patients with advanced HCC and baseline alpha-fetoprotein (AFP) levels≥400 ng/mL in two phase 3 studies (REACH and REACH-2).6 7 Further analysis of the REACH study demonstrated that in the subgroup with elevated AFP, the rate of deterioration of patients’ symptoms was significantly reduced.8 More recently, the REACH-2 trial, a follow-up study in patients with advanced HCC and elevated baseline AFP, confirmed the results from the REACH trial.7 In the current study, we sought to further assess the effects of ramucirumab on patient-reported outcomes (PROs), including HCC-related symptoms and HRQoL. To obtain a general estimate of treatment effect from a larger patient population, we examined PROs in the population of patients with baseline AFP ≥400 ng/mL pooled from the REACH and REACH-2 studies, in addition to confirming the consistency between the individual study populations. By investigating the impact of ramucirumab on PROs for patients with advanced HCC, we hope to demonstrate the effect of ramucirumab on additional outcomes that are important to both patients and clinicians while underscoring the value of considering such outcomes in studies that investigate novel agents for patients with advanced HCC.
Methods
Patients
Patient populations in REACH (NCT01140347; NCT02435433) and REACH-2 (NCT02435433) have been previously described in detail.6 7 Eligible patients had advanced HCC, Child-Pugh score <7 (Class A only), Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1, Barcelona Clinic Liver Cancer stage C or B and prior sorafenib (discontinued due to progression or intolerance). REACH patients in the current analysis were from a protocol-defined patient subpopulation with baseline AFP ≥400 ng/mL; REACH-2 patients were required to have a baseline AFP ≥400 ng/mL. All patients provided written, informed consent.
Study design
REACH and REACH-2 were global, double-blind, randomised, placebo-controlled phase 3 trials with similar protocol procedures, efficacy assessments and treatment regimens.6 7 Patients were randomised (1:1 REACH and 2:1 REACH-2) to receive ramucirumab 8 mg/kg or placebo intravenously once every 2 weeks until disease progression, unacceptable toxicity or withdrawal of consent. All patients in both treatment arms received best supportive care, as determined by the treating clinician(s), including concomitant treatments to address symptoms and side effects (online supplementary table S1) and palliative care consultation (data not summarised).
esmoopen-2020-000797supp001.pdf (355.2KB, pdf)
Patient-reported outcomes
Two patient-reported instruments were administered: the Functional Assessment of Cancer Therapy (FACT) Hepatobiliary Symptom Index (FHSI-8) and EuroQoL 5-Dimensions (EQ-5D). The FHSI-8 is a self-administered questionnaire used to assess the most frequent and concerning symptoms experienced by patients with hepatobiliary malignancies (lack of energy, nausea, pain, weight loss, back pain, fatigue, jaundice, stomach pain or discomfort).9–11 Each symptom was assessed using a 5-point response scale (from ‘not at all’ to ‘very much’), and scores were reversed and used to compute a total score (range 0–32), as per instrument guidelines, where a higher score represents lower symptom burden. The EQ-5D is a generic instrument for assessment of the effects of disease and treatment on HRQoL across many different disease states. The EQ-5D descriptive system comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression), each with 3 levels of severity, used in the derivation of an overall index score (where 1 represents best possible health; 0, death).12 EQ-5D index scores were used in REACH whereas a version of the EQ-5D with five severity levels (EQ-5D-5L) was used in REACH-2. For consistency, EQ-5D-5L scores were transformed to EQ-5D with the EQ-5D-5L crosswalk13 in the current analyses. Minimally important differences (MIDs) have been reported as 2–3 points for the FHSI-8 total score,14 and 0.06–0.08 points for the EQ-5D index score in all cancers.15 We have further conducted a psychometric analysis of REACH-2 data, which supported a 3-point meaningful change threshold for FHSI-8 total score in our trial population (data on file). We use these MIDs herein as criteria for clinically meaningful changes.
We administered the two surveys together at the same time points throughout both studies, administering the FHSI-8 first. We collected the surveys at baseline and end of treatment. In REACH, we also collected data at the start of cycles 4, 10 and 16 (ie, at 6 weeks following first treatment and every 12 weeks thereafter). In REACH-2, we collected data every 6 weeks. Following discontinuation from study therapy, the end-of-treatment visit occurred within 7 days, with PROs collected at that time.
