ABSTRACT
Disruption of the minor spliceosome due to mutations in RNU4ATAC is linked to primordial dwarfism in microcephalic osteodysplastic primordial dwarfism type 1, Roifman syndrome, and Lowry-Wood syndrome. Similarly, primordial dwarfism in domesticated animals is linked to positive selection in minor spliceosome components. Despite being vital for limb development and size regulation, its role remains unexplored. Here, we disrupt minor spliceosome function in the developing mouse limb by ablating one of its essential components, U11 small nuclear RNA, which resulted in micromelia. Notably, earlier loss of U11 corresponded to increased severity. We find that limb size is reduced owing to elevated minor intron retention in minor intron-containing genes that regulate cell cycle. As a result, limb progenitor cells experience delayed prometaphase-to-metaphase transition and prolonged S-phase. Moreover, we observed death of rapidly dividing, distally located progenitors. Despite cell cycle defects and cell death, the spatial expression of key limb patterning genes was maintained. Overall, we show that the minor spliceosome is required for limb development via size control potentially shared in disease and domestication.
KEY WORDS: Minor splicing, Limb development, Domestication, Primordial dwarfism
Summary: Defects in minor splicing result in short limbs that maintain patterning. This study shows how the minor spliceosome might regulate limb size but not morphogenesis in disease and domestication.
INTRODUCTION
The minor spliceosome, which consists of five small nuclear RNAs (snRNAs) – U11, U12, U4atac, U5 and U6atac – and associated proteins, splices <0.5% of introns, termed minor introns, found in <2% of genes, termed minor intron-containing genes (MIGs) (Levine and Durbin, 2001; Patel and Steitz, 2003; Olthof et al., 2019). In metazoans, the minor spliceosome, MIGs, and the position of minor introns within MIGs are all highly conserved (Russell et al., 2006; Basu et al., 2008; Szcześniak et al., 2013). This conservation could be explained by the enrichment of MIGs in essential functions required for cell survival (Baumgartner et al., 2019), which is consistent with embryonic lethality observed in multiple animal models with constitutive loss of minor spliceosome function (Otake et al., 2002; Baumgartner et al., 2018; Doggett et al., 2018). Consequently, it is unsurprising that human diseases with minor spliceosome loss-of-function mutations have not been discovered. However, mutations that result in partial loss of minor spliceosome function have been reported (Edery et al., 2011; He et al., 2011; Argente et al., 2014; Merico et al., 2015; Elsaid et al., 2017; Farach et al., 2018).
For example, microcephalic osteodysplastic primordial dwarfism type 1 (MOPD1), Roifman syndrome (RS) and Lowry-Wood syndrome (LWS) are linked to partial inhibition of the minor spliceosome (Edery et al., 2011; He et al., 2011; Merico et al., 2015; Farach et al., 2018). In these diseases, individuals harbor disparate mutations in RNU4ATAC, which encodes the U4atac snRNA, and display symptoms including microcephaly, micrognathia, vertebral deficits and primordial dwarfism (Putoux et al., 2016; Farach et al., 2018). The severity of primordial dwarfism observed in MOPD1, RS and LWS is observed along a gradation, with MOPD1 being the most severe, RS moderate and LWS mild (Putoux et al., 2016; Farach et al., 2018). Nonetheless, in all cases, the basic patterning of the limb skeletal elements, including the presence of a stylopod (humerus; femur), zeugopod (radius/ulna; tibia/fibula) and autopod (hand; foot), is maintained (Putoux et al., 2016; Farach et al., 2018).
As unique mutations in RNU4ATAC have been linked to MOPD1, RS, and LWS, the gradation of primordial dwarfism might be due to differential inhibition of the minor spliceosome (Putoux et al., 2016; Farach et al., 2018). This suggests that the activity of the minor spliceosome might serve as a rheostat for tissue size control, an idea bolstered by positive selection for genetic changes in MIGs and the minor spliceosome in domestication of several species (Baumgartner et al., 2019). In domestication, animals are selected for tameness, but secondarily show a constellation of phenotypic changes, referred to as domestication syndrome (DS) (Wilkins et al., 2014). Symptoms of DS include neoteny, reduction in brain size, reductions in jaw and tooth size, changes in the number of vertebrae, floppy ears, curling of the tail and reduction in limb size (Wilkins et al., 2014; Sánchez-Villagra et al., 2016). Notably, the phenotypes observed in DS parallel the symptoms observed in minor spliceosome-related diseases (Fig. S1) (Baumgartner et al., 2019). These observations led us to hypothesize that the activity of the minor spliceosome, and therefore MIG expression, plays a vital role in tissue size regulation, especially for the brain and the limb. Although we have previously reported that inhibition of the minor spliceosome in the developing mouse pallium resulted in cortical size reduction at birth (Baumgartner et al., 2018), the underlying mechanism through which disruption of the minor spliceosome results in micromelia remains unclear.
Here, we leverage our Rnu11Flx/Flx mouse to inhibit the minor spliceosome in the developing limb by using Prrx1-Cre, which is expressed at embryonic day (E) 9.5 in the forelimb and E10.5 in the hindlimb (Logan et al., 2002; Baumgartner et al., 2018). We show that limb size is reduced upon minor spliceosome inhibition, such that the severity corresponded to the developmental time at which U11 was lost. Moreover, we show that the U11-null forelimb contained basic proximo-distal (shoulder to fingertip) segmentation, whereas the U11-null hindlimb was patterned along the proximo-distal, antero-posterior (digit 1 to 5), and dorso-ventral (top of foot to sole) axes. We found that size reduction of the mutant limbs resulted from cell cycle defects and apoptosis of limb progenitor cells owing to elevated minor intron retention in MIGs crucial for cell cycle regulation. Notably, these cellular defects did not alter the spatial expression of certain essential limb patterning genes, including Shh, Fgf8, Hoxa11, Hoxa13 and others. In all, our study demonstrates potential cellular and molecular mechanisms underlying primordial dwarfism and micromelia in individuals with MOPD1, RS and LWS (Putoux et al., 2016; Farach et al., 2018). Finally, our findings implicate how the activity of the minor spliceosome could be used to achieve tissue size reduction without loss of basic patterning in domestication.
