Abstract
Synthetic hydrogels have been widely adopted as well-defined matrices for three-dimensional (3D) cell culture, with increasing interest in systems that enable the co-culture of multiple cell types for probing both cell-matrix and cell-cell interactions in studies of tissue regeneration and disease. We hypothesized that the unique dynamic covalent chemistry of self-healing hydrogels could be harnessed for not only the encapsulation and culture of human cells but also the subsequent construction of layered hydrogels for 3D co-cultures. To test this, we formed hydrogels using boronic acid-functionalized polymers and demonstrated their self-healing in the presence of physiologically-relevant cell culture media. Two model human cell lines, MDA-MB-231 breast cancer cells and CCL151 pulmonary fibroblasts, were encapsulated within these dynamic materials, and good viability was observed over time. Finally, self-healing of cut hydrogel ‘blocks’ laden with these different cell types was used to create layered hydrogels for the generation of a dynamic co-culture system. This work demonstrates the utility of self-healing materials for multi-dimensional cultures and establishes approaches broadly useful for a variety of biological applications.
Graphical Abstract
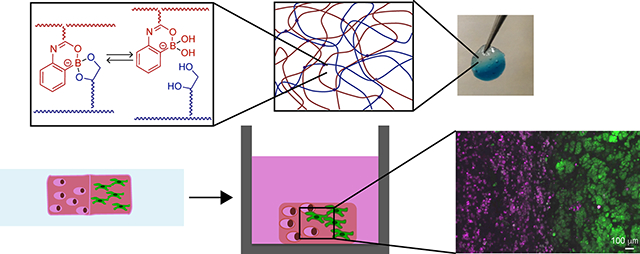
Dynamic covalent chemistries have been employed to create soft materials with unique and useful properties, including self-healing and response to external stimuli, that are relevant for a wide variety of applications, from coatings and sealants to drug delivery vehicles.1 Amongst these chemistries, phenylboronate ester complexation is particularly advantageous for use in biological applications, as the reaction occurs in aqueous conditions and has been observed to be biocompatible.2–4 Capitalizing upon this, materials formed via boronic acid-based chemistries have been utilized in several biological applications including biosensing, owing to their ability to bind diol functionalities presented by polysaccharides,5 and as injectable drug delivery vehicles, owing to their self-healing nature that enables material injection and reformation in situ.6,7 While these applications demonstrate the utility of boronic acid-based chemistries for the design of biomaterials, to date, few studies have investigated boronic acid-based materials for multi-dimensional cell culture applications, an area of growing interest for studying complex biological processes in vitro.8,9
Broadly, boronic acids are Lewis acids with pKa values that generally range from 4.5–10.10 In the presence of crosslinkers possessing 1,2- or 1,3-diols, boronic acid-based polymers can form dynamic covalent bonds that allow the formation of hydrogels at a solution pH similar to or greater than the pKa of the boronic acid.11 We have previously exploited this property to design boronic acid-based hydrogel materials that are capable of self-healing at a neutral pH.12 Specifically, copolymers of 2-acrylamidophenylboronic acid (2APBA) and poly(vinyl alcohol) (PVOH) were reacted under aqueous conditions; the intramolecular coordination between the carbonyl oxygen of the acrylamido moiety and the boron of the boronic acid group stabilize the boronate ester formed upon reaction of the boronic acid and diol presented by the 2APBA units and PVOH, respectively. The resulting material demonstrated that the boronate ester is favored in the equilibrium with the free boronic acid and diol at neutral pH, as opposed to the higher pH range of 8–9 that would be typical for an aryl boronic acid functional group. Approaches for the formation and healing of boronic acid-based hydrogels in more complex media are needed for the translation of such materials into cell culture applications, including the encapsulation and 3D culture of human cells.
Synthetic hydrogels have been instrumental in studying the effects of outside-in signaling on the function and fate of cells in different disease and regenerative processes.13–15 Often, these synthetic matrices are covalently crosslinked hydrogels, where matrix degradability, amongst other properties, has been observed to be a key feature for promoting many cellular functions, including spreading, proliferation, and migration.16–18 For example, matrix degradation was observed to be necessary for the lineage-specific differentiation of human mesenchymal stem cells in response to matrix modulus in 3D culture.19 In many synthetic systems, degradability is engineered into the backbone of the hydrogel network by including hydrolytically-degradable esters or proteinase-degradable peptides that allow irreversible cleavage and ‘remodeling’ of the synthetic matrix.17,18,20 While such degradation of the matrix is often beneficial, irreversible degradation of the covalent crosslinks that comprise the hydrogel ultimately leads to dissolution of the underlying polymer network where the timescale of matrix erosion must be tuned to match the application of interest.20,21 We hypothesized that the dynamic covalent nature of boronic acid-based hydrogels would allow for 3D cell culture within this synthetic matrix without the requirement of water- or proteinase-degradable moieties. Further, in addition to cell-matrix interactions, crosstalk between different cell types is common in many biological phenomena, and synthetic systems for the co-culture of multiple cell types are of increasing interest across fields.22–24 The ability of boronic acid-based hydrogels to self-heal could impart not only dynamic crosslinks but also allow for the construction of more complex material geometries by ‘healing’ hydrogel blocks of arbitrary shapes laden with complementary cells, facilely creating 3D co-cultures.