Statistical analyses
We performed a pooled data analysis for REACH (AFP ≥400 ng/mL) and REACH-2 by pooling together individual patient data from the two studies and analysing with stratification by study to account for clustering of patients within each study. Pooled analyses were prespecified prior to REACH-2 database lock. We calculated survey compliance as the percentage of completed assessments among the number of expected assessments (patients still on study without progression). We present descriptive statistics as mean±SD at baseline and at end of treatment for FHSI-8 total scores and EQ-5D index scores: inferential statistics were not performed as these data were confounded due to differential survival times in the two treatment groups and are subject to statistical concerns of nonrandom missing data. To mitigate concerns of bias associated with nonrandom missing data, time to first deterioration (TTD) was compared between treatment arms in FHSI-8 total and EQ-5D index scores with TTD prespecified as the time from the randomisation date to the first date with a clinically meaningful≥3-point and ≥0.06-point decrease from baseline, respectively.14 15 The TTD of each FHSI-8 symptom was compared between treatment arms with deterioration threshold prespecified as a decrease in one categorical response for an individual item. Patients who did not experience a deterioration were censored at the last assessment in TTD analyses. We estimated the TTD curves and medians using the Kaplan-Meier method. P values for treatment comparisons were derived from the log-rank test. A two-sided p<0.05 was considered statistically significant. We calculated HRs with 95% CI using a univariate Cox regression model. The individual studies were stratified by randomization stratification factors (REACH: aetiology; geographical region; REACH-2: macrovascular invasion; ECOG PS 0 vs 1; geographical region). The impact of missing data on the TTD analysis of FHSI-8 total scores was evaluated via a sensitivity analysis with missing data imputed by multiple imputation using Markov Chain Monte Carlo methodology on the REACH-2 analysis population, conducted as detailed above for the original analyses.
Results
The pooled analysis population included 542 patients (ramucirumab, n=316; placebo, n=226). Of these, 250 patients originated from REACH (ramucirumab, n=119; placebo, n=131) and 292 originated from REACH-2 (ramucirumab, n=197; placebo, n=95; table 1). At the time of data cut-off in each study, most patients were off treatment (ramucirumab, 94%–98%; placebo, 98%–100%), with the majority in both treatment groups discontinuing due to disease progression (ramucirumab, 69%–70%; placebo, 81%–87%; online supplementary figure S1). Demographics and baseline clinical characteristics were balanced between the treatment groups in each of the three analysis populations except median baseline AFP levels were slightly imbalanced at the per-study level (REACH (AFP ≥400 ng/mL): ramucirumab, 5293 ng/mL; placebo, 7022 ng/mL; REACH-2 ramucirumab, 3920 ng/mL; placebo, 2741 ng/mL); however, this was not observed once the data were pooled (ramucirumab, 4102 ng/mL; placebo, 4048 ng/mL; table 1).
