Abstract
Background
The nosocomial pathogen Enterococcus faecium can survive for prolonged periods of time on surfaces in the absence of nutrients. This trait is thought to contribute to the ability of E. faecium to spread among patients in hospitals. There is currently a lack of data on the mechanisms that are responsible for the ability of E. faecium to survive in the absence of nutrients.
Results
We performed a high-throughput transposon mutant library screening (Tn-seq) to identify genes that have a role in long-term survival during incubation in phosphate-buffered saline (PBS) at 20 °C. A total of 24 genes were identified by Tn-seq to contribute to survival in PBS, with functions associated with the general stress response, DNA repair, metabolism, and membrane homeostasis. The gene which was quantitatively most important for survival in PBS was usp (locus tag: EfmE745_02439), which is predicted to encode a 17.4 kDa universal stress protein. After generating a targeted deletion mutant in usp, we were able to confirm that usp significantly contributes to survival in PBS and this defect was restored by in trans complementation. The usp gene is present in 99% of a set of 1644 E. faecium genomes that collectively span the diversity of the species.
Conclusions
We postulate that usp is a key determinant for the remarkable environmental robustness of E. faecium. Further mechanistic studies into usp and other genes identified in this study may shed further light on the mechanisms by which E. faecium can survive in the absence of nutrients for prolonged periods of time.
Keywords: Enterococcus faecium, Starvation, Stress response, Functional genomics, Tn-seq
Background
Enterococcus faecium is a commensal of the human gut, but has emerged over the last few decades as an opportunistic pathogen which causes infections in hospitalized patients. E. faecium infections are often difficult to treat due to the high prevalence of resistance to antibiotics, including virtually all cephalosporins, aminoglycosides, clindamycin, and trimethoprim-sulfamethoxazole [1]. Additionally, resistance to the glycopeptide vancomycin is increasingly widespread in E. faecium strains, further complicating the treatment of infections. While the accumulation of antibiotic resistance determinants is a major contributor to E. faecium’s emergence as an important nosocomial pathogen, other adaptations, like the ability to form biofilms [2–4] and interact with host extracellular matrix and serum components [5], are also widespread in clinical isolates. Genomic analyses have revealed that the majority of recent clinical isolates of E. faecium belong to a defined sub-population which was termed clade A1, with strains from other niches, including animals and healthy humans mostly belonging to other clades in the E. faecium population [6–8].
E. faecium also has the ability to persist for long periods of time on synthetic surfaces like table tops, handrails, doorknobs and other medical surfaces [9–11]. The ability of E. faecium to spread via fomites is thus proposed to play a critical role in the inter-patient spread of E. faecium in hospital settings [12–14]. Many nosocomial pathogens are thought to spread via environmental contamination, but E. faecium can survive 3- to 5-times longer on inanimate objects compared to other Gram-positive nosocomial pathogens, such as Enterococcus faecalis, Staphylococcus aureus, and streptococci [15–17].
In natural environments, bacteria most often exist in a physiological state that is similar to the stationary phase of growth and are adapted to survive harsh conditions [18, 19]. While survival strategies like sporulation are limited to a subset of bacterial species, most bacteria possess the ability to survive in the complete absence of nutrients (e.g. in water or buffers) for weeks, months and in some cases even years [20, 21]. Research into the mechanisms by which bacteria manage to survive these nutrient-limited conditions is currently relatively scarce. Most stress responses upon nutrient deprivation involve the expression or activity of DNA repair systems, transcriptional regulation, metabolic pathways, and the biogenesis of cell walls and membranes [21, 22].
While survival in the absence of nutrients appears to be an important factor in the spread of E. faecium as a nosocomial pathogen, we currently lack an understanding of the genes involved in these processes. For this study, we have elucidated the E. faecium genes involved in survival upon starvation by high-throughput screening of a mariner transposon mutant library (Tn-seq) of a clinical E. faecium strain upon incubation in a buffer without carbon- or nitrogen-sources at room temperature for seven days. With this study, we generated the first insights into the mechanisms by which E. faecium can survive in the absence of nutrients.
Results
Effect of nutrients and temperature during prolonged incubation
The vancomycin-resistant, clade A1 strain E. faecium E745 was incubated at either 37 °C or 20 °C in the rich medium Brain Heart Infusion broth (BHI) or in phosphate-buffered saline (PBS) for up to 10 days (Fig. 1). By determining viable counts, we showed that cells in BHI were able to survive for a prolonged period of time. Indeed, the stationary phase cultures incubated in BHI at 20 °C, showed no reduction in viable counts during the course of the experiments, while at 37 °C the cultures were only losing their viability (0.9-log10 reduction) after more than 7 days. During incubation in the nutrient-free buffer PBS, significant reductions in viable counts were observed after 2 days of incubation at 37 °C and after 6 days at 20 °C, but a sub-population of cells remained viable until the end of the experiment.
Fig. 1.
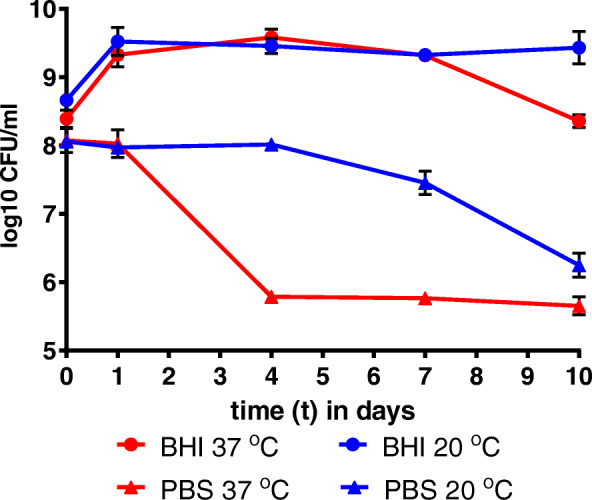
Survival of E. faecium E745 during starvation. E. faecium strain E745 was incubated for up to 10 days in BHI (circles) or PBS (triangles) at either 37 °C (red) or 20 °C (blue). Error bars show the standard deviation of data from three independent experiments and can be obscured by the symbol
E. faecium E745 genes required for survival during starvation
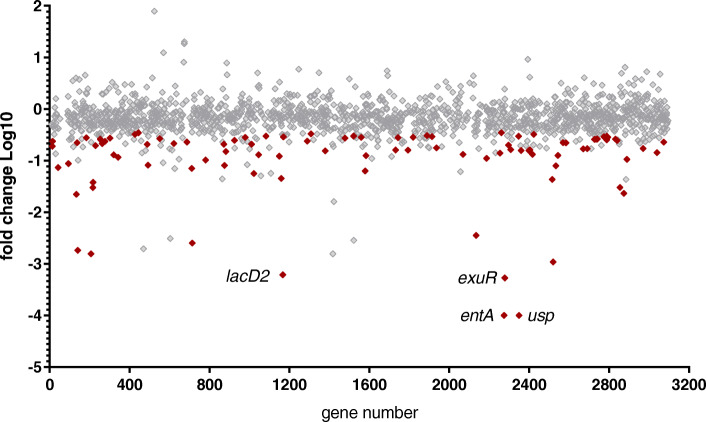
To investigate which genes were required for survival in PBS at 20 °C, we incubated a transposon mutant library of E745 in PBS for 7 days at 20 °C. The relative abundance of transposon mutants in 1631 chromosomal and plasmid genes of E. faecium E745 at day 7 was compared to the abundance of mutants present at the start of the experiment (Fig. 2). The abundance of transposon mutants in 24 genes was significantly reduced (Benjamini-Hochberg [BH] corrected P-value of < 0.05) by 10-fold or more, indicating that these genes contribute importantly to the survival of E. faecium in PBS (Table 1). We did not find any mutations that led to a higher abundance after incubation in PBS. The predicted functions of the 24 genes contributing to survival in PBS cover four groups, i.e. general stress response, DNA repair, metabolism, and membrane homeostasis. Transposon mutants in four genes had a greatly reduced (more than a 1000-fold) abundance upon 7-day incubation in PBS, indicating that these genes have a particularly large impact on survival in nutrient-limited conditions.