To test the feasibility of using boronic acid-based hydrogels for 3D cell culture, we first investigated the formation and self-healing of boronic acid-based hydrogels in the presence of cell culture media. Next, an approach was established for encapsulating different human cell types within these materials, allowing 3D culture and monitoring of cell viability over time. Finally, the utility of self-healing for fusing together cell-laden blocks of hydrogels was demonstrated for the creation of dynamic 3D co-cultures. Here, we specifically focused on the culture of human fibroblasts and human breast cancer cells. Individually, these cells are relevant for studies of wound healing, fibrosis, and cancer metastasis. Additionally, interactions between these two cell types are particularly interesting given recent work showing that wound healing environments may influence breast cancer progression.25 Our findings suggest that these boronic acid-based hydrogels are suitable materials for dynamic 3D cell culture and construction of complex material geometries. The approaches established here could be utilized in future studies to probe the role of cell-matrix and cell-cell interactions in more complex biological processes or even the delivery of cells in vivo.
We have previously demonstrated self-healing of boronic-acid hydrogels in neutral to acidic water (pH 4–7.4).12 For the encapsulation and culture of cells, however, hydrogel formation and healing must be performed in cell culture media. Cell culture media are typically aqueous solutions of salts and essential amino acids supplemented with serum isolated from animal sources, which contains a variety of proteins. As the diol reactivity of boronic acids allows for interactions with a number of proteins and polysaccharides,5 components in serum could interact to some degree with the boronic acid polymer with the potential to affect the ability of the hydrogels to form or heal.26
To examine this, statistical copolymers of 90 mol% N,N-dimethylacrylamide (DMA) and 10 mol% of the pinacol-protected ester of 2APBA (i.e., 2APBAE) were prepared (Mn = 34000 g/mol, Mw/Mn = 3.5). Commercially available PVOH (Mw = 31000–50000 g/mol, 87–89% hydrolyzed) was used as the diol-containing co-polymer. To assess hydrogel formation and healing, PVOH (5 wt%) and the 2APBA copolymer (5 wt%) were dissolved in media of increasing complexity: PBS, serum-free cell culture media, or serum-containing cell culture media (Figure 1A).
Figure 1.
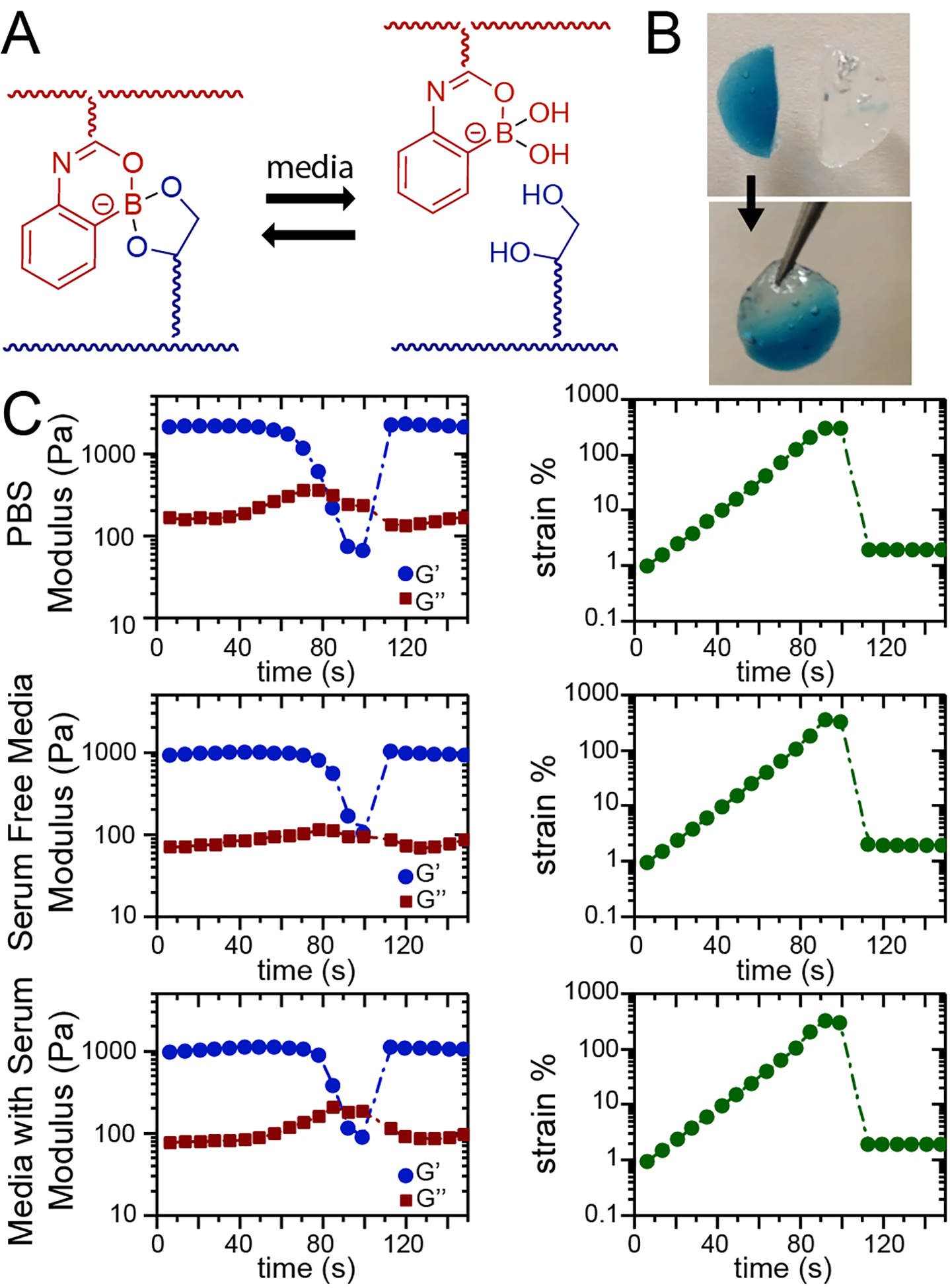
Boronic acid-based hydrogels self-heal in a variety of media. A) 2APBA and PVOH form a dynamic reversible covalent bond at neutral pH. Hydrogel healing in different media was assessed by B) a hang test and C) rheology using a strain sweep. During the strain sweep, the hydrogel network was ruptured as evidenced by the crossover of G´ and G´´, and upon reduction of strain, crossover of G´ and G´´ was observed, indicating hydrogel healing.