Table 1.
Demographics and baseline clinical characteristics of patients
| Pooled* | REACH (AFP ≥400 ng/mL)* | REACH-2* | |||||
| Ramucirumab N=316 |
Placebo N=226 |
Ramucirumab N=119 |
Placebo N=131 |
Ramucirumab N=197 |
Placebo N=95 |
||
| n (%), except where indicated | |||||||
| Sex | Male | 246 (77.8) | 189 (83.6) | 92 (77.3) | 110 (84.0) | 154 (78.2) | 79 (83.2) |
| Age (years) | Median | 64 | 62 | 62 | 59 | 64 | 64 |
| Race | Asian | 168 (53.2) | 123 (54.4) | 66 (55.5) | 78 (59.5) | 102 (51.8) | 45 (47.4) |
| ECOG PS | 0 | 173 (54.7) | 118 (52.2) | 60 (50.4) | 63 (48.1) | 113 (57.4) | 55 (57.9) |
| Geographical region 1 | Americas, Europe, Israel, Australia |
154 (48.7) | 108 (47.8) | 53 (44.5) | 58 (44.3) | 101 (51.3) | 50 (52.6) |
| Geographical region 2 | Asia (excluding Japan) |
101 (32.0) | 78 (34.5) | 46 (38.7) | 51 (38.9) | 55 (27.9) | 27 (28.4) |
| Geographical region 3 | Japan | 61 (19.3) | 40 (17.7) | 20 (16.8) | 22 (16.8) | 41 (20.8) | 18 (18.9) |
| Child-Pugh Score | A – 5 | 190 (60.1) | 135 (59.7) | 67 (56.3) | 81 (61.8) | 123 (62.4) | 54 (56.8) |
| Baseline BCLC Stage | B | 45 (14.2) | 29 (12.8) | 11 (9.2) | 9 (6.9) | 34 (17.3) | 20 (21.1) |
| FHSI-8 total score | Mean (SD) | 26.44 (4.82) | 26.29 (5.03) | 25.84 (5.03) | 25.92 (4.98) | 26.80 (4.66) | 26.78 (5.08) |
| EQ-5D index score† | Mean (SD) | 0.784 (0.190) | 0.806 (0.193) | 0.778 (0.215) | 0.807 (0.202) | 0.787 (0.174) | 0.806 (0.182) |
| Sorafenib discontinuation | Progressive disease |
274 (86.7) | 198 (87.6) | 108 (90.8) | 122 (93.1) | 166 (84.3) | 76 (80.0) |
| Intolerance | 42 (13.3) | 28 (12.4) | 11 (9.2) | 9 (6.9) | 31 (15.7) | 19 (20.0) | |
| Aetiology | Hepatitis B | 124 (39.2) | 102 (45.1) | 53 (44.5) | 66 (50.4) | 71 (36.0) | 36 (37.9) |
| Hepatitis C | 83 (26.3) | 56 (24.8) | 35 (29.4) | 28 (21.4) | 48 (24.4) | 28 (29.5) | |
| Significant Alcohol Use |
71 (22.5) | 43 (19.0) | 23 (19.3) | 22 (16.8) | 48 (24.4) | 21 (22.1) | |
| Macrovascular invasion | Present | 113 (35.8) | 77 (34.1) | 43 (36.1) | 44 (33.6) | 70 (35.5) | 33 (34.7) |
| Extrahepatic spread | Present | 226 (71.5) | 171 (75.7) | 85 (71.4) | 101 (77.1) | 141 (71.6) | 70 (73.7) |
| Baseline AFP (ng/mL) | Median | 4102 | 4048 | 5293 | 7022 | 3920 | 2741 |
| Minimum–maximum | 408–853 200 | 419–628 390 | 411–853 200 | 429–628 390 | 408–230 500 | 419–473 163 | |
*Data from references 6–8.
†For EQ-5D, for the pooled analysis, n=309 (ramucirumab) and n=218 (placebo); for REACH, n=116 (ramucirumab) and n=123 (placebo); for REACH-2, n=193 (ramucirumab) and n=95 (placebo).