Fig. 2.
Tn-seq analysis to identify genes with a role in survival during starvation. Each diamond represents a gene represented in the transposon library. The Y-axis indicates the relative abundance of each gene after incubation in PBS at 20 °C for 7 days compared to the abundance of each gene at the start of the experiment. A positive value indicates an enrichment of mutants during incubation in PBS while a negative value indicates genes that contribute to survival in PBS, with red diamonds representing genes that passed the threshold for statistical significance. The highlighted dots represent genes that are discussed in the text
Table 1.
Genes with a role in survival during starvation as determined by Tn-seq. Genes of which the transposon mutants were reduced by more than − 10-fold upon incubation in PBS for 7 days, using a BH-adjusted P-value of < 0.05 as cut-off for statistical significance
| Locus Tag | Name | Function | Fold Change | P-value (BH adjusted) |
|---|---|---|---|---|
| EfmE745_02439 | usp | Putative universal stress protein | <−2.5 × 105 | 0.023 |
| EfmE745_02361 | entA | Bacteriocin EntA | <−9.2 × 104 | 0.020 |
| EfmE745_02364 | exuR | DNA-binding transcriptional repressor | −1.9 × 103 | 0.034 |
| EfmE745_01205 | lacD2 | Tagatose 1,6-diphosphate aldolase 2 | −1.6 × 103 | 0.018 |
| EfmE745_02646 | sacY | Levansucrase and sucrase synthesis operon antiterminator | − 922.6 | 0.020 |
| EfmE745_00210 | – | hypothetical protein | − 639.1 | 0.042 |
| EfmE745_00142 | – | DNA alkylation repair enzyme | − 548.8 | 0.034 |
| EfmE745_00731 | licA_1 | Lichenan-specific phosphotransferase enzyme IIA component | − 396.6 | 0.039 |
| EfmE745_02217 | gbpA | GlcNAc-binding protein A precursor | −280.7 | 0.043 |
| EfmE745_00135 | – | Mga helix-turn-helix domain protein | −44.8 | 0.034 |
| EfmE745_03020 | – | hypothetical protein | −43.1 | 0.010 |
| EfmE745_00219 | rhaD | Rhamnulose-1-phosphate aldolase | −33.3 | 0.028 |
| EfmE745_02998 | – | hypothetical protein | −33.1 | 0.010 |
| EfmE745_00220 | rhaM | L-rhamnose mutarotase | −26.4 | 0.034 |
| EfmE745_02641 | – | putative HTH-type transcriptional regulator | −23.1 | 0.034 |
| EfmE745_01197 | – | hypothetical protein | −22.1 | 0.023 |
| EfmE745_01055 | – | ABC-2 family transporter protein | −17.8 | 0.040 |
| EfmE745_01627 | – | PTS system glucitol/sorbitol-specific transporter subunit IIA | −15.9 | 0.016 |
| EfmE745_00728 | cshA | DEAD-box ATP-dependent RNA helicase | −14.1 | 0.038 |
| EfmE745_00043 | tmpC | Membrane lipoprotein TmpC precursor | −13.6 | 0.016 |
| EfmE745_02660 | drrA | Daunorubicin/doxorubicin resistance ATP-binding protein | −12.6 | 0.046 |
| EfmE745_00895 | rnmV | Ribonuclease M5 | −12.4 | 0.038 |
| EfmE745_00502 | metP | Methionine import system permease protein | −12.3 | 0.033 |
| EfmE745_00095 | – | Arsenical resistance operon trans-acting repressor ArsD | −11.4 | 0.029 |
The usp gene (locus-tag: EfmE745_02439) was identified as the most important gene involved in survival in PBS. Inspection of the mariner transposon insertion sites in usp revealed no unusual patterns in the abundance or placement of the transposon insertion sites, supporting the validity of this hit (Fig. 3a). The usp gene encodes a 17.4 kDa putative universal stress protein with no clear function, although homologs are widespread among all domains of life [23, 24]. The second-highest hit in the Tn-seq analyses was found to be in the gene that encodes the previously characterised enterococcal bacteriocin EntA (locus tag: EfmE745_02361) [25, 26]. Like for usp, transposon insertions in entA were no longer detected in the library at day 7. Bacteriocins are small secreted proteins or peptides produced by bacteria to reduce competition of unrelated bacterial strains. A unique feature of bacteriocins is that they are always co-expressed with their associated immunity gene to avoid auto-inhibition [27]. When we inspected the distribution of transposon insertions in entA, we noted that there was only one insertion site in entA which is located close to the 3′ end of the gene (Fig. 3b). As the presumptive bacteriocin immunity gene was found to be overlapping with entA and was also disrupted by the transposon insertion, there is a distinct possibility that this transposon mutant is killed due to the production of the EntA bacteriocin during nutrient deprivation, by the other mutants in the population in which these genes were not disrupted. For this reason, we did not include entA in further functional analyses (described below). The transposon mutants in EfmE745_02364 (exuR) and EfmE745_01205 (lacD2) (Fig. 3c and d) were also highly reduced upon incubation in PBS, and transposon insertion sites in these genes showed no unusual patterns.