Hydrogels in different media were formed in situ on a rheometer by mixing with a pipette 100 μL each of 2APBA copolymer and PVOH solutions directly on the Peltier plate of the rheometer, followed by lowering of the top flat plate (20 mm diameter). Following established protocols for the evaluation of self-healing, a time sweep at constant frequency (ω = 10 rad/s) was performed while linearly increasing the applied strain until failure of the hydrogel, as indicated by the crossover of G´´ > G´. Upon reduction of the strain, hydrogel healing was monitored (time sweep at 1% strain), as evidenced by another crossover of G´ and G´´. Successful self-healing was observed in all media, as evidenced by both a hang test (Figure 1B) and these rheometric studies (Figure 1C). Self-healing of hydrogels with an increased polymer concentration (10 wt%) were also investigated, but inconsistent results in self-healing in physiologically relevant media were observed by hang tests. For all studies moving forward, 5 wt% hydrogels were investigated, which produced matrices with storage moduli of approximately 1 kPa. The resulting hydrogels were free-standing and had moduli in the range of various soft tissues,27 including lung tissue and tumors (Young’s modulus E ~ 1.5 to 5+ kPa (G ~ 0.5 to 1.5 assuming a Poisson’s ratio of 0.5)),28–30 and of other synthetic matrices used for 3D cell culture, including for studies of fibroblasts31,32 and breast cancer cells.33 Further, these hydrogels were robust not only upon formation but also over longer times: little change in their mass (mDry) or mass swelling (q) was observed over the time course (Figure S1). Overall, these investigations supported the stability and potential utility of these self-healing materials for cell culture studies.
We next examined if the mechanism of hydrogel formation was permissive to the encapsulation of human cells. To promote cell adhesion, fibronectin was included within the pre-gelation solution, where we hypothesized that the boronic acid moiety may interact with free alcohol groups presented by fibronectin for retention of the protein within the hydrogel.5,34 A fibronectin concentration of 300 nM was selected, which is in the range of concentrations reported for encapsulation in synthetic hydrogels to promote cell adhesion (typically, 100 nM to 300 nM).35–37 To examine fibronectin retention over time, we encapsulated rhodamine-labeled fibronectin along with human lung fibroblasts (CCL-151, 5,000 cells/μL) during hydrogel formation and used confocal microscopy to measure fluorescence within the hydrogels. Fibronectin was observed throughout the hydrogel over the course of 7 days, where over 50% of the fibronectin originally encapsulated was estimated to be retained at day 7 (Figure S2). These observations supported that fibronectin was present at relevant levels within these dynamic covalent hydrogels over the course of cell culture. Cell viability within these synthetic matrices subsequently was examined. 2APBA copolymer (5 wt%) and PVOH (5 wt%) were dissolved in serum-free cell culture media, and cells (CCL-151 lung fibroblasts or MDA-MB-231 breast cancer cells, 10,000 cells/μL) were resuspended in the 2APBA solution and supplemented with fibronectin (600 nM). This cell suspension was gently mixed with the PVOH solution in a 1:1 ratio using a pipette to form a hydrogel within a well plate (here, an 8-well chamber slide for facile imaging). The resulting hydrogel contained 300 nM fibronectin and a final cell concentration of 5,000 cells/μL. Encapsulated cells were cultured under sterile conditions over 1 week in serum containing media (37°C and 5% CO2).
Cell viability was assessed with a live/dead cytotoxicity assay and confocal microscopy (Figure 2). Both cell types exhibited good viability over the course of 7 days, indicating cytocompatibility of the materials and encapsulation method and that the decrease in fibronectin had no significant impact on cell viability. Control experiments confirmed that cell viability was not impacted by the presence of encapsulated fibronectin in these materials (0 vs. 300 nM, Figure S3); however, as cell migration has been observed to weakly depend on RGD concentration in other synthetic hydrogels, fibronectin was included in all subsequent studies.38
Figure 2.