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; EQ-5D, EuroQoL five dimensions; FHSI-8, Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index; n, number of patients in specified category; N, number of patients in the intent-to-treat population;SD, standard deviation.
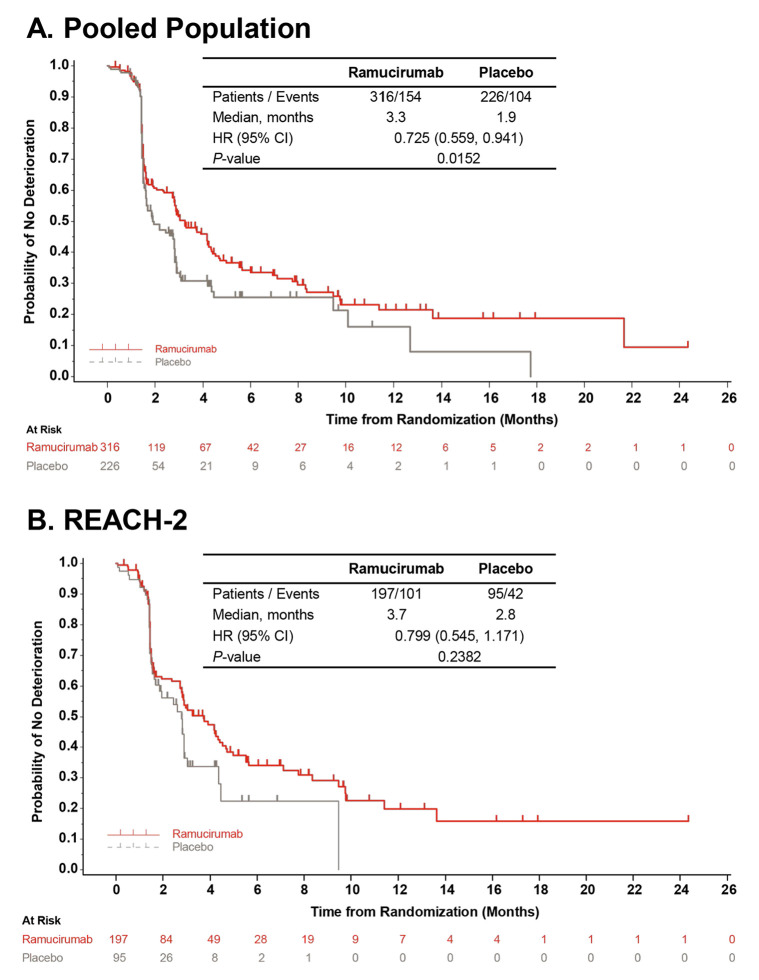
We observed similar compliance with the FHSI-8 and EQ-5D instruments across the two treatment groups in all analysis populations, with rates of ≥95% at baseline and ≥65% at end of treatment (online supplementary table S2; Chau et al).8 In the pooled population, the TTD in HCC symptoms based on total FHSI-8 scores was prolonged for patients assigned to ramucirumab compared with patients assigned to placebo (median 3.3 vs 1.9 months, respectively; HR 0.725; 95% CI, 0.559 to 0.941; p=0.0152; figure 1A). The findings from the individual study populations were consistent with the pooled data although the differences between treatment groups for TTD in FHSI-8 total scores did not reach statistical significance (ramucirumab vs placebo: REACH (AFP ≥400 ng/mL) median 2.9 vs 1.6 months; HR 0.690; 95% CI 0.470 to 1.014; p=0.054; Online supplementary figure S2A; REACH-2 median 3.7 vs 2.8 months; HR 0.799; 95% CI 0.545 to 1.171; p=0.2382; figure 1B). We observed similar findings in the sensitivity analysis on the REACH-2 cohort (ramucirumab: median 3.0 months; placebo: median 2.8 months; HR 0.816; 95% CI 0.572 to 1.163; p=0.2610), indicating that missing data did not have a statistical impact on the TTD analysis. Sensitivity analyses of FHSI-8 outcomes in REACH also found no effect of missing data on the overall findings.8
Figure 1.
Time to first deterioration in FHSI-8 total score. Data shown are from (A) pooled and (B) REACH-2 populations. The curves and medians were estimated using the Kaplan-Meier method, HR (95% CI) was estimated using a Cox regression model. In case of no FHSI-8 deterioration, the subject was censored at the time of the last FHSI-8 recording. FHSI-8, Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index.
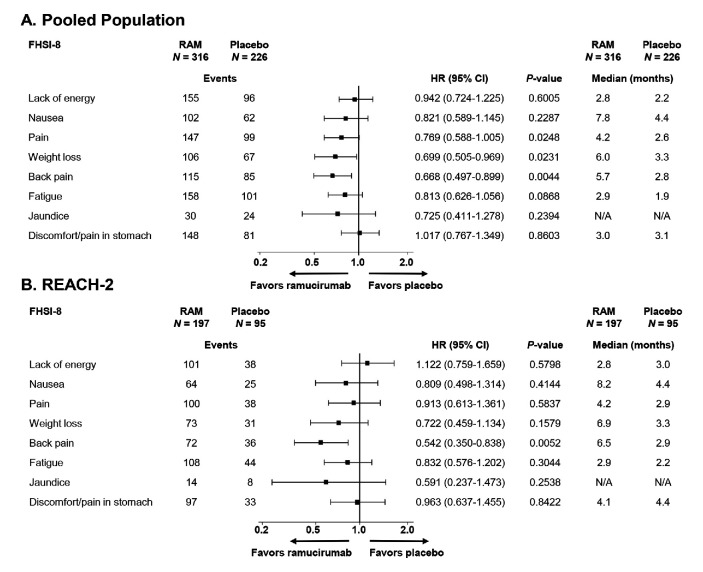
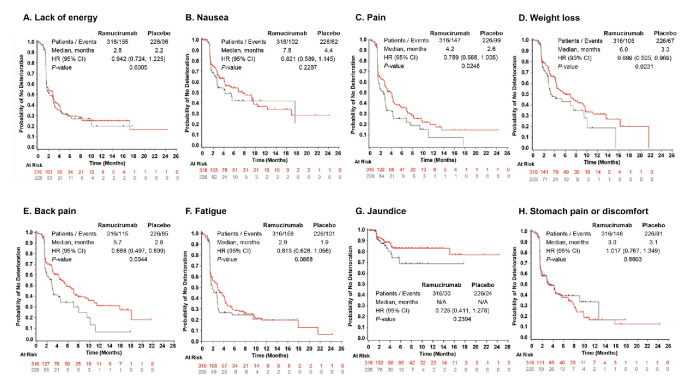
For the individual FHSI-8 symptom items (figure 2A; figure 3A–H), in the pooled population, we found statistically non-significant delays in the time to deterioration for symptoms of nausea and fatigue and statistically significant delays in TTD for the symptoms of weight loss (ramucirumab: median 6.0 months; placebo: median 3.3 months; HR 0.699; 95% CI, 0.505 to 0.969; p=0.0231), pain (ramucirumab: median 4.2 months; placebo: median 2.6 months; HR 0.769; 95% CI, 0.588 to 1.005; p=0.0248), and back pain (ramucirumab: median 5.7 months; placebo: median 2.8 months; HR 0.668; 95% CI, 0.497 to 0.899; p=0.0044; figure 3C–E). The REACH (AFP ≥400 ng/mL) subpopulation also demonstrated a correlation between ramucirumab and improved symptoms, with the most notable being delayed worsening of pain (ramucirumab: median 3.3 months; placebo: median 1.8 months; HR 0.635; 95% CI 0.422 to 0.955, p=0.0259; Online supplementary figure S2B). Similarly, in REACH-2, we found patients assigned to ramucirumab experienced delays to symptom worsening for the symptoms of nausea, pain, back pain, weight loss and fatigue, although back pain was the only individual symptom to reach significance for prolonged TTD (ramucirumab: median 6.5 months; placebo: median 2.9 months; HR 0.542 95% CI 0.350 to 0.838; p=0.0052; figure 2B).