Fig. 3.
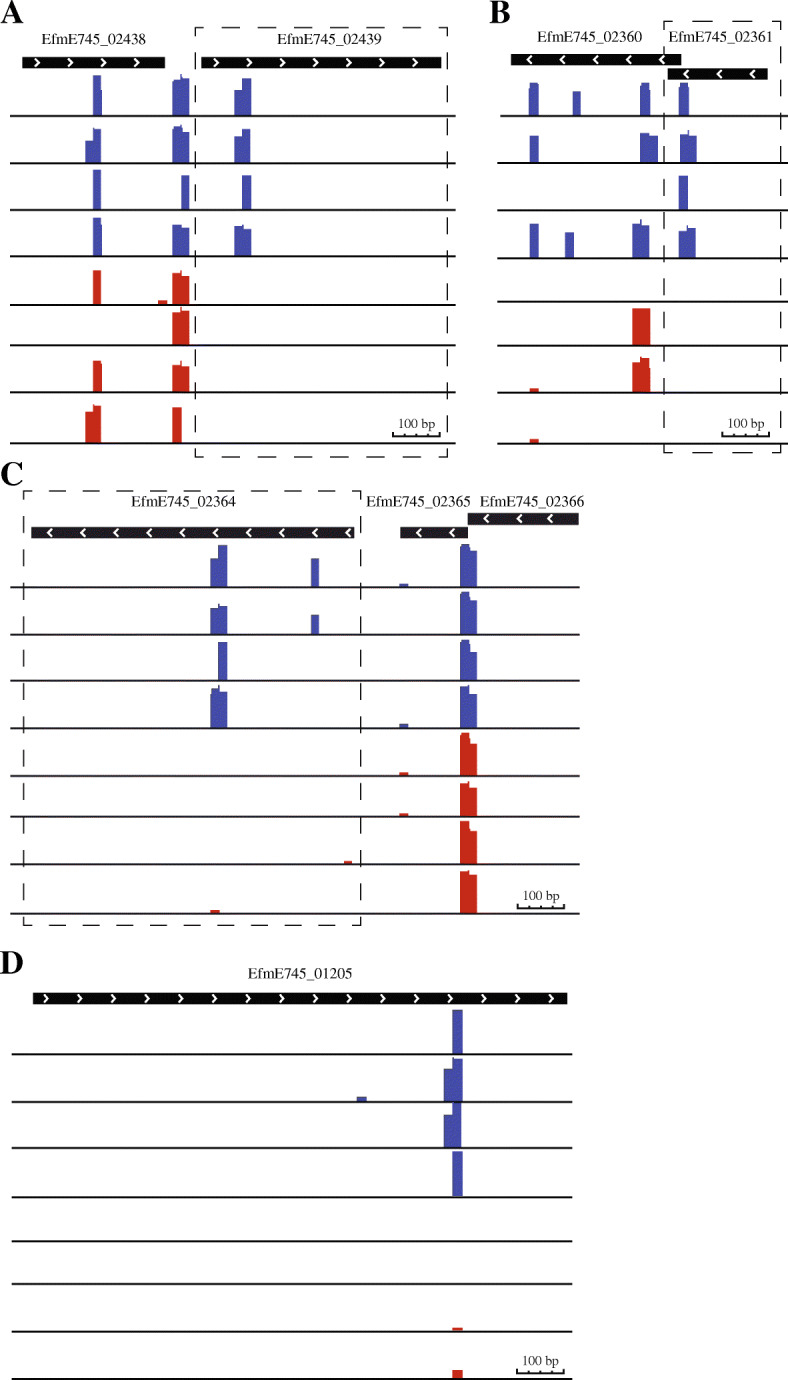
Visual representation of the transposon insertion sites in usp, entA, exuR and lacD2. Genes and gene direction are depicted by the black bars with arrows. Transposon insertion abundance is shown by the bars below the genes on a Log10 scale. Blue and red bars denote the abundance of transposon insertion mutants at day 0 and at day 7, respectively. Abundance of transposon insertion are shown for EfmE745_02439 (usp), EfmE745_02361 (entA), EfmE745_02364 (exuR) and EfmE745_01205 (lacD2) in panel a, b, c and panel d, respectively
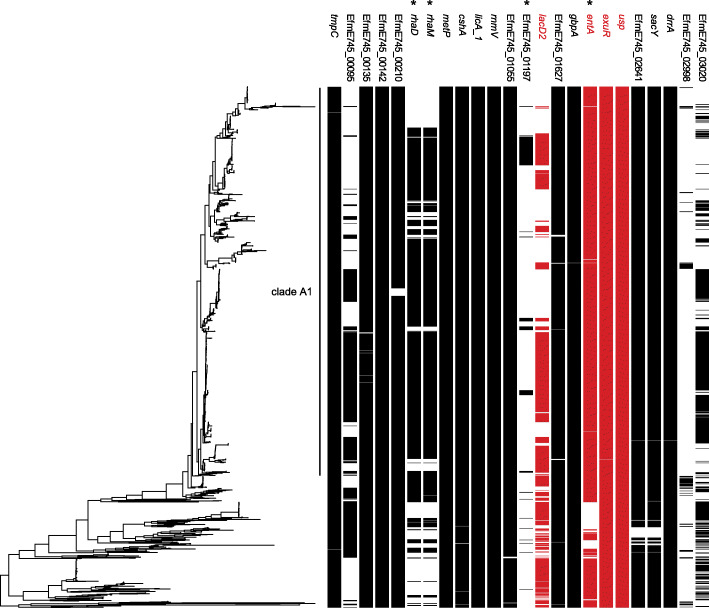
Next we studied the presence of all 24 genes identified by Tn-seq in the whole genome sequences of a collection of 1644 E. faecium strains isolated from healthy humans, patients, pets, pigs, and poultry (Fig. 4), previously described in [8]. We found that usp and exuR are present in 99, and 100% respectively of the isolates tested and these genes can be thus considered part of E. faecium core genome. The lacD2 gene was present in 53% of genome sequences in this collection. Four genes, including entA, were found to be significantly enriched (χ2 test, P < 0.05 with Benjamini-Hochberg-correction for multiple testing) in clade A1 strains.
Fig. 4.