RESULTS
Differential effect of U11 loss on the developing forelimb and hindlimb
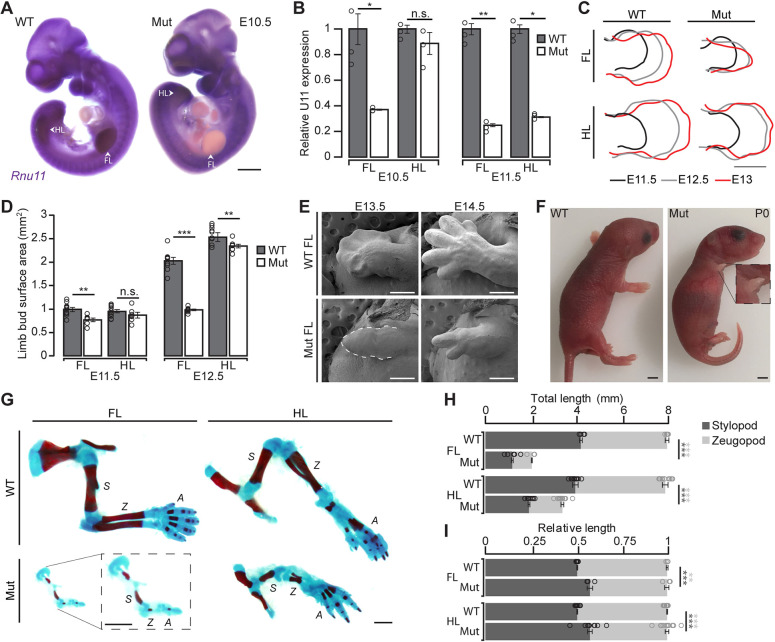
To test how inhibition of the minor spliceosome results in micromelia, we generated Rnu11Flx/Flx::Prrx1-Cre+::CAG-loxpSTOPloxp-tdTomato+ (mutant) embryos that we compared with wild-type (WT) littermates. We used tdTomato expression as a reporter of Cre activity to corroborate the previously described expression difference of Prrx1 in the forelimb and hindlimb buds (Logan et al., 2002). As expected, we found tdTomato signal only in the forelimb at E10.5 but in both the forelimb and hindlimb at E11.5 (Fig. S2A,B). Moreover, by whole-mount in situ hybridization (WISH), we confirmed the loss of U11 snRNA in the E10.5 mutant forelimb, but not hindlimb (Fig. 1A). Quantitative reverse transcription-PCR (qRT-PCR) revealed a significant 62.9% reduction of U11 in the E10.5 mutant forelimb, whereas the hindlimb did not show a similar loss (Fig. 1B). At E11.5, qRT-PCR for U11 showed that it was significantly downregulated 75.1% in the mutant forelimb and 68.8% in the mutant hindlimb (Fig. 1B). This temporal difference in U11 loss was reflected in the early onset of visible morphological defects in the mutant forelimb at E11.5, with increased severity observed at E12.5 and E13 (Fig. 1C; Fig. S3A). Quantification of limb bud surface area confirmed that the E11.5 mutant forelimb was significantly smaller than the E11.5 WT forelimb, and that this phenotype was exacerbated by E12.5 (Fig. 1D). Although the mutant hindlimb did not show gross morphological defects by E13, quantification revealed that the overall size of the mutant hindlimb was significantly reduced by E12.5 (Fig. 1C,D; Fig. S3A). There was no left/right bias observed in the mutant forelimb or hindlimb phenotypes (Fig. S3B).
Fig. 1.
U11-null limbs have stunted growth but maintain basic segmentation. (A) WISH for Rnu11 in E10.5 WT and mutant (Mut) embryos with arrows pointing to forelimb (FL) and hindlimb (HL) buds. (B) Quantification of WT and mutant Rnu11 expression from dissected E10.5 and E11.5 FL and HL buds through qRT-PCR (n=3). (C,D) Limb bud traces from WT and mutant FL and HL at E11.5 (black), E12.5 (gray) and E13 (red) (C) with quantification of surface area (D). (E) SEM images of WT and mutant FL buds at E13.5 and E14.5. Dashed line used to show E13.5 Mut FL perimeter. (F) P0 images of WT and mutant pups, with higher magnification image of mutant FL. (G) Skeletal preparation for WT and mutant FL and HL at P0, with higher magnification image of mutant FL. (H-I) Quantification of total (H) and relative (I) long bone length for WT and mutant FL and HL at P0. A, autopod; S, stylopod; Z, zeugopod. Data are mean±s.e.m. For details of statistical methods, see Table S6. *P<0.05, **P<0.01, ***P<0.001. Scale bars: 100 μm in A,C,E,G; 1 mm in F.
U11-null limbs are reduced in size but show basic patterning at birth
Based on the reduced size of the mutant limb buds, we investigated limb morphogenesis through scanning electron micrograph (SEM) imaging at E13.5 and E14.5. At E13.5, the mutant forelimb did not have the obvious flattened, paddle-like shape observed in the WT forelimb (Fig. 1E). Moreover, digital condensations observed in the E13.5 WT forelimb were not visible in the mutant forelimb (Fig. 1E). In contrast, at E14.5, the mutant forelimb showed two digital processes, compared with the five digits observed in the WT control (Fig. 1E). As digit formation was observed in the mutant forelimb at E14.5, but not E13.5, we sought to determine whether digit condensation was occurring earlier but was unable to be captured by SEM. Therefore, we performed Hematoxylin and Eosin (H&E) staining on E12.5 WT and mutant forelimb bud sections, which revealed regions of chondrogenesis in the E12.5 mutant forelimb (Fig. S4). Unlike the mutant forelimb, the mutant hindlimb showed morphology comparable with the WT control at both time points imaged through SEM (Fig. S5).
The presence of digits in the E14.5 mutant forelimb led us to hypothesize that the other elements along the proximo-distal axis, including the stylopod (humerus) and zeugopod (radius/ulna) were also formed. To test this hypothesis, we performed skeletal preparations on WT and mutant mice at birth [postnatal day (P) 0], which showed that, although severely stunted, the P0 mutant forelimb had basic segmentation, as it contained a stylopod, a single zeugopod element and a single digit (Fig. 1F-H). Moreover, the mutant hindlimb, which was smaller compared with the WT, consisted of a femur, tibia, fibula and foot (Fig. 1F-H). Although the mutant hindlimb lacked a medial cuneiform and had a stunted digit 1, basic patterning across the proximo-distal and antero-posterior axes was preserved as it contained a stylopod, two zeugopod elements and an autopod with five digits (Fig. 1F,G; Fig. S6A).
Given the reduction in overall limb size, we aimed to determine whether the proximal and distal long bones of the mutant limbs were proportionally reduced in size. We found that the stylopod-zeugopod ratio was significantly altered in the mutant forelimb and hindlimb (Fig. 1I). In both, the stylopod comprised a significantly greater amount of total limb length, indicating that the mutant limbs were mesomelic (Fig. 1I). Furthermore, in MOPD1, osteogenic defects causing narrow bones with reduced bone density have been reported (Krøigård et al., 2016). Therefore, we quantified the length and width of the Alizarin Red-stained (ossified) region of the long bones of the WT and mutant limbs at P0. We discovered that the stylopod and zeugopod were significantly narrower and less ossified in both the mutant forelimb and hindlimb relative to WT controls (Fig. S6B-D).