Figure 2.
Time to deterioration of FHSI-8 symptom items in patients in the (A) pooled; and (B) REACH-2 populations. Data in panel A are shown longitudinally in online supplementary figure S2. FHSI-8, Functional Assessment of Cancer Therapy hepatobiliary symptom index; N, number of patients in intent-to-treat population; N/A, not available (medians could not be calculated due to low number of events); RAM, ramucirumab.
Figure 3.
Longitudinal depiction of time to deterioration of FHSI-8 symptom items in patients with advanced hepatocellular carcinoma and baseline AFP ≥400 ng/mL. Data represent pooled individual patient data from REACH-2 and REACH (AFP ≥400 ng/mL) populations. FHSI-8 symptom items included (A) lack of energy; (B) nausea; (C) pain; (D) weight loss; (E) back pain; (F) fatigue; (G) jaundice and (H) stomach pain or discomfort. The curves and medians were estimated using the Kaplan-Meier method, HR (95% CI) was estimated using a COX regression model. AFP, alpha-fetoprotein; FHSI-8, Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index; N/A, medians could not be calculated due to low number of events.
In the pooled population, the TTD in HRQoL based on the EQ-5D index score was not significantly different for patients assigned to ramucirumab (median 2.9 months) compared with patients assigned to placebo (median 1.9 months; HR 0.858; 95% CI 0.662 to 1.111; p=0.2485; online supplementary figure S3A). A delay in TTD with ramucirumab treatment was observed in the REACH-2 population, but the difference between treatment groups did not reach significance (median ramucirumab: 4.2 months; placebo: 2.9 months; HR 0.811; 95% CI 0.543 to 1.211; p=0.2898; online supplementary figure S3B).
In the pooled and REACH-2 populations, patients in both arms reported clinically meaningful decreases from baseline in total FHSI-8 score and EQ-5D at end of treatment (online supplementary table S3). In the REACH (AFP ≥400 ng/mL) population, for FHSI-8, only patients in the placebo treatment arm reported an average meaningful decrease (mean change: ramucirumab: −2.21 (±5.63); placebo: −3.73 (±5.88)) whereas the changes in EQ-5D scores were similar in the two treatment groups (mean change: ramucirumab: −0.120 (±0.320); placebo: −0.191 (±0.297)).8 In both the REACH (AFP ≥400 ng/mL)8 and REACH-2 populations (online supplementary table S4), FHSI-8 total scores were maintained over the course of treatment in both treatment groups, with scores declining only at end of treatment.