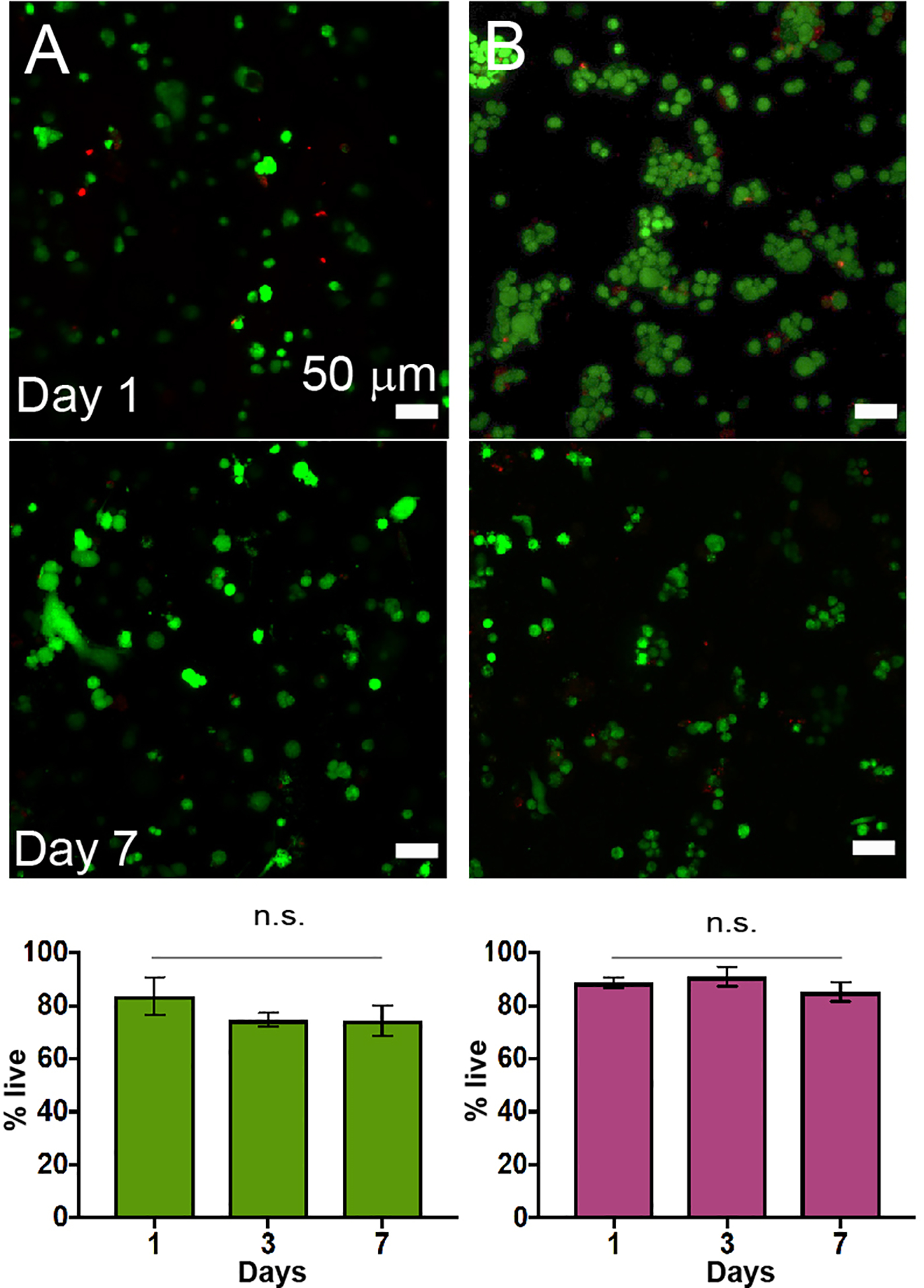
A) Human pulmonary fibroblasts (CCL151) and B) human breast cancer cells (MDA MB 231) were encapsulated in 5 wt% boronic acid-based hydrogels formed in serum-free media supplemented with 300 nM fibronectin, where good cell viability was observed over the course of 7 days (representative images shown; quantification for n=3).
Motility of both fibroblasts and breast cancer cells was observed within these matrices (5 wt% with 300 nM fibronectin) (Supplemental Videos 1 and 2, respectively). Interestingly, both cell types remained rounded over 7 days in culture, where only short periods of cell elongation were observed qualitatively during migration of some fibroblasts. Based on these observations, we speculate that movement of cells through these dynamic matrices may be occurring, at least in part, by an amoeboid mechanism, where cells squeeze through pores while maintaining a rounded morphology; this mechanism of migration is consistent with observations of cancer cells in vivo and cancer cells and fibroblasts during in vitro culture within enzymatically-degradable hydrogels when proteolysis was inhibited.38–40 Broadly, these results supported the relevance of these materials for a variety of 3D cell culture experiments.