U11 loss resulted in elevated minor intron retention in MIGs that regulate cell cycle
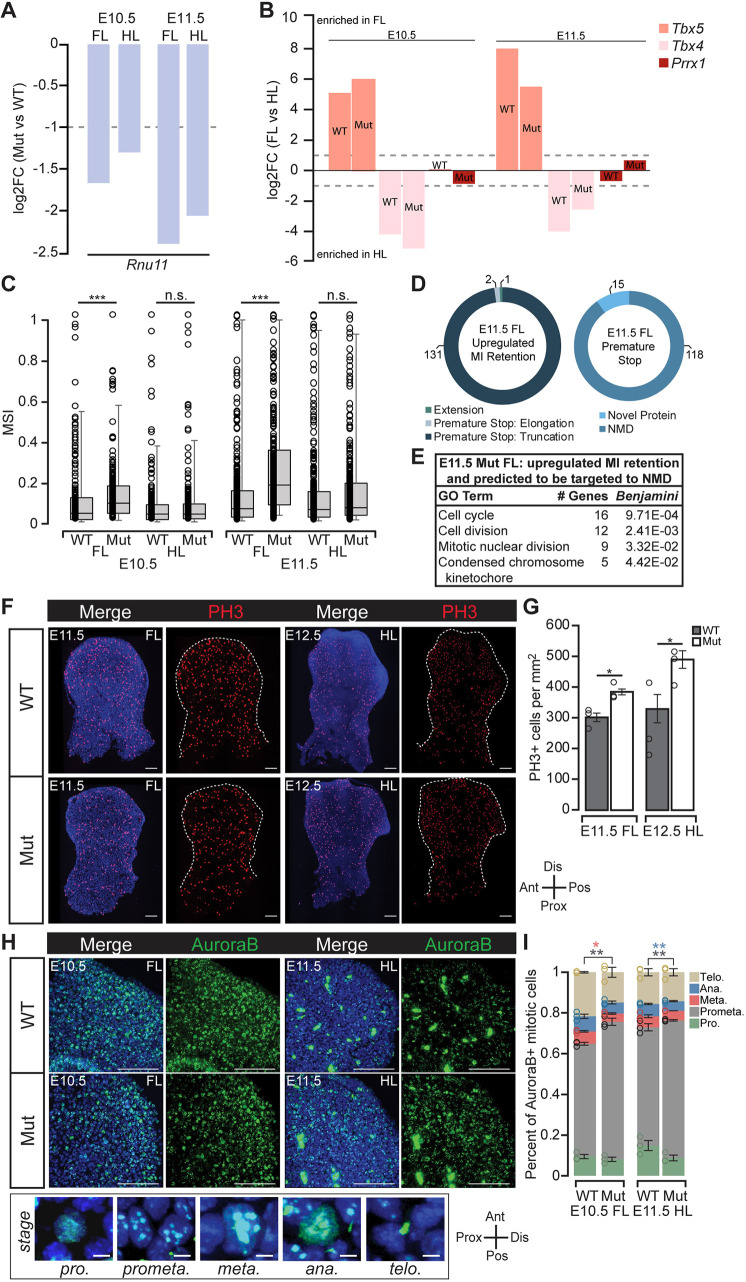
To identify the underlying molecular pathways disrupted in the mutant limbs, we performed total RNA-seq using E10.5 and E11.5 WT and mutant forelimb and hindlimb samples. We found that U11 was reduced by 73.5% at E10.5 and 81.2% at E11.5 in the mutant forelimb compared with the respective WT controls (Fig. 2A). Similarly, we found that U11 was reduced by 62.5% at E10.5 and 75.8% at E11.5 in the mutant hindlimb compared with the respective WT controls (Fig. 2A). The incomplete loss of U11 expression is consistent with Prrx1-Cre being expressed in the limb bud mesoderm but not in the overlying limb bud ectoderm (Logan et al., 2002). Moreover, forelimb and hindlimb specificity was reflected in the significant enrichment of Tbx5, a forelimb marker, in all forelimb samples, significant enrichment of Tbx4, a hindlimb marker, in all hindlimb samples, and non-differential expression (NonDE) of Prrx1 in all sample comparisons (Fig. 2B) (Zuniga, 2015).
Fig. 2.
U11 loss resulted in minor intron retention in MIGs that regulate cell cycle and caused defective mitosis. (A) Bar chart of confident log2 fold change (FC) of Rnu11 in the mutant (Mut) forelimb (FL) and hindlimb (HL) (compared with WT age- and tissue-matched controls) at E10.5 and E11.5 quantified through RNA-seq, with dashed line representing threshold for significance. (B) Bar chart of confident log2FC of Tbx5, Tbx4 and Prrx1 in WT and mutant FL and HL at E10.5 and E11.5 quantified through RNA-seq, with dashed lines representing thresholds for significance. (C) Box-plot representing 10th-90th percentile mis-splicing index (MSI) for all MIGs that show minor intron retention in WT and mutant FL and HL at E10.5 and E11.5. Horizontal line shows median. Bottom whisker shows 10th-25th percentile and top whisker shows 75th-90th percentile MSI. (D) Pie chart depicting consequence of minor intron retention on the ORF of MIGs with significantly elevated minor intron retention in the E11.5 mutant FL (left) and whether a premature stop codon is predicted to trigger NMD or result in novel protein production (right). (E) GO enrichment for MIGs that have significantly upregulated minor intron (MI) retention and are predicted to be targeted to NMD in the E11.5 mutant FL. (F,G) IF for PH3 counterstained with DAPI in WT and mutant E11.5 FL and E12.5 HL (F) with quantification (G) normalized to limb bud area. Dotted line indicates limb bud perimeter. (H,I) IF for AuroraB counterstained with DAPI in WT and mutant E10.5 FL and E11.5 HL (H) with quantification (I) of the percentage of cells in each mitotic stage per sample. Bottom panels show representative staining of AuroraB for prophase (pro.), prometaphase (prometa.), metaphase (meta.), anaphase (ana.) and telophase (telo.). Ant, anterior; Dis, distal; Pos, posterior; Prox, proximal. Data are mean± s.e.m. (G,I). For details of statistical methods, see Table S6. *P<0.05, **P<0.01, ***P<0.001. Scale bars: 100 μm.
As U11 loss is expected to inhibit the minor spliceosome, we performed minor intron retention analysis on our RNA-seq data. We discovered elevated minor intron retention in the E10.5 mutant forelimb compared with its WT counterpart as indicated by a significantly elevated median mis-splicing index (MSI) (Fig. 2C). In contrast, there was no significant difference in minor intron retention between the E10.5 WT and mutant hindlimb (Fig. 2C). Similarly, at E11.5, we found a significantly elevated median MSI in the mutant forelimb but not in the mutant hindlimb (Fig. 2C). For each sample, we next identified the individual minor introns that were retained at significantly higher levels compared with the corresponding WT dataset. We found 21 minor introns to be significantly retained in the E10.5 mutant forelimb, whereas only two minor introns showed significantly elevated retention in the E10.5 mutant hindlimb compared with their respective WT controls (Table S1). At E11.5, we found 134 minor introns to be significantly retained in the mutant forelimb and 15 minor introns to be significantly retained in the mutant hindlimb compared with their respective WT controls (Table S1). Altogether, retention of these minor introns affected splicing of 152 MIGs in at least one of the mutant conditions.
As minor intron retention can introduce a premature stop codon and result in nuclear degradation or nonsense mediated decay (NMD), we analyzed the effect of minor intron retention on the open reading frame (ORF) of these 152 MIGs (Baumgartner et al., 2018; Olthof et al., 2019). Indeed, we found that minor intron retention almost invariably resulted in the introduction of a premature stop codon (150/152; 98.6%) (Tables S2,S3). Of these, we found that 134 events were predicted to result in NMD, whereas only 16 would encode novel proteins (Table S3). We next used DAVID to identify the pathways that may be compromised upon loss-of-function of these MIGs. Only the 118 MIGs in the E11.5 mutant forelimb predicted to undergo NMD had significant enrichment, including cell cycle, cell division, mitotic nuclear division and condensed chromosome kinetochore (Fig. 2D,E; Table S4). This therefore suggested potential cell cycle defects in the U11-null limbs.