Presence of genes involved in long-term survival under nutrient-limiting conditions in E. faecium. Whole genome sequence based phylogenetic tree of 1644 E. faecium strains representing the global E. faecium population. The presence of the different E745 genes was plotted along the phylogeny using the R package ggtree. Highlighted in red are EfmE745_01205 (lacD2), EfmE745_02361 (entA), EfmE745_02364 (exuR) and EfmE745_02439 (usp). Genes that are significantly (χ2 test, P < 0.05 with Benjamini-Hochberg-correction for multiple testing) enriched in clade A1 strains versus non-clade A1 strrains are indicated by an asterisk
To further test the importance of these genes we have attempted to create gene deletion mutants in usp, exuR and lacD2, but only managed to construct deletions in usp and exuR. These mutants were tested for their ability to survive in PBS for a 7-day period.
The universal stress protein Usp, but not ExuR, contributes to survival of E. faecium during prolonged starvation
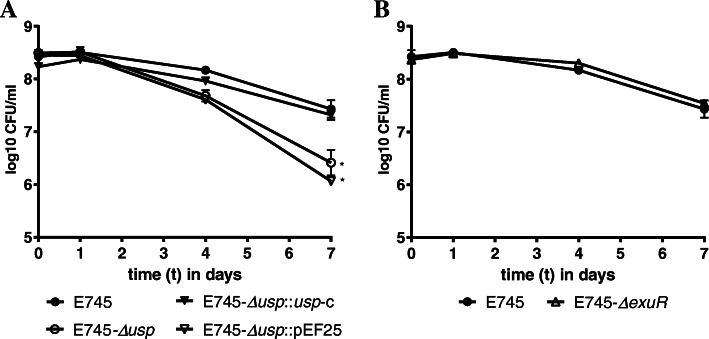
To determine whether usp has an impact on the survival of E745 we created the deletion mutant E745-Δusp and complemented the deletion by supplying the usp gene in trans on a plasmid (strain E745-Δusp::usp-C). We incubated E745, E745-Δusp, E745-Δusp::usp-C, and E745-Δusp::pEF25 (the usp deletion mutant transformed with the empty vector used for in trans complementation) in PBS at 20 °C and determined viability of the cell suspension over a 7-day period (Fig. 5a). The E. faecium E745 strain showed a similar survival response as seen in Fig. 1. However, the E745-Δusp and E745-Δusp::pEF25 cell suspension lost their viability quicker than E745, with a statistically significant (P < 0.05; one way ANOVA) 1-log lower viable count after 7 days than the wild-type. The complemented strain, E745-Δusp::usp-C, responded similar to E745 with no statistically significant difference detected at any time point. These results confirm the role of usp in survival of E. faecium E745 in the absence of nutrients. E745-ΔexuR was found to survive equally well as the wild-type strain during starvation (Fig. 5b), suggesting that exuR does not have a role in survival during starvation.
Fig. 5.
Phenotypic analysis of usp and exuR deletion mutants. Data for usp and exuR deletion mutants are shown in panel a and b, respectively, and represent three biologically independent experiments. All strains were incubated in PBS at 20 °C for up to 7 days. * indicates a statistical difference (P < 0.05) between survival of E. faecium E745 and Δusp and Δusp::pEF25 at day 7, as determined by one way ANOVA. Error bars show the standard deviation of data and can be obscured by the symbol
Discussion
Most bacteria have a ‘feast and famine’ lifestyle where long periods of severe nutrient limitation are interrupted by short bursts of nutrient availability [21]. For E. faecium, we propose that this cycle is particularly relevant for the spread of this opportunistic pathogen in hospitals, where it can survive on inanimate objects until it is transferred to a human host. Indeed, recent work has suggested that this is a trait that characterizes the genus Enterococcus and has been selected for over hundreds of millions of years of microbial evolution [28]. The finding that the genes most likely to contribute to the survival of E. faecium during starvation are part of the core genome of this species, support the crucial role of starvation tolerance in the evolution of E. faecium. While E. faecium has a low intrinsic virulence, it has become a significant threat to immunocompromised patients in hospitals due to its ability to rapidly acquire genes involved in antibiotic resistance and other traits that contribute to its ability to successfully colonize patients [29]. The mechanisms by which it can survive and spread in the hospital environment have so far received less attention.
In this study we investigated the potential of vancomycin-resistant Enterococcus faecium to survive in the absence of nutrients. This trait may contribute to the ability of E. faecium to survive on inanimate objects in hospitals and thus can impact its success as a nosocomial pathogen. We noted that in the complete absence of nutrients, during incubation in PBS, E. faecium cultures exhibit a small loss in viability over a period of 7 days at 20 °C, while at 37 °C a significant loss of viability has occurred after four days, although a sub-population of cells remains viable, which could represent persister cells [30]. We note that the E. faecium cells may be entering a ‘viable but non-culturable’ state after prolonged incubation in our experiments [31]. The faster decrease in viability at 37 °C, compared to 20 °C, may be due to increased enzymatic activities and higher metabolic turnover at the higher temperature [32].
To obtain a better understanding into the genes that are involved in the response upon prolonged starvation, we performed Tn-seq on a transposon mutant library that was starved for nutrients for 7 days at 20 °C. This analysis revealed a variety of genes that were potentially involved in retaining viability. Genes could be assigned to four major functional groups, i.e. DNA repair, metabolism, cell membrane and cell wall homeostasis. DNA repair, or lack thereof, can contribute to survival during starvation as reactive oxygen species and other DNA damaging molecules accumulate [33]. Metabolic adaptations during starvation are essential as shifts in metabolism, including changes in transport systems, are required for optimal acquisition of nutrients and the excretion of metabolic waste products [34, 35]. Changes to the cytoplasmic membrane and cell wall have also been described to contribute to the ability of cells to withstand harsh conditions, including starvation [28]. The final category of genes we identified in our Tn-seq analysis have roles in the general stress response, i.e. the cell’s extended toolbox for responding to adverse conditions [23, 24]. Our Tn-seq data suggest that most genes involved in survival during nutrient deprivation are part of the core genome of E. faecium. However, four genes are enriched in clade A1 strains which could suggest that these genes may play a role in the emergence of strains from this clade as nosocomial pathogens.
We studied the role of the general stress protein Usp (locus tag: EfmE745_02439) which belongs to this last functional group and confirmed that it has a significant impact on survival of E. faecium during starvation. Universal stress proteins (USPs) are mostly studied in E. coli where they protect the cell against adverse conditions. However, the exact mechanism(s) by which they act are currently unknown. This could be a result of the many different subtypes found, and the complex responses they contribute to. USPs are found throughout all branches of the tree of life and are important for survival under adverse conditions in many species [23, 24]. In this study we expand these observations to E. faecium.