Discussion
The current analysis demonstrates that ramucirumab treatment is associated with a delay in the deterioration of disease-related symptoms in patients with advanced HCC and baseline AFP ≥400 ng/mL. In the pooled analysis of individual patient data from the study populations, comparison of TTD in disease-related symptoms based on FHSI-8 total score revealed a prolongation with single-agent ramucirumab compared with placebo. We also observed significant delays in deterioration for the individual symptoms of weight loss, pain and back pain with ramucirumab. Taken together with previously published reports on OS and PFS benefits,6–8 these data highlight that second-line ramucirumab treatment has a significant benefit to both survival and cancer-related symptoms among patients with advanced HCC and elevated baseline AFP.
Patients with advanced HCC often experience substantial symptom burden and worsening of their HRQoL over time as the disease progresses or due to treatment-related toxicities or other complications.3 Our data demonstrate that mean FHSI-8 scores were maintained relative to placebo, while patients received ramucirumab, with significant declines observed primarily at the end-of-treatment visits in both ramucirumab and placebo groups. These data support the conclusion that the deterioration of symptoms occurred as a result of disease progression (the most common reason to end treatment) rather than due to treatment-associated toxicity. A relationship between deterioration of symptoms and disease progression is supported by the TTD analyses, which showed a significant delay in median TTD in FHSI-8 total score with ramucirumab compared with placebo, mirroring the improvements previously observed in PFS in patients with advanced HCC and elevated AFP.6 7 TTD in EQ-5D index scores was not substantially different between treatment groups, suggesting no worsening of HRQoL with ramucirumab treatment. As expected, the discrepancy observed is likely attributed to the sensitivity of instruments, specifically that the FHSI-8 focuses on eight hepatobiliary disease-specific symptoms,10 while the EQ-5D is a generic non-disease-specific tool intended to complement other HRQoL measurement methods.12 Nevertheless, results on the FHSI-8 and EQ-5D were consistent across all three analysis populations examined and are particularly noteworthy in the context of the historically poor survival outcomes seen in advanced HCC. These findings are also in agreement with our previous demonstration of a linear relationship between the deterioration in FHSI-8 total scores and change in tumour size in REACH8 and the current demonstration that ramucirumab has significant and clinically meaningful benefits in delaying the worsening in the symptoms of weight loss, pain and back pain, specific symptoms which have been related to HCC tumour burden.16 17
In the first-line HCC setting, two recent studies also reported improved PROs for the experimental arms, highlighting the relevance and importance of considering patient-reported HRQoL outcomes in HCC phase 3 trials.4 5 These observations are relevant to the goal of therapy in this patient population, which is to extend survival while maintaining patients’ well-being, and underscore the need for further work that addresses the impact of new therapies on the symptoms and HRQoL of patients with advanced HCC. To date, no other second-line systemic agents have demonstrated similar benefits in terms of HCC symptoms, however, direct comparison is difficult due to use of different HRQoL measurement tools and interpretation of what constitutes meaningful benefit.18–20 Thus, our current findings represent a thorough assessment of disease-specific symptoms in patients with advanced HCC and highlight the value of measuring cancer-related symptoms with PRO instruments in this patient population for which survival benefits of new treatments may be limited.
A strength of the current study is that REACH and REACH-2 had similar designs, assessments and treatments, and the two studies observed consistent results in the relevant patient population with elevated baseline AFP. Further, these similarities allowed for pooling of REACH-2 and REACH (AFP ≥400 ng/mL) data, which enabled a better-powered assessment of ramucirumab treatment due to the larger dataset. In addition, compliance in completing the PRO assessments was relatively high at end of treatment (>65%) despite the rapidly progressive nature of the disease, which could have influenced the ability to collect data. However, the study had a number of limitations, common to studies of this type. Importantly, informative censoring bias was potentially a factor as, by study design, some PROs were not collected due to disease progression or death, when scores would be expected to be poor. In addition, patients had to meet the studies’ eligibility criteria, limiting the generalisability of these results to the broader patient population with advanced HCC. Further, in the current study, differences in the version of the EQ-5D used in each study resulted in the need for converting the scores in order to combine them, and assessments were made at different times during the two studies, which precluded the pooling of some data for specific timepoint analyses. Additionally, the integrated analyses were not included in an a priori gate-keeping approach and, as such, are not controlled for multiple hypothesis testing. Finally, many other symptoms and concerns (eg, depression, anxiety, cognition) and important factors that can affect HCC symptom burden (eg, hospitalisations, concomitant medication usage, financial toxicity, physical function) are not reported here and should be explored in future work.