U11 loss resulted in defective mitosis, prolonged S-phase, and slowing of cell cycle
To test whether cell cycle was affected in the E11.5 mutant forelimb, we first performed immunofluorescence (IF) for phospho-histone H3 (PH3), which marks all mitotic cells. Indeed, we observed an increase in PH3+ cells at E11.5 in the mutant forelimb, which was not found to occur at E10.5 (Fig. 2F,G; Fig. S7A,B). Similarly, we observed an increase in the number of PH3+ cells in the mutant hindlimb at E12.5, but not at E11.5 (Fig. 2F,G; Fig. S7A,C). The increase in mitotic cells in the E11.5 mutant forelimb and E12.5 mutant hindlimb could be caused by a delay in progenitor cell progression through the mitotic phases, as we previously found in the U11-null pallium (Baumgartner et al., 2018). Therefore, we performed IF for AuroraB, as its subcellular localization can be used to identify prophase, prometaphase, metaphase, anaphase and telophase (Fig. 2H). Although the total number of cells in mitosis was not different between the E10.5 WT and mutant forelimb, the distribution of cells within each phase was shifted such that, in the mutant, there were significantly more cells in prometaphase and significantly fewer cells in metaphase (Fig. 2H,I). Similarly, in the E11.5 mutant hindlimb, we found significantly more cells in prometaphase and significantly fewer cells in anaphase (Fig. 2H,I). Taken together, these data suggested that U11 loss caused defective mitotic progression and an accumulation of PH3+ cells 24 h later, indicating that cell cycle speed might be generally reduced in limb progenitor cells.
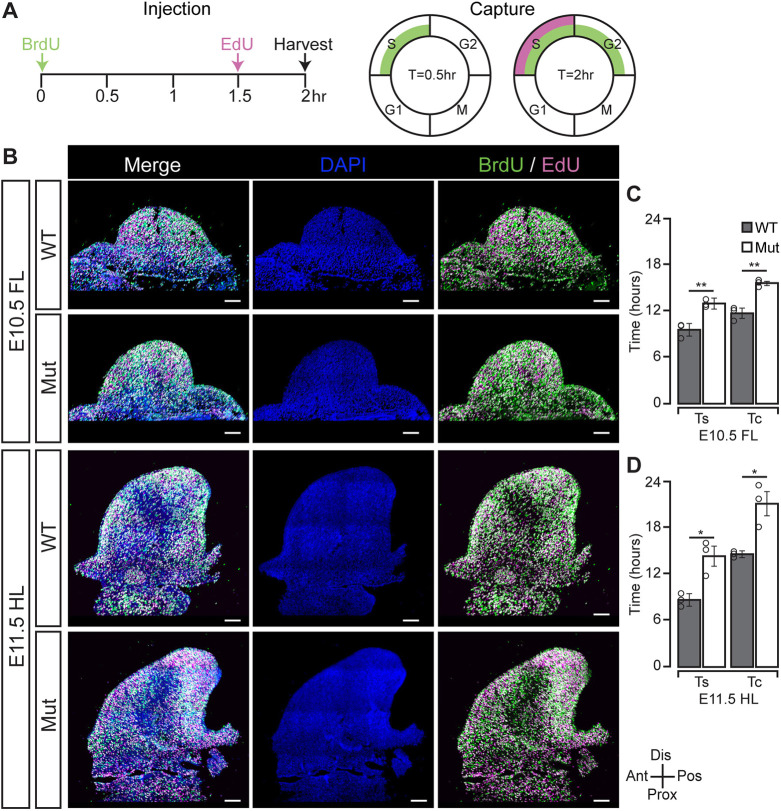
To determine the cell cycle speed of limb progenitor cells, we employed a BrdU/EdU pulse-chase paradigm previously used in the developing limb (Boehm et al., 2010). According to this paradigm, pregnant dams were injected with BrdU 2 h before harvest so that all cells in S-phase within 30 min of injection were marked (Fig. 3A) (Boehm et al., 2010). Subsequently, EdU injection 30 min before harvest marked all cells in S-phase at the time of harvest (Fig. 3A) (Boehm et al., 2010). This double-labeling technique allowed for the identification of cells that did not enter S-phase in the 2 h period, stayed in S-phase during the 2 h period, or left S-phase before the EdU injection (Fig. 3A) (Boehm et al., 2010). In turn, quantification of these populations of cells allowed for the estimation of S-phase length and total cell cycle time (Boehm et al., 2010). Using this strategy, we discovered a significant increase in the length of S-phase and total cell cycle time in the E10.5 mutant forelimb compared with its WT counterpart (Fig. 3B,C). Specifically, S-phase was increased by 3.3 h, whereas cell cycle time was increased by 4.1 h (Fig. 3C). Similarly, we found a significant increase in S-phase by 5.9 h and cell cycle time by 6.8 h in the E11.5 mutant hindlimb relative to its WT counterpart (Fig. 3B,D). Thus, in both the E10.5 mutant forelimb and E11.5 mutant hindlimb, mitotic delay and prolonged S-phase contribute to decreased cell cycle speed, but the lengthening of S-phase was the primary driver (Figs 2I; 3C,D). Defective cell cycle progression and subsequently reduced cell cycle speed led us to hypothesize that rapidly dividing cells would undergo apoptosis, as we have previously observed (Baumgartner et al., 2018).
Fig. 3.
U11 loss resulted in an elongation of S-phase and a reduction of cell cycle speed. (A) Adapted schematic of injection paradigm to quantify cell cycle speed as described by Boehm et al. (2010). BrdU and EdU take 30 min to be detected, and are thus captured at T=0.5 h and T=2 h, respectively. (B) IF for BrdU and enzymatic detection for EdU with DAPI counterstain in WT and mutant (Mut) E10.5 forelimb (FL) and E11.5 hindlimb (HL). (C,D) Quantification of total S-phase time (Ts) and total cell cycle time (Tc) for WT and mutant E10.5 FL (C) and E11.5 HL (D). Ant, anterior; Dis, distal; Pos, posterior; Prox, proximal. Data are mean±s.e.m. For details of statistical methods, see Table S6. *P<0.05, **P<0.01. Scale bars: 50 μm.