The dynamic covalent chemistry of these boronic acid-based materials should allow not only cell encapsulation and culture but also provide opportunities for the construction of more complex material geometries through self-healing. Here, we aimed to examine the relevance of this approach for creating dynamic co-cultures, using human fibroblasts and human breast cancer cells as model cell types of interest for studies of cancer. For example, the active remodeling of the extracellular environment, a function performed by fibroblasts and other stromal cells, has been implicated in breast cancer metastasis, and recent studies of cancer-associated fibroblasts suggest that cell-cell signaling between fibroblasts and breast cancer cells may promote progression.41
To create dynamic co-cultures by exploiting hydrogel self-healing, cells of each respective type were labeled with a cell tracker dye (lung fibroblasts, green; breast cancer cells, red) and separately encapsulated during the formation boronic-acid hydrogels, as previously described. Using a sterile blade, hydrogels laden with each cell type were cut in half, and the two halves containing complementary cell types placed in direct contact to allow healing (Figure 3A). These resulting hydrogel co-cultures were healed in an incubator at 37°C and 5% CO2 for one hour to form two new hydrogel constructs, each with one half containing fibroblasts and the other half containing breast cancer cells.
Figure 3.
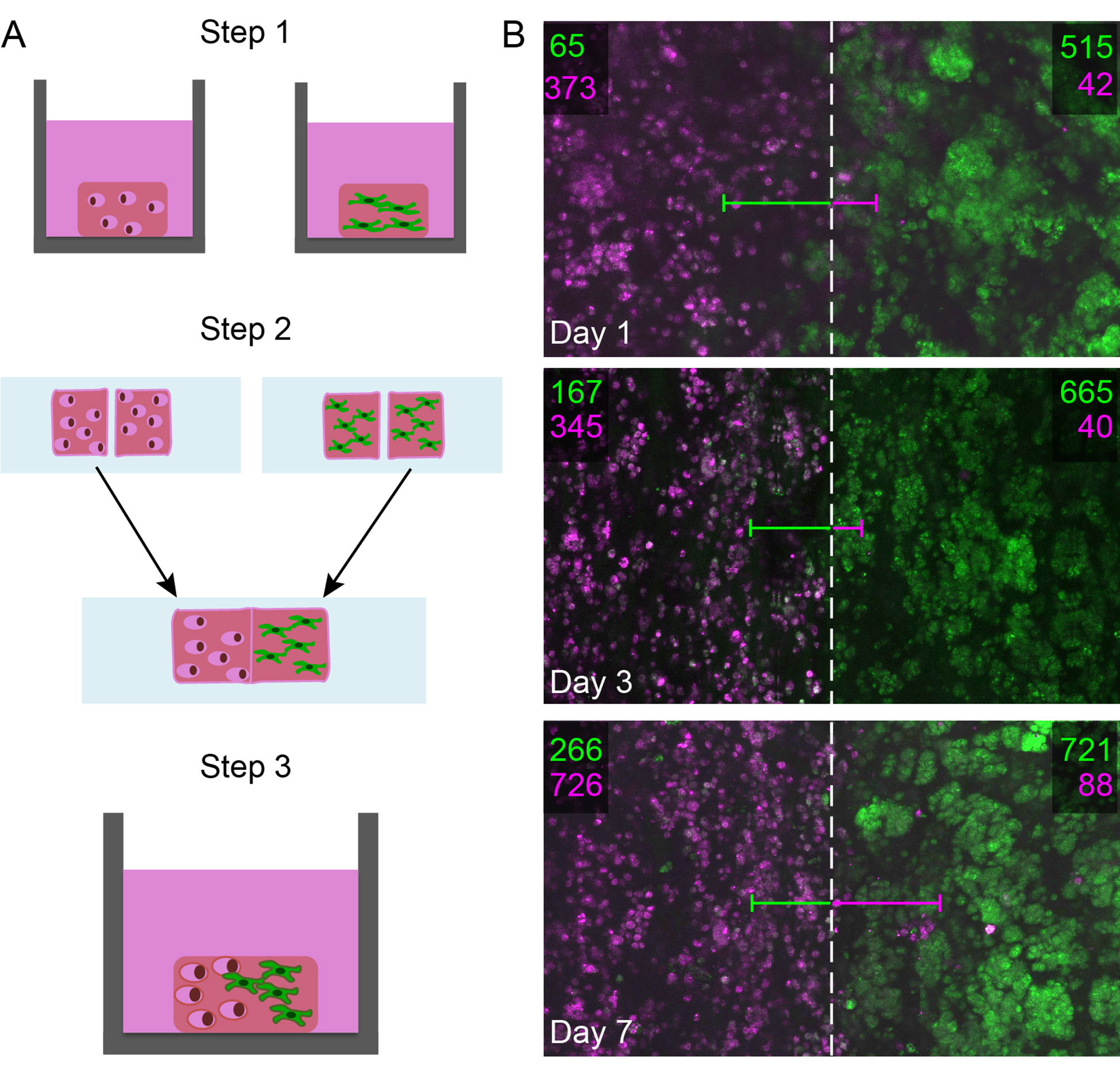
A) Human pulmonary fibroblasts (CCL151, green) and human breast cancer cells (MDA MB 231, pink) were co-cultured in self-healing boronic acid-based hydrogels. B) Confocal z-stacks centered at the healed interface were taken over a large area of the healed hydrogel using a tile scan. The number of cells in a 1000 μm long region of interest on either side of the healing interface was quantified in three dimensions, where the number of fibroblasts is shown in green and the number of breast cancer cells is shown in pink for each side. The average distance each cell type had traveled across the interface into the opposite half is represented by each line (green line for fibroblasts and pink line for breast cancer cells). Projections of the confocal z-stack tile scan are shown here for a top down view of the 2000-μm long total region of interest (interface noted with a dashed lined).