The exuR gene (locus-tag: EfmE745_02364) appeared to be important for survival upon nutrient starvation on the basis of our Tn-seq screening, but we were unable to confirm this finding by studying an exuR deletion mutant. The E. coli ExuR homologue represses genes involved in the metabolism of D-galacturonate and D-glucuronate [36, 37]. ExuR expression is upregulated in the absences of glucose which allows E. coli to use different carbon sources for its growth and survival. We speculate that ExuR in E. faecium is involved in rerouting carbohydrate metabolism, similar to E. coli. It is possible that the derepression of pathways involved in alternative carbon source utilization in the ΔexuR mutant might negatively affect the growth of cells during the recovery step in the glucose-rich medium BHI, which we performed to minimize a Tn-seq signal originating from dead cells in the cell suspension.
Conclusions
In this study, we have identified 24 E. faecium genes that could potentially affect its survival in the absence of nutrients. Our Tn-seq data suggest that most genes involved in survival during nutrient deprivation are part of the core genome of E. faecium. However, four genes are enriched in clade A1 strains which could suggest that these genes may play a role in the emergence of strains from this clade as nosocomial pathogens. We have confirmed the role of the usp gene, but future functional characterization of other genes identified here may further increase our understanding of the mechanisms by E. faecium can survive outside human or animal hosts.
Methods
Bacterial strains, plasmids, growth conditions, and oligonucleotides
The vancomycin-resistant, clade A1 E. faecium strain E745 [38] was used throughout this study. E. faecium E745 was isolated from a rectal swab of a patient, during routine surveillance of a VRE outbreak in a Dutch hospital, and its genome was previously sequenced to completion [38]. E. faecium was grown at 37 °C in brain heart infusion broth (BHI; Oxoid), unless otherwise mentioned. The E. coli strains EC1000 [39] and DH5α were grown in Lysogeny Broth (LB). Antibiotics were used at the following concentrations: erythromycin 50 μg ml− 1, chloramphenicol 10 μg ml− 1, spectinomycin 200 μg ml− 1 and gentamicin 300 μg ml− 1 for E. faecium and spectinomycin 100 μg ml− 1, chloramphenicol 4 μg ml− 1, erythromycin 100 μg ml− 1 and gentamicin 50 μg ml− 1 for E. coli. The transposon library in E. faecium E745 was previously described [38]. The vectors pWS3 [40], pVDM1001 [41], pCRE-Lox [42], pEF25 [43] and pGPA1 [38] were obtained from our laboratory’s culture collection. Genomic DNA isolation was performed using the Wizard Genomic DNA Purification kit (Promega).
The sequences of all oligonucleotides used in this study are provided in Table 2. The sequences of synthesized DNA fragments are listed in Additional file 1.
Table 2.
Oligonucleotides used in this study. Relevant restriction sites are underlined
| Name | Sequence (5′- 3′) |
|---|---|
| oVDM2001 | AAACACGTTCTTTCCAAACCGTTTCATCCTTTGAG |
| oVDM2002 | AAAACTCAAAGGATGAAACGGTTTGGAAAGAACGT |
| oVDM2003 | CAAACAACGGCATTAACGGAGATTACCAT |
| oVDM2004 | GTGTTTGATAAAAAACTTTGTCTACATGAC |
| oVDM2005 | CTGCATATGCTGTTTTAGGCG |
| oVDM2006 | GTGTAATTCTGTCAGAGAGC |
| oVDM2007 | AAAAAAAACCCGGGCAATTGGGGCTATCCATCGGGAAATGAATGCC |
| oVDM2008 | AAAAAAAACTCGAGTTATGACGACGGACTTCTCGC |
| oVDM2009 | GAGGGAATTCTACCGTTCGTATAGCATACATTATACGAAGTTATGATAAACCCAGCGAACCATTTGAGG |
| oVDM2010 | CTCCGAATTCTACCGTTCGTATAATGTATGCTATACGAAGTTATTCAATCTTTATAAGTCCTTTTATAA |
| oVDM2011 | GGAGGCAGATTGCCTTGAAT |
| oVDM2012 | TCCAATAATTCGGCTCTCT |
| oVDM2013 | GACTCGTTTATGTTTTGTTCCGTC |
| oVDM2014 | GTATTTTCCAAGATCGTTCG |
| oVDM2015 | AAAAAAGTCGACACAAGAAAACGGTTCTGTTTCTGCGAGC |
| oVDM2016 | AAAAAAACCCGGGTTAAGCTTTGGTGTGGTCTTTAGCT |
| oVDM2017 | CGGAGCCACGGCGGCCGAA |
| oVDM2018 | CTGCGCGTAATCTGCTGCTTCGA |
Isolation and transformation of plasmids
Plasmid isolation from overnight E. coli cultures was performed using the GeneJET plasmid miniprep kit (Thermo Fischer Scientific, Bleiswijk, The Netherlands) according to the manufacturer’s instructions. Transformation of plasmids into E. faecium E745 was performed as previously described [42].
Determination of viability during starvation
E. faecium E745 cultures were grown overnight in 3 ml BHI at 37 °C with shaking at 150 rpm. An aliquot of 20 μl of an overnight culture was transferred to 20 ml BHI which was again grown at 37 °C with shaking at 150 rpm until the culture reached an optical density at 600 nm (OD600) of 0.3–0.4. Aliquots (3 ml) of these cultures were then centrifuged (3000 g, 5 min), washed once in PBS (137 mM NaCl; 2.7 mM KCl; 10 mM Na2HPO4; 2 mM KH2PO4; pH 7.4) and resuspended in 3 ml PBS and incubated at either 37 °C or 20 °C with continuous shaking at 150 rpm. Controls remained in BHI and were incubated at the same temperatures with shaking. At days 0, 1, 4, 7, and 10 samples were taken and serially diluted in PBS, after which viable counts were determined using the Miles and Misra method [44].
Tn-seq analysis of conditionally essential genes in E. faecium E745 during prolonged starvation
For the identification of genes that were conditionally essential for prolonged starvation in E. faecium, we grew the previously constructed mariner transposon mutant library in E. faecium E745 [38] in 10-ml BHI supplemented with gentamicin overnight at 37 °C, after which 20 μl of the culture was transferred to 10 ml pre-warmed BHI supplemented with 200 μg ml− 1 gentamicin, which was incubated at 37 °C until the culture reached an OD600 of 0.3. After pelleting the cells by centrifugation (3000 g, 5 min), washing once with PBS, and resuspension of the cells in 10 ml PBS, a 3 ml aliquot was transferred to a new tube and incubated at 20 °C for 7 days. The remaining suspension was used for the isolation of genomic DNA. At day 7, the cell suspensions were washed once with PBS and then transferred to 3 ml prewarmed BHI and incubated at 37 °C until OD600 0.3 to recover viable cells. The experiments with the transposon mutant library were performed with four independent replicates.