Loss of U11 resulted in death of a subset of distally concentrated limb progenitor cells
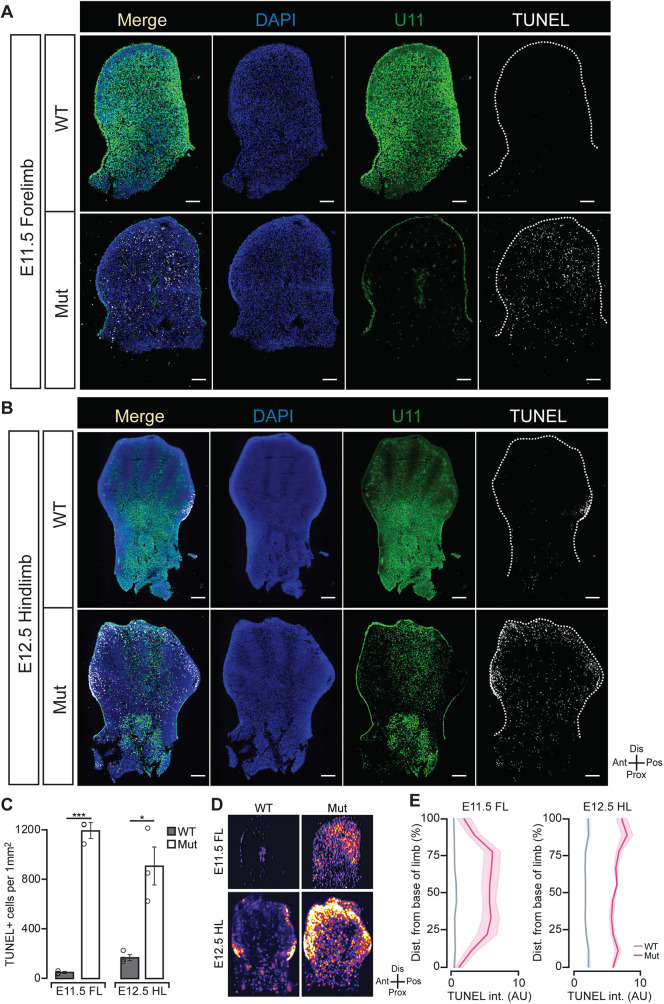
To identify the U11-null limb progenitor cells that undergo apoptosis, we performed fluorescence in situ hybridization (FISH) for U11 followed by terminal dUTP nick end labeling (TUNEL) to mark dying cells. Across the dorso-ventral axis, different sections of the limb showed varying patterns of TUNEL. Therefore, we sought to obtain a three-dimensional perspective of apoptosis by processing all sections of the developing limb, compiling them, and reconstructing a 2D heat map for TUNEL using ImageJ. Using this strategy, we found mesodermal U11 loss with a significant increase in cell death concentrated distally and posteriorly in the E11.5 mutant forelimb, but not in the E10.5 mutant forelimb (Fig. 4A,C-E; Fig. S8A,B). Similarly, the mutant hindlimb showed significant cell death concentrated distally and anteriorly at E12.5, but not at E11.5 (Fig. 4B-E; Fig. S8A,C). Thus, in both the mutant forelimb and hindlimb, progenitor cell death was concentrated in the domain of highest proliferation 24 h after cell cycle defects (Figs 2I; 3C,D; 4C) (Boehm et al., 2010).
Fig. 4.
U11 loss caused distally concentrated cell death. (A,B) Fluorescent in situ hybridization (FISH) for Rnu11 with terminal dUTP-nick end labeling (TUNEL) counterstained with DAPI in WT and mutant (Mut) E11.5 forelimb (FL) (A) and E12.5 hindlimb (HL) (B). Dotted line indicates limb bud perimeter. (C) Quantification of TUNEL+ cells normalized to limb bud area. (D) Heat map of serially overlaid images from A and B, with brighter color representing higher concentration of TUNEL+ signal. (E) Quantification of TUNEL intensity from D in arbitrary units (AU) in relation to the distance (dist.) from the base of the limb bud. Ant, anterior; Dis, distal; Pos, posterior; Prox, proximal. Data are mean±s.e.m. (error bars in C, shaded region in E). For details of statistical methods, see Table S6. *P<0.05, **P<0.01, ***P<0.001. Scale bars: 100 μm.
The spatial expression of key limb patterning genes was maintained in the E11.5 mutant forelimb
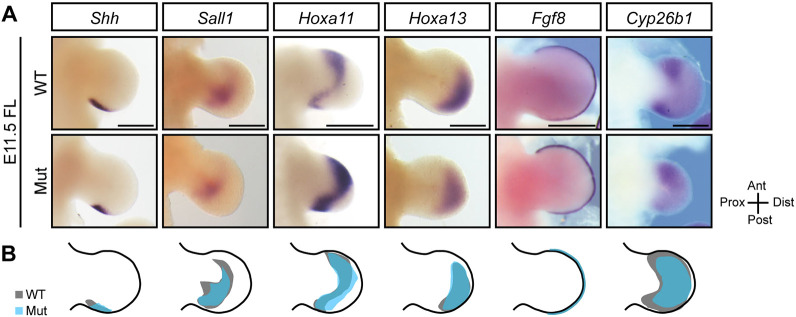
Although elevated minor intron retention in the U11-null forelimb led to cell cycle defects at E10.5 and cell death at E11.5, we observed timely chondrogenesis at E12.5, obvious digits at E14.5, and basic proximo-distal segmentation at P0 (Fig. 1E,G; Fig. 2I; Fig. 3C,D; Fig. 4C; Fig. S4). This led us to explore whether these hallmarks of limb development were achieved because the spatial expression of certain essential limb patterning genes were maintained in the mutant forelimb. For this, we performed WISH for Shh, which is crucial for establishing both proximo-distal and antero-posterior patterning; Hoxa11, as it is expressed by zeugopod progenitors; Hoxa13, as it is expressed by autopod progenitors; Sall1, owing to its interaction with Shh and Hoxa13; and Fgf8 and Cyp26b1, for their crucial roles in proximo-distal patterning (Tabin and Wolpert, 2007; Kawakami et al., 2009; Zuniga, 2015). This analysis showed that Shh, Sall1, Hoxa11, Hoxa13, Fgf8 and Cyp26b1 possess expression domains in the E11.5 mutant forelimb that are comparable with the E11.5 WT forelimb (Fig. 5A,B). Thus, our WISH analysis revealed that the cellular defects caused by U11 loss did not alter the spatial expression domains of key limb patterning genes.
Fig. 5.
U11 loss did not alter the expression domains of key limb patterning genes in the E11.5 mutant forelimb. (A) WISH for Shh, Sall1, Hoxa11, Hoxa13, Fgf8 and Cyp26b1 in the WT and mutant (Mut) E11.5 forelimb (FL). (B) Schematic showing traced WT limb bud and gene expression domain (black) with mutant gene expression domain (blue) overlaid. Scale bars: 50 μm.
DISCUSSION
The regulation of tissue size is a fundamental question in biology as, in mammals, the adult body mass ranges from ∼1.5 g in the Etruscan shrew to ∼1,500,000 g in the blue whale (Penzo-Mendez and Stanger, 2015). A direct example of tissue size reduction is observed in the limb size of domesticated animals, such as dogs, cats and cows, compared with their wild ancestors (Sánchez-Villagra et al., 2016). Comparisons of genome-wide association studies between domesticated animals and their wild counterparts have revealed shared selection for genetic changes in MIGs and components of the minor spliceosome (Baumgartner et al., 2019). This finding suggests that tissue size could be altered by modulating expression of certain MIGs and/or the minor spliceosome, which is reinforced by graded primordial dwarfism observed in MOPD1, RS and LWS (Putoux et al., 2016; Farach et al., 2018).
