Figure 5. MD simulations suggest that the Dm BicD-CTD/F684I mutant switches between homotypic and asymmetric registries.
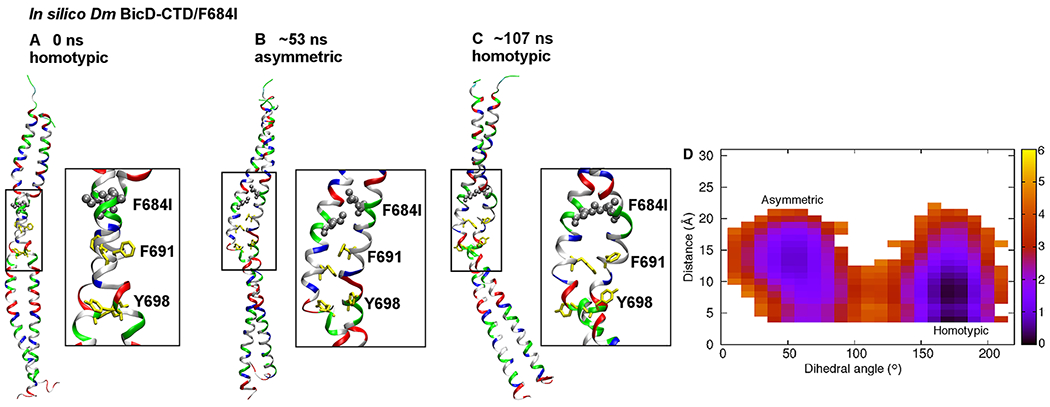
(A) Cartoon representation of the equilibrated structure of Dm BicD-CTD/F684I,30 with homotypic registry, colored by residue type (blue: positively charged, red: negatively charged, green: polar, white: non-polar). F684 was mutated to isoleucine (silver spheres). F691 and Y698 are shown in yellow stick representation. A close-up of the boxed area is shown on the right. The equilibration comprised of a 10 ns simulation with restraints on the backbone heavy atoms, and a 50 ns unrestrained simulation. (B) Structure of the F684I mutant of Dm BicD-CTD after ~53 ns of an MD simulation. Note that the N-terminal region of the coiled-coil switches to a heterotypic registry; therefore, the overall coiled-coil registry is asymmetric. (C) Structure of the F684I mutant of Dm BicD-CTD after 107 ns of the same MD simulation. Note that the structure switches back to a homotypic coiled-coil registry. However, the solvent-exposed F691 sidechains are oriented towards the same side, as opposed to opposite sides in A. This leads to a slight distortion of the coiled coil around the F691 residues. (D) Free energy in kcal/mol as a function of the C-Cα-Cβ-Cγ dihedral angle of F691 of chain A (plotted along the horizontal axis), and the distance between the sidechain N atom of K678 of chain A and the Cδ atom of E673 of chain B (plotted along the vertical axis). The distance between the sidechain N atom and Cδ was chosen, since both oxygen atoms of the carboxyl group can engage in salt bridge formation. The free energy is depicted using a color map that ranges from 0 to 6 kcal/mol. The free energy difference between the minima is ~1 kcal/mol, with a free energy barrier of ~4-5 kcal/mol. See Figure S5.