To assess cell response within these dynamic co-cultures over time, the hydrogels were cultured in serum-containing cell culture media and imaged using confocal microscopy over a week. At time points of interest (days 1, 3, 7), a confocal tile scan was performed, centered on the interface of the two gel halves, allowing examination of cell number and positions within a large volume (~2000 μm wide and 100 μm thick). Image analysis (Figure S4 and S5) was used to create two regions of interest on either side of the interface: one side originally containing all fibroblasts (green) and the other originally containing all breast cancer cells (pink), each region 1000 μm wide. The number of fibroblasts and breast cancer cells within each region of interest over time was counted to assess any changes in cell number and location as indirect measures of cell proliferation and migration (Figure 3B). Additionally, the distance between the center of each cell body and the healed interface was quantified for the complementary cell type to assess how far the cells on average had moved into the new region of interest.
In the dynamic co-cultures, both fibroblasts and breast cancer cells were able to cross the healed interface into the new region of interest. Increasing numbers of ‘non-resident’ cells to the region were observed, suggesting directional migration, which was not observed in the absence of co-culture (Figure S6). Qualitatively, a smaller number of breast cancer cells were observed migrating long distances in comparison to a greater number of fibroblasts observed migrating short distances close to the healed interface, resulting in different temporal trends in the average distance traveled. Additionally, increased numbers of the original cell type ‘resident’ in respective regions of interest were observed over time, suggesting the cells also were proliferating which was confirmed with an EdU assay (Figure S7). These studies demonstrated the utility of self-healing for the creation of more complex culture environments for probing dynamic cell-cell and cell-matrix interactions.
In sum, we have established approaches for forming and self-healing boronic acid-based hydrogel materials in cell culture media and demonstrated their utility for 3D culture of human cells, including generation of dynamic co-cultures. These results highlight the opportunity that self-healing materials,42,43 particularly boronic acid-based hydrogels,9 present for 3D culture of a variety of cell types, individually or in concert. With this system now established, future studies tracking the movement of cells or cell-cell signaling molecules within these materials could garner interesting insights into the mechanism and regulators of cell migration and other functions of relevance to the study of disease or regenerative processes. Additionally, fundamental studies of cell response to cell-matrix interactions within this dynamic covalent system could be insightful, including examination of mechanotransduction and related cell responses in this temporally-evolving hydrogel structure for comparison to its irreversibly-degradable or physical hydrogel counterparts.44 In particular, stress relaxation of synthetic extracellular matrices, in addition to other matrix properties, is emerging as an important regulator of cell fate45,46 and is a prominent feature imparted by the dynamic covalent chemistry of these boronic acid-based hydrogels, providing opportunities for unique investigations. Further, new materials systems could be designed to integrate this dynamic covalent chemistry with cell-degradable crosslinks for the creation of hybrid materials with emergent properties for a variety of applications.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank the Bio-Imaging Center at the Delaware Biotechnology Institute for instrument usage.
Funding Sources
The authors gratefully acknowledge support for related work from the National Science Foundation (DMR-1410223 and DMR-1253906), the Pew Charitable Trusts (00026178), and the Delaware COBRE programs with grants from the National Institute of General Medicine Sciences (NIGMS P20GM104316 and 5 P30 GM110758-02) from the National Institutes of Health.
ABBREVIATIONS
- 3D
Three-dimensional
- 2APBA
2-acrylamidophenylboronic acid
- PVOH
poly(vinyl alcohol)
- DMA
N,N-Dimethylacrylamide
- PBS
phosphate buffered saline
Footnotes
Supporting Information
Experimental details and supporting figures
REFERENCES
- (1).Wei Z; Yang JH; Zhou J; Xu F; Zrínyi M; Dussault PH; Osada Y; Chen YM Self-Healing Gels Based on Constitutional Dynamic Chemistry and Their Potential Applications. Chem. Soc. Rev 2014, 43 (23), 8114–8131. [DOI] [PubMed] [Google Scholar]
- (2).Kane RC; Bross PF; Farrel AT; Pazdur R Velcade: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist 2003, No. 8, 508–513. [DOI] [PubMed] [Google Scholar]