An important characteristic of limb size reduction in DS and minor spliceosome-related disease is the maintenance of limb patterning (Sánchez-Villagra et al., 2016). In agreement, we show here that the U11-null forelimb and hindlimb are reduced in size but maintain basic segmentation and patterning, respectively (Fig. 1G,H). In the mutant forelimb, the discordance between our observation of a single digit at P0 and two digits through SEM at E14.5 is most likely due to the penetrance of Prrx1-Cre activity (Fig. 1E,G). Regardless, this does not challenge the notion that U11 loss does not disrupt the basic segmentation of the limb (Fig. 1B,G). Moreover, the difference in the phenotype observed between the mutant forelimb and hindlimb is most likely due to the difference in the onset of Prrx1-Cre expression, which is delayed in the mutant hindlimb (Fig. S2A,B). Therefore, there is a more substantial depletion of early progenitor cells in the mutant forelimb, which results in an insufficient number of cells available to form a limb along all three axes. Indeed, this concept of a progenitor cell number threshold for skeletal element formation has been previously proposed by Wolpert et al. (1979). Conversely, in the mutant hindlimb, delayed progenitor cell loss may have resulted in sufficient progenitor cells available for it to achieve patterning along all three axes (Fig. 1G; Fig. S2A,B). However, the loss of the medial cuneiform and stunted digit 1 bolsters the idea that there is a critical number of cells required to form each skeletal structure (Fig. 1G; Fig. S6A) (Wolpert et al., 1979). Another possibility is that the severe phenotype observed in the U11-null forelimb is due to minor spliceosome inhibition and subsequent cell cycle defects and cell death at a time when limb patterning is being established. Extending this idea to the mutant hindlimb would predict a less severe phenotype as minor spliceosome inhibition is delayed, and therefore its consequences occur after crucial patterning events have been achieved. Indeed, the hindlimb is reduced in size but maintains basic patterning (Fig. 1F-H). Shared in both the mutant forelimb and hindlimb, U11 loss caused mesomelia and reductions in bone thickness and ossification, the latter of which are observed in individuals with MODP1 (Fig. 1I, Fig. S6C,D) (Krøigård et al., 2016). Thus, our studies reveal a requirement for the minor spliceosome not only in general limb development but also bone formation and metabolism.
In MOPDI, RS and LWS, the underlying mutations in RNU4ATAC are predicted to inhibit minor spliceosome function (Edery et al., 2011; He et al., 2011; Merico et al., 2015; Farach et al., 2018). Our ablation of Rnu11 in the developing limb also achieves inhibition of the minor spliceosome. Therefore, the molecular defects reported herein could potentially inform the limb defects observed in MOPDI, RS and LWS. Consistent with previous reports, we show that U11 loss resulted in the upregulation of minor intron retention in a subset of MIGs (Fig. 2C,D; Table S1) (Baumgartner et al., 2018; Olthof et al., 2019). Some of these MIGs are crucial for cell cycle regulation, including Cdc45, Dna2 and Spc24, which suggested that the limb defects observed in our mutant mice stem from cell cycle dysregulation (Fig. 2E; Table S1) (McCleland et al., 2004; Aparicio et al., 2006; Duxin et al., 2009). In fact, mutations in Cdc45 and Dna2, which both show elevated minor intron retention in the E11.5 mutant forelimb, are linked to primordial dwarfism in Meier-Gorlin syndrome and Seckel syndrome, respectively (Table S1) (Shaheen et al., 2014; Fenwick et al., 2016). These findings point to a potential role for mis-splicing of these two genes in driving the micromelia observed in individuals with MOPD1, RS and LWS (Shaheen et al., 2014; Fenwick et al., 2016; Putoux et al., 2016; Farach et al., 2018).
The minor splicing defect identified in MIGs responsible for cell cycle regulation is in agreement with our observation that U11-null progenitor cells are stuck in the prometaphase-to-metaphase transition (Fig. 2I), which is also similar to our previous findings in the U11-null pallium (Baumgartner et al., 2018). Moreover, upregulation of minor intron retention in certain MIGs, including Dna2 and E2f6, predicted potential S-phase defects, which was reflected in the lengthening of S-phase in the mutant forelimb and hindlimb (Fig. 3C,D; Table S1) (Giangrande et al., 2004; Duxin et al., 2009). Together, these cell cycle defects reduced overall progenitor cell cycle speed (Fig. 3C,D). In addition, we found massively elevated cell death, which was concentrated in the distal, posterior compartment of the mutant forelimb and distal, anterior compartment of the mutant hindlimb (Fig. 4C,D). These data suggested that, although there is a large population of U11-null limb progenitor cells, distal progenitor cells are more susceptible to the consequences of U11 loss (Fig. 4D,E). We propose that this is because these cells are the most rapidly dividing, as previously reported (Boehm et al., 2010), and thus do not have enough time to accommodate aberrant MIG expression. This idea agrees with our previous report of U11 loss in the developing pallium, which triggered death of the rapidly dividing radial glial cells but not the intermediate progenitor cells or neurons (Baumgartner et al., 2018). One alternative explanation is that the differentiation state of the progenitor cells in the distal versus proximal domains of the limb confers differential susceptibility to U11 loss. This idea is based on the observation that the distal limb progenitor cells are the most undifferentiated (Tabin and Wolpert, 2007). Nonetheless, the combined effect of cell cycle defects and apoptosis resulted in a severe depletion of progenitor cells, thereby limiting the number of cells available to form all the structures of the limb (Figs 2G,I; 3C,D; 4C).
Given that the U11-null limbs experienced cell cycle defects and cell death, we were surprised to find maintenance of spatial gene expression for key limb patterning genes (Fig. 5A,B). This result reinforced our observation that the mutant forelimb maintains a normal developmental trajectory, given that we found timely chondrogenesis at E12.5 and visible digits at E14.5 (Fig. 1E; Fig. S4). Thus, although inhibition of the minor spliceosome reduces the number of progenitor cells available to form a limb, it does not appear to affect spatial gene regulatory networks that govern limb morphogenesis (Fig. 5A,B). Based on these observations, we emphasize the notion that the minor spliceosome may be an ideal target for selection in evolutionary-driven tissue size scaling, such as DS.
The skeletal phenotypes of the P0 mutant limbs are similar to those reported in DicerFlx/Flx::Prrx1-Cre+ and CtcfFlx/Flx::Prrx1-Cre+ mice (Harfe et al., 2005; Soshnikova et al., 2010). Specifically, each shows stunted limb size, defects in antero-posterior expansion, but maintenance of skeletal segmentation along the proximo-distal axis (Fig. 1F,G) (Harfe et al., 2005; Soshnikova et al., 2010). These phenotypes reinforce a previously reported notion that proximo-distal segmentation occurs first in limb development and is followed by the expansion/bifurcation of these segments (Richardson et al., 2004). This model is borne out in our finding of a single zeugopod element and digit in the U11-null mutant forelimb rather than two zeugopod elements (Fig. 1G). Moreover, similar phenotypes were observed through X-irradiation experiments of the developing chick limb bud in which, despite loss of progenitor cells, limb segment specification along the proximo-distal axis was maintained (Galloway et al., 2009). It is of great interest to determine whether there exist shared mechanisms between species that maintain basic limb segmentation/patterning in response to a developmental insult.
Overall, we show how minor spliceosome inhibition, as well as the developmental insult in the diseases MOPD1, RS and LWS, could cause primordial dwarfism. Moreover, we speculate that selection for changes in genes that could modulate the activity of the minor spliceosome might similarly result in short limbs that maintain patterning in domesticated species.
MATERIALS AND METHODS
Animal husbandry
Mouse husbandry and procedures were carried out in accordance with protocols approved by the University of Connecticut Institutional Animal Care and Use Committee, which operates under the guidelines of the US Public Health Service Policy for laboratory animal care. The Rnu11 conditional knockout mouse used in this study was generated and described by Baumgartner et al. (2018). Prrx1-Cre was used to ablate Rnu11 in the developing limbs (Logan et al., 2002). The experiments described above used male and female C57/Bl6 Rnu11Flx/Flx::Prrx1-Cre−::CAG-loxpSTOPloxp-tdTomato+ (WT) mice and Rnu11Flx/Flx::Prrx1-Cre+::CAG-loxpSTOPloxp-tdTomato+ (mutant) mice. Heterozygous (Rnu11WT/Flx::Prrx1-Cre+::CAG-loxpSTOPloxp-tdTomato+) males were used for breeding and were indistinguishable from littermate controls. For embryonic harvests, E0.5 was considered noon the morning a vaginal plug was observed.