In conclusion, the current findings demonstrate that ramucirumab treatment is associated with a benefit in delaying the deterioration of disease-related symptoms and maintaining HRQoL in patients with advanced HCC and baseline AFP ≥400 ng/mL. By demonstrating the potential for second-line ramucirumab therapy to address both the symptoms and HRQoL of patients with HCC, our results have far-reaching implications to help guide patient-clinician discussions about the risks and benefits of this therapy.
Acknowledgments
We thank the patients and their families/caregivers, the study investigators and their staff, the independent data monitoring committee, and the REACH and REACH-2 clinical trial teams. Medical writing assistance (Kaye L. Stenvers, PhD) and editorial assistance (Angela Lorio and Dana Schamberger) was provided by Syneos Health and funded by Eli Lilly and Company.
Footnotes
AXZ and RDN contributed equally.
Presented at: A portion of the data was presented at European Society for Medical Oncology 2018 Congress as Zhu et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: Patient-reported outcome results across two phase 3 studies (REACH-2 and REACH). Ann Oncol. 2018;29(suppl 8):abstract 622PD. 10.1093/annonc/mdy282.006.
Contributors: All authors contributed to at least one of the following: study conception, design, data acquisition, analysis and/or interpretation. All authors contributed to drafting of the manuscript and/or critical revision of the work for important intellectual content. All authors read and approved the final manuscript.
Funding: This study was funded by Eli Lilly and Company. No grant number is applicable.
Competing interests: AXZ: Consulting or advisory role: Eisai, Bristol-Myers Squibb, Merck, Novartis, Sanofi, AstraZeneca, Bayer, Exelixis, Eli Lilly and Company; Research funding: Eli Lilly and Company, Bayer, Bristol-Myers Squibb, Novartis, Merck. RN: None. PRG: Consulting/advisory boards and personal fees: AstraZeneca, Bayer Schering Pharma, Bristol-Myers Squibb, Ipsen, Eli Lilly and Company MSD, Merck, Novartis, Roche, Sirtex Medical, Sillajen. RSF: Consulting: AstraZeneca, Bayer, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Merck, Novartis, Roche/Genentech. JML: Consulting: Eli Lilly and Company, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Eisai, Celsion Corporation, Exelixis, Merck, Ipsen, Glycotest, Navigant, Leerink Swann, Midatech, Fortress Biotech, Sprink Pharmaceuticals, Nucleix, CanFite. Research funding; Bayer HealthCare Pharmaceuticals, Eisai, Bristol-Myers Squibb, Ipsen. J-FB: Personal and consulting fees: Eli Lilly and Company, Bayer, BMS, Eisai, Ipsen, Onxeo, during the conduct of the study. TO: Advisory role: Eli Lilly and Company, Nippon Boehringer Ingelheim, Dainippon Simitomo Pharma, Ono Pharmaceutical, Nano Carrier, Zeria Pharmaceutical, Daiichi Sankyo. Research grant: Eli Lilly and Company, Novartis Pharma K.K., Kowa K.K., Takeda Bio Development Center Limited, Nippon Boehringer Ingelheim, Dainippon Simitomo Pharma, Pfizer Jana, Bayer Yakuhin, Chugai Pharmaceutical, Yakuruto Honsha, Ono Pharmaceutical, Eisaid, AstraZeneca K.K., Merck Serono, OncoTherapy Science, Kyowa Hakko Kirin, Shizuoka Industry, Baxter, Nano Carrier, Zeria Pharmaceutical, Glaxo Smith Kline K.K., Nobelpharma. Honoraria/personal fees: Eli Lilly and Company, Novartis Pharma K.K., Dainippon Simitomo Pharma, Pfizer Jana, Bayer Yakuhin, Chugai Pharmaceutical, Yakuruto Honsha., Ono Pharmaceutical, Eisai Co, AstraZeneca K.K., Merck Serono, Baxter, Nobelpharma, Bristol-Myers Squibb Company, Nipponchemofa, EA Pharma, FUJIFILM RI Pharma, Astellas Pharma, Nippon Kayaku, Daiichi Sankyo, Celgene, K.K., MSD, K.K., Teijin Pharma. IC: Advisory board: Sanofi Oncology, Eli Lilly and Company, Bristol-Myers Squibb, MSD, Bayer, Roche, Merck Serono, Five Prime Therapeutics; Astra-Zeneca, Oncologie International, Pierre Fabre; Research funding: Eli-Lilly and Company, Janssen-Cilag, Sanofi Oncology, Merck-Serono. Honorarium: Eli Lilly and Company. DC: Consultant for Eli Lilly and Company, during the conduct of the study; outside the submitted work. Consultant for Abbvie; BMS; Pfizer; Novartis; Bioverativ; Ipsen; FACIT.org, president. AG, JG, LB, PBA; CW: Employee and minor shareholder of Eli Lilly and Company. YH is a former employee of Eli Lilly and Company. MK: Consulting or advisory role: Bayer, Bristol-Myers Squibb, Eisai, MSD, Ono Pharmaceutical. Personal fees/Honoraria: Bayer, Eisai, MSD. Research funding: Abbvie, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharma, Daiichi Sankyo, EA Pharma, Eisai, Gilead, Medico’s Hirata, Otsuka, Takeda, Taiho Pharmaceutical.