- (3).Sharma N; Sharma D An Upcoming Drug for Onychomycosis: Tavaborole. Natl. J. Pharmacol. Pharmacother 2015, 6 (4), 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Munson E; Huband MD; Castanheira M; Fedler KA; Flamm RK Determination of MIC and Disk Diffusion Quality Control Guidelines for Meropenem-Vaborbactam, a Novel Carbapenem/Boronic Acid β-Lactamase Inhibitor Combination. Diagn. Microbiol. Infect. Dis 2017, 90 (4), 324–328. [DOI] [PubMed] [Google Scholar]
- (5).Wang HC; Lee AR Recent Developments in Blood Glucose Sensors. J. Food Drug Anal 2015, 23 (2), 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wang X; Zhen X; Wang J; Zhang J; Wu W; Jiang X Doxorubicin Delivery to 3D Multicellular Spheroids and Tumors Based on Boronic Acid-Rich Chitosan Nanoparticles. Biomaterials 2013, 34 (19), 4667–4679. [DOI] [PubMed] [Google Scholar]
- (7).Brooks WLA; Sumerlin BS Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev 2016, 116 (3), 1375–1397. [DOI] [PubMed] [Google Scholar]
- (8).Tang S; Ma H; Tu H-C; Wang H-R; Lin P-C; Anseth KS Adaptable Fast Relaxing Boronate-Based Hydrogels for Probing Cell-Matrix Interactions. Adv. Sci 2018, 2018, 1800638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chen Y; Diaz-Dussan D; Wu D; Wang W; Peng Y-Y; Asha AB; Hall DG; Ishihara K; Narain R Bioinspired Self-Healing Hydrogel Based on Benzoxaborole-Catechol Dynamic Covalent Chemistry for 3D Cell Encapsulation. ACS Macro Lett. 2018, 904–908. [DOI] [PubMed] [Google Scholar]
- (10).Cambre JN; Sumerlin BS Biomedical Applications of Boronic Acid Polymers. Polymer (Guildf). 2011, 52 (21), 4631–4643. [Google Scholar]
- (11).Guan Y; Zhang Y Boronic Acid-Containing Hydrogels: Synthesis and Their Applications. Chem. Soc. Rev 2013, 42 (20), 8106–8121. [DOI] [PubMed] [Google Scholar]
- (12).Deng CC; Brooks WLA; Abboud KA; Sumerlin BS Boronic Acid-Based Hydrogels Undergo Self-Healing at Neutral and Acidic PH. ACS Macro Lett. 2015, 4 (2), 220–224. [DOI] [PubMed] [Google Scholar]
- (13).Kharkar PM; Kiick KL; Kloxin AM Designing Degradable Hydrogels for Orthogonal Control of Cell Microenvironments. Chem. Soc. Rev 2013, 42 (17), 7335–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Guvendiren M; Burdick JA Engineering Synthetic Hydrogel Microenvironments to Instruct Stem Cells. Curr. Opin. Biotechnol 2013, 24 (5), 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Owen SC; Shoichet MS Design of Three-Dimensional Biomimetic Scaffolds. J. Biomed. Mater. Res. - Part A 2010, 94 (4), 1321–1331. [DOI] [PubMed] [Google Scholar]
- (16).Trappmann B; Baker BM; Polacheck WJ; Choi CK; Burdick JA; Chen CS Matrix Degradability Controls Multicellularity of 3D Cell Migration. Nat. Commun 2017, 8 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Lutolf MP; Hubbell JA Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol 2005, 23 (1), 47–55. [DOI] [PubMed] [Google Scholar]
- (18).Schultz KM; Kyburz KA; Anseth KS Measuring Dynamic Cell–Material Interactions and Remodeling during 3D Human Mesenchymal Stem Cell Migration in Hydrogels. Proc. Natl. Acad. Sci 2015, 112 (29), E3757–E3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Khetan S; Guvendiren M; Legant WR; Cohen DM; Chen CS; Burdick JA Degradation-Mediated Cellular Traction Directs Stem Cell Fate in Covalently Crosslinked Three-Dimensional Hydrogels. Nat. Mater 2013, 12 (5), 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Patterson J; Hubbell JA Enhanced Proteolytic Degradation of Molecularly Engineered PEG Hydrogels in Response to MMP-1 and MMP-2. Biomaterials 2010, 31 (30), 7836–7845. [DOI] [PubMed] [Google Scholar]
- (21).Gobin AS; West JL Cell Migration through Defined, Synthetic Extracellular Matrix Analogues. FASEB J. 2002, 16 (7), 751–753. [DOI] [PubMed] [Google Scholar]
- (22).Wang R; Xu J; Juliette L; Castilleja A; Love J; Sung SY; Zhau HE; Goodwin TJ; Chung LWK Three-Dimensional Co-Culture Models to Study Prostate Cancer Growth, Progression, and Metastasis to Bone. Semin. Cancer Biol 2005, 15 (5 SPEC. ISS.), 353–364. [DOI] [PubMed] [Google Scholar]
- (23).Aguirre A; Planell JA; Engel E Dynamics of Bone Marrow-Derived Endothelial Progenitor Cell/Mesenchymal Stem Cell Interaction in Co-Culture and Its Implications in Angiogenesis. Biochem. Biophys. Res. Commun 2010, 400 (2), 284–291. [DOI] [PubMed] [Google Scholar]
- (24).Shephard P; Martin G; Smola-Hess S; Brunner G; Krieg T; Smola H Myofibroblast Differentiation Is Induced in Keratinocyte-Fibroblast Co-Cultures and Is Antagonistically Regulated by Endogenous Transforming Growth Factor-β and Interleukin-1. Am. J. Pathol 2004, 164 (6), 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Insua-Rodríguez J; Oskarsson T The Extracellular Matrix in Breast Cancer. Adv. Drug Deliv. Rev 2016, 97, 41–55. [DOI] [PubMed] [Google Scholar]