After genomic DNA extraction, Tn-seq libraries were prepared as described previously [38]. Tn-seq libraries were sequenced on one lane of a HiSeq 2500 with 50 nt single-end reads. The sequence reads of the Tn-seq experiments have been made available in the European Nucleotide Archive with accession number PRJEB37076.
Tn-seq data analysis
Tn-seq data analysis was performed as described previously [38]. In short, Illumina sequence reads were demultiplexed, based on their barcode, using Galaxy [45], and 16-nucleotide fragments of each read, corresponding to E745 genomic sequences flanking the transposon, were mapped to the E745 genome using Bowtie 2 [46]. The number of reads per gene were determined using the Integrative Genome Viewer (IGV [47];). Reads that mapped to the final 10% of a gene were discarded as these insertions may not inactivate gene function [48]. Read counts per gene were then normalized in each replicate by calculating the RPKM (Reads Per Kilobase per Million input reads), with subsequent statistical analysis of these values being performed with Cyber-T [49, 50]. Genes were determined to be significantly contributing to survival in PBS if the Benjamini-Hochberg-corrected P-value was < 0.05 and the difference in abundance of a gene between day 0 and day 7 was > 10 or < − 10.
Construction of targeted deletion mutants
The usp deletion mutant was created using CRISPR-Cas9-mediated genome editing as previously described [41]. In short, a CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat) targeting usp was inserted into pVDM1001 by annealing oVDM2001 and oVDM2002 together and ligating this DNA fragment into BsaI-digested pVDM1001 creating pVDM-xusp. Next, a DNA fragment (gBlock, Integrated DNA Technologies; Leuven, Belgium) was synthesized containing the 463 bp upstream region of usp fused together with the 513 bp downstream region of usp (Additional file 1). This DNA fragment was amplified using oVDM2003 and oVDM2004 and was subsequently ligated in SmaI-digested pVDM-xusp creating pVDM-Δusp. This plasmid was then transformed into E. faecium E745 which already carried pVPL3004 [51] with selection for transformants on BHI agar containing 200 μg ml− 1 spectinomycin and 50 μg ml− 1 erythromycin at 30 °C for 48 h. The deletion of usp was confirmed by PCR using oVDM2005 and oVDM2006. The plasmids pVPL3004 and pVDM-Δusp were cured from E. faecium E745-Δusp by sub-culturing in BHI for 72 h.
The 848-bp DNA fragment containing the promotor of usp and the complete usp gene for in trans complementation (Additional file 1) was synthesized, and subsequently this fragment was amplified by PCR using oVDM2015 and oVDM2016, and subsequently digested with XmaI and SalI. The digested fragment was ligated into pEF25 to form pEF25-usp-C. This vector was subsequently transformed into E745-Δusp and transformants were selected on BHI agar supplemented with 200 μg ml− 1 spectinomycin. The presence of the vector in E. faecium E745-Δusp was confirmed by PCR using oVDM2017 and oVDM2018 and the resulting complemented strain was named E745-Δusp::usp-C.
We were unable to generate an exuR deletion mutant with the CRISPR-based genome editing approach described above and we thus used our previously described [42] allelic replacement method, with minor modifications, to generate this mutant. A DNA fragment (Integrated DNA Technologies; Leuven, Belgium) was ordered containing the 496 bp upstream region of exuR fused to the 515 bp region downstream of exuR (Additional file 1). The DNA fragment was amplified using oVDM2007 and oVDM2008, digested with XhoI and XmaI and subsequently ligated into similarly digested pWS3 to create pVDM-exuR. The gentamicin resistance cassette from pGPA1 was amplified using oVDM2009 and oVDM2010, followed by digestion with EcoRI. This DNA fragment was then ligated into similarly digested pVDM-exuR, resulting in pVDM-exuR + G. E745 was transformed with pVDM-exuR + G and transformants were selected at 30 °C on BHI supplemented with 200 μg ml− 1 spectinomycin. The presence of pVDM-exuR + G in the transformants was confirmed by PCR using oVDM2011 and oVDM2012. To induce a double crossover event to replace exuR with the gentamicin resistance cassette, a colony harbouring pVDM-exuR + G was used to inoculate 200 ml BHI medium and then subcultured for 72 h at 37 °C after which the culture was plated on BHI with 300 μg ml− 1 gentamicin. At least 100 colonies were transferred to BHI agar containing either 300 μg ml− 1 gentamicin or 200 μg ml− 1 spectinomycin. Colonies that were resistant to gentamicin but not spectinomycin were checked for successful double crossover events using oVDM2013 and oVDM2014. The resulting mutant was named E745::ΔexuR + G and was consequently transformed with pCRE-lox to remove the gentamicin cassette from the genome, as described previously [42]. Removal of the gentamicin cassette was confirmed using PCR using oVDM2013 and oVDM2014 and curing of pCRE-lox after removal of the gentamicin cassette was performed as described previously [42].
Presence and absence of genes involved in survival during starvation in E. faecium genomes
Abricate (https://github.com/tseemann/abricate, version 0.8) was used to identify presence of the 24 E. faecium E745 genes that had the largest role in survival in PBS, as determined by Tn-seq, against the draft assemblies from 1644 E. faecium genomes that represent the global diversity of the species E. faecium [8]. We considered a minimum identity and coverage of 95 and 80%, respectively, to consider a gene as present in a particular draft assembly. To visualize the presence and absence of these genes in the context of the phylogeny of E. faecium, we used the neighbour-joining tree described in [8], based on a RAxML tree-based of 955 E. faecium core genes, and used the R package ggtree (version 1.14.6) to plot the presence of the different E745 genes.
Supplementary information
Additional file 1. Sequences of synthesized DNA fragments for the deletion of usp and exuR and the in trans complementation of usp.
Acknowledgements
Not applicable.