WISH
WISH analysis was carried out as described previously (Riddle et al., 1993). Embryos were halved with tungsten needles (Fine Science Tools, 10130-10) after rehydration. Probes were generated using the primers listed in Table S5.
qRT-PCR
Gene expression was quantified through qRT-PCR as described previously with normalization to Rn7sk for Rnu11 (Baumgartner et al., 2018). Primers used are listed in Table S5.
SEM
SEM was performed as described previously with help from the University of Connecticut's Bioscience Electron Microscopy Core Facility (Martin, 1990).
Skeletal preparation
Skeletal preparation was performed as described previously (McLeod, 1980). Percent ossification was calculated by dividing the Alizarin Red-stained region by the total long bone length.
H&E staining
H&E was performed on 10 μm limb bud sections as described previously (Baumgartner et al., 2018).
Bioinformatics analysis & data accessibility
Total RNA-seq was performed on E10.5 and E11.5 WT and mutant forelimb and hindlimb (n=3 for each sample). RNA extraction was carried out using TRIzol (Thermo Fisher Scientific, 15596018) per the manufacturer's instructions. RNA sample preparation and sequencing were executed by the University of Connecticut's Center for Genome Innovation. Library preparation was carried out using the Illumina TruSeq Stranded Total RNA Library Sample Prep Kit (RS-122-2201) with RiboZero for ribosomal RNA depletion. Sequencing was performed using Illumina NextSeq 500. Reads were mapped to mm10 using Hisat2 (Kim et al., 2015). Gene expression calls were determined through IsoEM2 (Mandric et al., 2017). Differential gene expression was determined using IsoDE2 (Mandric et al., 2017). Minor intron retention and ORF analyses were performed as described previously (Baumgartner et al., 2018). DAVID was employed for functional enrichment analysis of gene sets with significance determined by Benjamini–Hochberg adjusted P-value<0.05. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE146424.
IF
We used 10 μm limb bud cryosections for IF as described previously (Karunakaran et al., 2015). Primary antibodies were diluted to 1:50 (MoBu-1 clone mouse anti-BrdU, Santa Cruz Biotechnology, sc-51514), 1:100 (mouse anti-Aurora-B/AIM1, BD Biosciences, 611082) or 1:500 (rabbit anti-PH3, Bethyl Laboratories, IHC-00061).
BrdU & EdU pulse
Timed-pregnant dams were injected with BrdU and EdU (100 nmol per gram bodyweight) per the paradigm described in Boehm et al. (2010) and represented in Fig. 3A. BrdU was detected by IF using the MoBu-1 clone mouse anti-BrdU antibody. Detection of EdU was performed using the Click-iT EdU Alexa Fluor 647 imaging kit (Thermo Fisher Scientific, C10340) in accordance with the manufacturer's instructions.
FISH
FISH for U11 was performed on 10 μm limb bud cryosections as described previously (Baumgartner et al., 2018).
TUNEL
TUNEL was performed on 10 μm limb bud cryosections using the in situ cell death detection kit, TMR Red (Roche Diagnostics), in accordance with the manufacturer's instructions.
Imaging & quantification
For imaging of H&E, IF for PH3, and FISH for U11 with TUNEL, Keyence BZ-X710 was used at 10× (H&E) or 20× (IF, FISH) with automatic settings for light (H&E) or fluorescence (IF, FISH) intensity (set to the WT) and image stitching. All sections on a processed slide, which contained n=1 WT and mutant, were imaged with the same settings. To generate a heat map of TUNEL signal, all sections processed for TUNEL from a single limb bud were concatenated, stacked and 3D projected, which was used for a 3D surface plot to produce a heat map that is a 2D rendering of 3D TUNEL+ signal intensity. This was performed for a minimum of three limb buds per WT and mutant forelimb at E10.5 and E11.5 as well as WT and mutant hindlimb at E11.5 and E12.5. For imaging of IF for AuroraB and IF for BrdU with EdU Click-iT reaction, slides were imaged using a Leica SP2 confocal microscope as described previously (Baumgartner et al., 2018). Further image processing and quantification was carried out using IMARIS v.8.3.1 (Bitplane) and Adobe Photoshop CS4 as described previously (Baumgartner et al., 2018). SEM imaging was performed using a Nova NanoSEM 450 electron nanoscope through the University of Connecticut's Bioscience Electron Microscopy Core Facility. All representative images and traces were performed for minimum n=3, with exception for the SEM images in Fig. 1E and Fig. S5, which were performed for n=1.
RNA isolation and cDNA prep
Limb buds (n=3) from E10.5 and E11.5 WT and mutant embryos were dissected for RNA isolation and cDNA preparation as described by Baumgartner et al. (2018). We used 100 ng of RNA for cDNA synthesis.
Statistical methods
Detailed information for all statistical tests performed can be found in Table S6.
Supplementary Material
Acknowledgements
We thank Dr Bo Reese from the University of Connecticut’s Center for Genome Innovation for assistance with RNA-seq; Dr Xuanhao Sun and Dr Maritza Abril from the University of Connecticut's Bioscience Electron Microscopy Facility for assistance with SEM imaging; Dr Ion Mandoiu from the University of Connecticut's Computer Science and Engineering Department for the establishment of bioinformatic platforms; and Dr Anouk Olthof for help with bioinformatic analyses.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.D.D., R.N.K.; Methodology: K.D.D., R.N.K.; Software: R.N.K.; Validation: K.D.D., C.L., A.M.V., R.N.K.; Formal analysis: K.D.D., R.N.K.; Investigation: K.D.D., C.L., G.S.A., R.N.K.; Resources: R.N.K.; Data curation: K.D.D., R.N.K.; Writing - original draft: K.D.D., R.N.K.; Writing - review & editing: K.D.D., R.N.K.; Visualization: K.D.D., R.N.K.; Supervision: R.N.K.; Project administration: R.N.K.; Funding acquisition: K.D.D., R.N.K.
Funding
Funding for this study comes from the National Institute of Neurological Disorders and Stroke (R01NS102538 and R21NS096684 to R.N.K.) and the National Science Foundation Graduate Research Fellowship Program (2018257410 to K.D.D.). Deposited in PMC for release after 12 months.