- (26).Frantzen F; Grimsrud K; Heggli DE; Sundrehagen E Protein-Boronic Acid Conjugates and Their Binding to Low-Molecular-Mass Cis-Diols and Glycated Hemoglobin. J. Chromatogr. B Biomed. Sci. Appl 1995, 670 (1), 37–45. [DOI] [PubMed] [Google Scholar]
- (27).Markert CD; Guo X; Skardal A; Wang Z; Bharadwaj S; Zhang Y; Bonin K; Guthold M Characterizing the Micro-Scale Elastic Modulus of Hydrogels for Use in Regenerative Medicine. J. Mech. Behav. Biomed. Mater 2013, 27, 115–127. [DOI] [PubMed] [Google Scholar]
- (28).Booth AJ; Hadley R; Cornett AM; Dreffs AA; Matthes SA; Tsui JL; Weiss K; Horowitz JC; Fiore VF; Barker TH; Moore BB; Martinez FJ; Niklason LE; White ES Acellular Normal and Fibrotic Human Lung Matrices as a Culture System for in Vitro Investigation. Am. J. Respir. Crit. Care Med 2012, 186 (9), 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Levental I; Georges PC; Janmey PA Soft Biological Materials and Their Impact on Cell Function. Soft Matter 2007, 3 (3), 299–306. [DOI] [PubMed] [Google Scholar]
- (30).Paszek MJ; Zahir N; Johnson KR; Lakins JN; Rozenberg GI; Gefen A; Reinhart-King CA; Margulies SS; Dembo M; Boettiger D; Hammer DA; Weaver VM Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8 (3), 241–254. [DOI] [PubMed] [Google Scholar]
- (31).Mabry KM; Lawrence RL; Anseth KS Dynamic Stiffening of Poly(Ethylene Glycol)-Based Hydrogels to Direct Valvular Interstitial Cell Phenotype in a Three-Dimensional Environment. Biomaterials 2015, 49, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Bott K; Upton Z; Schrobback K; Ehrbar M; Hubbell JA; Lutolf MP; Rizzi SC The Effect of Matrix Characteristics on Fibroblast Proliferation in 3D Gels. Biomaterials 2010, 31 (32), 8454–8464. [DOI] [PubMed] [Google Scholar]
- (33).Huang H; Ding Y; Sun XS; Nguyen TA Peptide Hydrogelation and Cell Encapsulation for 3D Culture of MCF-7 Breast Cancer Cells. PLoS One 2013, 8 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Whyte GF; Vilar R; Woscholski R Molecular Recognition with Boronic Acids-Applications in Chemical Biology. J. Chem. Biol 2013, 6 (4), 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Smithmyer M; Spohn J; Kloxin AM Probing Fibroblast Activation in Response to Extracellular Cues with Whole Protein- or Peptide-Functionalized Hydrogels. ACS Biomater. Sci. Eng 2018, acsbiomaterials.8b00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Weber LM; Hayda KN; Anseth KS Cell-Matrix Interactions Improve Beta-Cell Survival and Insulin Secretion in Three-Dimensional Culture. Tissue Eng. Part A 2008, 14 (12), 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kloxin AM; Benton JA; Anseth KS In Situ Elasticity Modulation with Dynamic Substrates to Direct Cell Phenotype. Biomaterials 2010, 31 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Schwartz MP; Fairbanks BD; Rogers RE; Rangarajan R; Zaman MH; Anseth KS A Synthetic Strategy for Mimicking the Extracellular Matrix Provides New Insight about Tumor Cell Migration. Integr. Biol 2010, 2 (1), 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Wolf K; Mazo I; Leung H; Engelke K; Von Andrian UH; Deryugina EI; Strongin AY; Bröcker EB; Friedl P Compensation Mechanism in Tumor Cell Migration: Mesenchymal-Amoeboid Transition after Blocking of Pericellular Proteolysis. J. Cell Biol 2003, 160 (2), 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Friedl P; Wolf K Plasticity of Cell Migration: A Multiscale Tuning Model. J. Cell Biol 2010, 188 (1), 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yu Y; Xiao CH; Tan LD; Wang QS; Li XQ; Feng YM Cancer-Associated Fibroblasts Induce Epithelial-Mesenchymal Transition of Breast Cancer Cells through Paracrine TGF-β Signalling. Br. J. Cancer 2014, 110 (3), 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Phadke A; Zhang C; Arman B; Hsu C-C; Mashelkar RA; Lele AK; Tauber MJ; Arya G; Varghese S Rapid Self-Healing Hydrogels. Proc. Natl. Acad. Sci 2012, 109 (12), 4383–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Appel EA; Tibbitt MW; Webber MJ; Mattix BA; Veiseh O; Langer R Self-Assembled Hydrogels Utilizing Polymer-Nanoparticle Interactions. Nat. Commun 2015, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Rosales AM; Anseth KS The Design of Reversible Hydrogels to Capture Extracellular Matrix Dynamics. Nat. Rev. Mater 2016, 1 (2), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Chaudhuri O; Gu L; Darnell M; Klumpers D; Bencherif SA; Weaver JC; Huebsch N; Mooney DJ Substrate Stress Relaxation Regulates Cell Spreading. Nat. Commun 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chaudhuri O; Gu L; Klumpers D; Darnell M; Bencherif SA; Weaver JC; Huebsch N; Lee HP; Lippens E; Duda GN; Mooney DJ Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater 2016, 15 (3), 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