Abbreviations
- PBS
Phosphate-buffered saline
- BHI
Brain Heart Infusion broth
- BH
Benjamini-Hochberg
- USPs
Universal Stress Proteins
- LB
Lysogeny Broth
- OD600
Optical density at 600 nm
- RPKM
Reads Per Kilobase per Million input reads
- IGV
Integrative Genome Viewer
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeat
Authors’ contributions
VdM performed experiments and analysed the data. SAA contributed bioinformatic analyses. RW and WvS designed the study. VdM and WvS drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a VIDI grant (917.13.357) from the Netherlands Organization for Scientific Research (VIDI: 917.13.357) and a Royal Society Wolfson Research Merit Award (WM160092) to W.v.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets generated in the current study are available in the European Nucleotide Archive with accession number PRJEB37076. The genome sequence of E. faecium E745 used in this study is available through the European Nucleotide Archive with accession number GCA_001750885.1. Requests for E. faecium E745 can be made by contacting the corresponding author.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12864-020-06984-2.
References
- 1.García-Solache M, Rice LB. The Enterococcus: a model of adaptability to its environment. Clin Microbiol Rev. 2019;32:1–28. doi: 10.1128/CMR.00058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heikens E, Singh KV, Jacques-Palaz KD, van Luit-Asbroek M, Oostdijk EAN, Bonten MJM, et al. Contribution of the enterococcal surface protein Esp to pathogenesis of Enterococcus faecium endocarditis. Microbes Infect. 2011;13:1185–1190. doi: 10.1016/j.micinf.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heikens E, Bonten MJM, Willems RJL. Enterococcal surface protein esp is important for biofilm formation of Enterococcus faecium E1162. J Bacteriol. 2007;189:8233–8240. doi: 10.1128/JB.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paganelli FL, Huebner J, Singh KV, Zhang X, van Schaik W, Wobser D, et al. Genome-wide screening identifies Phosphotransferase system permease BepA to be involved in Enterococcus faecium endocarditis and biofilm formation. J Infect Dis. 2016;214:189–195. doi: 10.1093/infdis/jiw108. [DOI] [PubMed] [Google Scholar]
- 5.Zhao M, Sillanpää J, Nallapareddy SR, Murray BE. Adherence to host extracellular matrix and serum components by Enterococcus faecium isolates of diverse origin. FEMS Microbiol Lett. 2009;301:77–83. doi: 10.1111/j.1574-6968.2009.01806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebreton F, van Schaik W, McGuire AM, Godfrey P, Griggs A, Mazumdar V, et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio. 2013;4:00534–00513. doi: 10.1128/mBio.00534-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raven KE, Reuter S, Reynolds R, Brodrick HJ, Russell JE, Török ME, et al. A decade of genomic history for healthcare-associated Enterococcus faecium in the United Kingdom and Ireland. Genome Res. 2016;26:1388–1396. doi: 10.1101/gr.204024.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arredondo-Alonso S, Top J, Schürch AC, McNally A, Puranen S, Pesonen M, et al. Plasmids shaped the recent emergence of the major nosocomial pathogen Enterococcus faecium. MBio. 2020;11:530725. doi: 10.1128/mBio.03284-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagenvoort JHT, De Brauwer EIGB, Penders RJR, van der Linden CJ, Willems RJ, Top J, et al. Environmental survival of vancomycin-sensitive ampicillin-resistant Enterococcus faecium (AREfm) Eur J Clin Microbiol Infect Dis. 2015;34:1901–1903. doi: 10.1007/s10096-015-2430-x. [DOI] [PubMed] [Google Scholar]
- 10.Wagenvoort JHT, De Brauwer EIGB, Penders RJR, Willems RJ, Top J, Bonten MJ. Environmental survival of vancomycin-resistant Enterococcus faecium. J Hosp Infect. 2011;77:282–283. doi: 10.1016/j.jhin.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein RA, Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39:1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farr BM. Prevention and control of methicillin-resistant Staphylococcus aureus infections. Curr Opin Infect Dis. 2004;17:317–322. doi: 10.1097/01.qco.0000136926.52673.cd. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45:255–262. doi: 10.1053/jhin.2000.0768. [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Howden BP, Stinear TP. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr Opin Microbiol. 2018;41:76–82. doi: 10.1016/j.mib.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 15.de Regt MJA, van der Wagen LE, Top J, Blok HEM, Hopmans TEM, Dekker AW, et al. High acquisition and environmental contamination rates of CC17 ampicillin-resistant Enterococcus faecium in a Dutch hospital. J Antimicrob Chemother. 2008;62:1401–1406. doi: 10.1093/jac/dkn390. [DOI] [PubMed] [Google Scholar]
- 16.Wagenvoort JH, Penders RJ. Long-term in-vitro survival of an epidemic MRSA phage-group III-29 strain. J Hosp Infect. 1997;35:322–325. doi: 10.1016/s0195-6701(97)90229-2. [DOI] [PubMed] [Google Scholar]
- 17.Wagenvoort JHT, Joosten EJAJ. An outbreak Acinetobacter baumannii that mimics MRSA in its environmental longevity. J Hosp Infect. 2002;52:226–227. doi: 10.1053/jhin.2001.1294. [DOI] [PubMed] [Google Scholar]
- 18.Finkel SE. Long-term survival during stationary phase: evolution and the GASP phenotype. Nat Rev Microbiol. 2006;4:113–120. doi: 10.1038/nrmicro1340. [DOI] [PubMed] [Google Scholar]
- 19.Leclercq R, Oberlé K, Galopin S, Cattoir V, Budzinski H, Petit F. Changes in enterococcal populations and related antibiotic resistance along a medical center-wastewater treatment plant-river continuum. Appl Environ Microbiol. 2013;79:2428–2434. doi: 10.1128/AEM.03586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao CH, Shollenberger LM. Survivability and long-term preservation of bacteria in water and in phosphate-buffered saline. Lett Appl Microbiol. 2003;37:45–50. doi: 10.1046/j.1472-765x.2003.01345.x. [DOI] [PubMed] [Google Scholar]
- 21.Navarro Llorens JM, Tormo A, Martínez-García E. Stationary phase in gram-negative bacteria. FEMS Microbiol Rev. 2010;34:476–495. doi: 10.1111/j.1574-6976.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 22.Boutte CC, Crosson S. Bacterial lifestyle shapes stringent response activation. Trends Microbiol. 2013;21:174–180. doi: 10.1016/j.tim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kvint K, Nachin L, Diez A, Nyström T. The bacterial universal stress protein: function and regulation. Curr Opin Microbiol. 2003;6:140–145. doi: 10.1016/s1369-5274(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 24.Seifart Gomes C, Izar B, Pazan F, Mohamed W, Mraheil MA, Mukherjee K, et al. Universal stress proteins are important for oxidative and acid stress resistance and growth of Listeria monocytogenes EGD-e in vitro and in vivo. PLoS One. 2011;6:e24965. [DOI] [PMC free article] [PubMed]