Patient consent for publication: Not required.
Ethics approval: All studies were conducted with the approval of independent ethics committees at all participating centres and in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
References
- 1.GLOBOCON Liver cancer fact sheet, 2018. Available: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf [Accessed 17 Jan 2019].
- 2.World Health Organization Who cancer fact sheet, 2018. Available: https://www.who.int/news-room/fact-sheets/detail/cancer [Accessed 19 Jan 2019].
- 3.Li L, Yeo W. Value of quality of life analysis in liver cancer: a clinician's perspective. World J Hepatol 2017;9:867–83. 10.4254/wjh.v9.i20.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galle PR, Finn RS, Qin S, et al. Patient-Reported outcomes (PROs) from the phase III IMbrave150 trial of atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first-line treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC) [abstract]. JCO 2020;38:476 10.1200/JCO.2020.38.4_suppl.476 [DOI] [Google Scholar]
- 5.Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (nivo) vs sorafenib (sor) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) [abstract]. Ann Oncol 2019;30:v874–5. 10.1093/annonc/mdz394.029 [DOI] [Google Scholar]
- 6.Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859–70. 10.1016/S1470-2045(15)00050-9 [DOI] [PubMed] [Google Scholar]
- 7.Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282–96. 10.1016/S1470-2045(18)30937-9 [DOI] [PubMed] [Google Scholar]
- 8.Chau I, Peck-Radosavljevic M, Borg C, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer 2017;81:17–25. 10.1016/j.ejca.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 9.Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol 2002;20:2229–39. 10.1200/JCO.2002.07.093 [DOI] [PubMed] [Google Scholar]
- 10.Yount S, Cella D, Webster K, et al. Assessment of patient-reported clinical outcome in pancreatic and other hepatobiliary cancers: the FACT Hepatobiliary Symptom Index. J Pain Symptom Manage 2002;24:32–44. 10.1016/s0885-3924(02)00422-0 [DOI] [PubMed] [Google Scholar]
- 11.Gable J, Ayer D, Girvan A. Qualitative Patient Interviews to Support the FACT Hepatobiliary Symptom Index-8 Among Patients With Hepatocellular Carcinoma and Elevated Baseline Alpha-fetoprotein [abstract]. ISPOR European Congress; November 10-14, Barcelona, Spain, 2018. [Google Scholar]
- 12.EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 13.van Hout B, Janssen MF, Feng Y-S, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708–15. 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 14.Steel JL, Eton DT, Cella D, et al. Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatobiliary carcinoma. Ann Oncol 2006;17:304–12. 10.1093/annonc/mdj072 [DOI] [PubMed] [Google Scholar]
- 15.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70. 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christian-Miller N, Frenette C. Hepatocellular cancer pain: impact and management challenges. J Hepatocell Carcinoma 2018;5:75–80. 10.2147/JHC.S145450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun VC-Y, Sarna L. Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs 2008;12:759–66. 10.1188/08.CJON.759-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 19.Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54–63. 10.1056/NEJMoa1717002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abou-Alfa GK, Mollon P, Meyer T, et al. Quality-Adjusted life years assessment using cabozantinib for patients with advanced hepatocellular carcinoma (aHCC) in the CELESTIAL trial. JCO 2019;37:207 10.1200/JCO.2019.37.4_suppl.207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000797supp001.pdf (355.2KB, pdf)