- 25.O’Keeffe T, Hill C, Ross RP. Characterization and heterologous expression of the genes encoding enterocin a production, immunity, and regulation in Enterococcus faecium DPC1146. Appl Environ Microbiol. 1999;65:1506–1515. doi: 10.1128/aem.65.4.1506-1515.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henning C, Gautam D, Muriana P. Identification of multiple bacteriocins in Enterococcus spp. using an Enterococcus-specific bacteriocin PCR array. Microorganisms. 2015;3:1–16. doi: 10.3390/microorganisms3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickey RM, Twomey DP, Ross RP, Hill C. Potential of the enterocin regulatory system to control expression of heterologous genes in Enterococcus. J Appl Microbiol. 2003;95:390–397. doi: 10.1046/j.1365-2672.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 28.Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, Gilmore MS. Tracing the enterococci from paleozoic origins to the hospital. Cell. 2017;169:849–861. doi: 10.1016/j.cell.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman Prieto AM, van Schaik W, Rogers MRC, Coque TM, Baquero F, Corander J, et al. Global emergence and dissemination of enterococci as nosocomial pathogens: attack of the clones? Frontiers Microbiol. 2016;7:788. doi: 10.3389/fmicb.2016.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van den Bergh B, Fauvart M, Michiels J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol Rev. 2017;41:219–251. doi: 10.1093/femsre/fux001. [DOI] [PubMed] [Google Scholar]
- 31.Del Mar LM, Bonato B, Signoretto C, Canepari P. Vancomycin resistance is maintained in enterococci in the viable but nonculturable state and after division is resumed. Antimicrob Agents Chemother. 2003;47:1154–1156. doi: 10.1128/AAC.47.3.1154-1156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arana I, Muela A, Orruño M, Seco C, Garaizabal I, Barcina I. Effect of temperature and starvation upon survival strategies of Pseudomonas fluorescens CHA0: comparison with Escherichia coli. FEMS Microbiol Ecol. 2010;74:500–509. doi: 10.1111/j.1574-6941.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- 33.Saint-Ruf C, Pesut J, Sopta M, Matic I. Causes and consequences of DNA repair activity modulation during stationary phase in Escherichia coli. Crit Rev Biochem Mol Biol. 2007;42:259–270. doi: 10.1080/10409230701495599. [DOI] [PubMed] [Google Scholar]
- 34.Crosse AM, Greenway DLA, England RR. Accumulation of ppGpp and ppGp in Staphylococcus aureus 8325-4 following nutrient starvation. Lett Appl Microbiol. 2000;31:332–337. doi: 10.1046/j.1472-765x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- 35.Liebeke M, Dörries K, Zühlke D, Bernhardt J, Fuchs S, Pané-Farré J, et al. A metabolomics and proteomics study of the adaptation of Staphylococcus aureus to glucose starvation. Mol BioSyst. 2011;7:1241–1253. doi: 10.1039/c0mb00315h. [DOI] [PubMed] [Google Scholar]
- 36.Robert-Baudouy J, Portalier R, Stoeber F. Regulation of hexuronate system genes in Escherichica coli K-12: multiple regulation of the uxu operon by exuR and uxuR gene products. J Bacteriol. 1981;145:211–220. doi: 10.1128/jb.145.1.211-220.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hugovieux-Cotte-Pattat N, Robert-Baudouy J. Regulation and transcription direction of exuR, a self-regulated repressor in Escherichia coli K-12. J Mol Biol. 1982;156:221–228. doi: 10.1016/0022-2836(82)90468-5. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, de Maat V, Guzmán Prieto AM, Prajsnar TK, Bayjanov JR, de Been M, et al. RNA-seq and Tn-seq reveal fitness determinants of vancomycin-resistant Enterococcus faecium during growth in human serum. BMC Genomics. 2017;18:893. doi: 10.1186/s12864-017-4299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leenhouts K, Buist G, Bolhuis A, ten Berge A, Kiel J, Mierau I, et al. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol Gen Genet. 1996;253:217–224. doi: 10.1007/s004380050315. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Vrijenhoek JEP, Bonten MJM, Willems RJL, van Schaik W. A genetic element present on megaplasmids allows Enterococcus faecium to use raffinose as carbon source. Environ Microbiol. 2011;13:518–528. doi: 10.1111/j.1462-2920.2010.02355.x. [DOI] [PubMed] [Google Scholar]
- 41.de Maat V, Stege PB, Dedden M, Hamer M, van Pijkeren J-P, Willems RJL, et al. CRISPR-Cas9-mediated genome editing in vancomycin-resistant Enterococcus faecium. FEMS Microbiol Lett. 2019;366:fnz256. doi: 10.1093/femsle/fnz256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Paganelli FL, Bierschenk D, Kuipers A, Bonten MJM, Willems RJL, et al. Genome-wide identification of ampicillin resistance determinants in Enterococcus faecium. PLoS Genet. 2012;8:e1002804. doi: 10.1371/journal.pgen.1002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Top J, Sinnige JC, Majoor EAM, Bonten MJM, Willems RJL, Van Schaik W. The recombinase IntA is required for excision of esp-containing ICEEfm1 in Enterococcus faecium. J Bacteriol. 2011;193:1003–1006. doi: 10.1128/JB.00952-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. J Hyg (Lond) 1938;38:732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afgan E, Baker D, Batut B, Van Den Beek M, Bouvier D, Ech M, et al. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6. [DOI] [PMC free article] [PubMed]
- 48.Chao MC, Abel S, Davis BM, Waldor MK. The design and analysis of transposon insertion sequencing experiments. Nat Rev Microbiol. 2016;14:119–128. doi: 10.1038/nrmicro.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- 50.Kayala MA, Baldi P. Cyber-T web server: differential analysis of high-throughput data. Nucleic Acids Res. 2012;40 Web Server issue:W553–W559. doi: 10.1093/nar/gks420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh JH, Van Pijkeren JP. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res. 2014;42:1–11. doi: 10.1093/nar/gku623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Sequences of synthesized DNA fragments for the deletion of usp and exuR and the in trans complementation of usp.
Data Availability Statement
The datasets generated in the current study are available in the European Nucleotide Archive with accession number PRJEB37076. The genome sequence of E. faecium E745 used in this study is available through the European Nucleotide Archive with accession number GCA_001750885.1. Requests for E. faecium E745 can be made by contacting the corresponding author.