Data availability
Data have been deposited in GEO under accession number GSE146424.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.190967.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.190967.reviewer-comments.pdf
References
- Aparicio T., Ibarra A. and Mendez J. (2006). Cdc45-MCM-GINS, a new power player for DNA replication. Cell Div. 1, 18 10.1186/1747-1028-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente J., Flores R., Gutiérrez-Arumí A., Verma B., Martos-Moreno G. A., Cuscó I., Oghabian A., Chowen J. A., Frilander M. J. and Pérez-Jurado L. A. (2014). Defective minor spliceosome mRNA processing results in isolated familial growth hormone deficiency. EMBO Mol. Med. 6, 299-306. 10.1002/emmm.201303573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M. K., Makalowski W., Rogozin I. B. and Koonin E. V. (2008). U12 intron positions are more strongly conserved between animals and plants than U2 intron positions. Biol. Direct 3, 19 10.1186/1745-6150-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner M., Olthof A. M., Aquino G. S., Hyatt K. C., Lemoine C., Drake K., Sturrock N., Nguyen N., Al Seesi S. and Kanadia R. N. (2018). Minor spliceosome inactivation causes microcephaly, owing to cell cycle defects and death of self-amplifying radial glial cells. Development 145, dev166322 10.1242/dev.166322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner M., Drake K. and Kanadia R. N. (2019). An integrated model of minor intron emergence and conservation. Front. Genet. 10, 1113 10.3389/fgene.2019.01113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm B., Westerberg H., Lesnicar-Pucko G., Raja S., Rautschka M., Cotterell J., Swoger J. and Sharpe J. (2010). The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol. 8, e1000420 10.1371/journal.pbio.1000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett K., Williams B. B., Markmiller S., Geng F.-S., Coates J., Mieruszynski S., Ernst M., Thomas T. and Heath J. K. (2018). Early developmental arrest and impaired gastrointestinal homeostasis in U12-dependent splicing-defective Rnpc3-deficient mice. RNA 24, 1856-1870. 10.1261/rna.068221.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxin J. P., Dao B., Martinsson P., Rajala N., Guittat L., Campbell J. L., Spelbrink J. N. and Stewart S. A. (2009). Human Dna2 is a nuclear and mitochondrial DNA maintenance protein. Mol. Cell. Biol. 29, 4274-4282. 10.1128/MCB.01834-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery P., Marcaillou C., Sahbatou M., Labalme A., Chastang J., Touraine R., Tubacher E., Senni F., Bober M. B., Nampoothiri S. et al. (2011). Association of TALS developmental disorder with defect in minor splicing component U4atac snRNA. Science 332, 240-243. 10.1126/science.1202205 [DOI] [PubMed] [Google Scholar]
- Elsaid M. F., Chalhoub N., Ben-Omran T., Kumar P., Kamel H., Ibrahim K., Mohamoud Y., Al-Dous E., Al-Azwani I., Malek J. A. et al. (2017). Mutation in noncoding RNA RNU12 causes early onset cerebellar ataxia. Ann. Neurol. 81, 68-78. 10.1002/ana.24826 [DOI] [PubMed] [Google Scholar]
- Farach L. S., Little M. E., Duker A. L., Logan C. V., Jackson A., Hecht J. T. and Bober M. (2018). The expanding phenotype of RNU4ATAC pathogenic variants to Lowry Wood syndrome. Am. J. Med. Genet. A 176, 465-469. 10.1002/ajmg.a.38581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick A. L., Kliszczak M., Cooper F., Murray J., Sanchez-Pulido L., Twigg S. R. F., Goriely A., McGowan S. J., Miller K. A., Taylor I. B. et al. (2016). Mutations in CDC45, encoding an essential component of the pre-initiation complex, cause Meier-Gorlin syndrome and craniosynostosis. Am. J. Hum. Genet. 99, 125-138. 10.1016/j.ajhg.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway J. L., Delgado I., Ros M. A. and Tabin C. J. (2009). A reevaluation of X-irradiation-induced phocomelia and proximodistal limb patterning. Nature 460, 400-404. 10.1038/nature08117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande P. H., Zhu W., Schlisio S., Sun X., Mori S., Gaubatz S. and Nevins J. R. (2004). A role for E2F6 in distinguishing G1/S- and G2/M-specific transcription. Genes Dev. 18, 2941-2951. 10.1101/gad.1239304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe B. D., McManus M. T., Mansfield J. H., Hornstein E. and Tabin C. J. (2005). The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc. Natl. Acad. Sci. USA 102, 10898-10903. 10.1073/pnas.0504834102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Liyanarachchi S., Akagi K., Nagy R., Li J., Dietrich R. C., Li W., Sebastian N., Wen B., Xin B. et al. (2011). Mutations in U4atac snRNA, a component of the minor spliceosome, in the developmental disorder MOPD I. Science 332, 238-240. 10.1126/science.1200587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran D. K. P., Chhaya N., Lemoine C., Congdon S., Black A. and Kanadia R. (2015). Loss of citron kinase affects a subset of progenitor cells that alters late but not early neurogenesis in the developing rat retina role of citron kinase in retinal neurogenesis. Invest. Ophthalmol. Vis. Sci. 56, 787-798. 10.1167/iovs.14-15272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Uchiyama Y., Rodriguez Esteban C., Inenaga T., Koyano-Nakagawa N., Kawakami H., Marti M., Kmita M., Monaghan-Nichols P., Nishinakamura R. et al. (2009). Sall genes regulate region-specific morphogenesis in the mouse limb by modulating Hox activities. Development 136, 585-594. 10.1242/dev.027748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Langmead B. and Salzberg S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357-360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krøigård A. B., Frost M., Larsen M. J., Ousager L. B. and Frederiksen A. L. (2016). Bone structure in two adult subjects with impaired minor spliceosome function resulting from RNU4ATAC mutations causing microcephalic osteodysplastic primordial dwarfism type 1 (MOPD1). Bone 92, 145-149. 10.1016/j.bone.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Levine A. and Durbin R. (2001). A computational scan for U12-dependent introns in the human genome sequence. Nucleic Acids Res. 29, 4006-4013. 10.1093/nar/29.19.4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M., Martin J. F., Nagy A., Lobe C., Olson E. N. and Tabin C. J. (2002). Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77-80. 10.1002/gene.10092 [DOI] [PubMed] [Google Scholar]
- Mandric I., Temate-Tiagueu Y., Shcheglova T., Al Seesi S., Zelikovsky A. and Ma˘ndoiu I. I. (2017). Fast bootstrapping-based estimation of confidence intervals of expression levels and differential expression from RNA-Seq data. Bioinformatics 33, 3302-3304. 10.1093/bioinformatics/btx365 [DOI] [PubMed] [Google Scholar]
- Martin P. (1990). Tissue patterning in the developing mouse limb. Int. J. Dev. Biol. 34, 323-336. [PubMed] [Google Scholar]
- McCleland M. L., Kallio M. J., Barrett-Wilt G. A., Kestner C. A., Shabanowitz J., Hunt D. F., Gorbsky G. J. and Stukenberg P. T. (2004). The vertebrate Ndc80 complex contains Spc24 and Spc25 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr. Biol. 14, 131-137. 10.1016/j.cub.2003.12.058 [DOI] [PubMed] [Google Scholar]
- McLeod M. J. (1980). Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299-301. 10.1002/tera.1420220306 [DOI] [PubMed] [Google Scholar]
- Merico D., Roifman M., Braunschweig U., Yuen R. K. C., Alexandrova R., Bates A., Reid B., Nalpathamkalam T., Wang Z., Thiruvahindrapuram B. et al. (2015). Compound heterozygous mutations in the noncoding RNU4ATAC cause Roifman Syndrome by disrupting minor intron splicing. Nat. Commun. 6, 8718 10.1038/ncomms9718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olthof A. M., Hyatt K. C. and Kanadia R. N. (2019). Minor intron splicing revisited: identification of new minor intron-containing genes and tissue-dependent retention and alternative splicing of minor introns. BMC Genomics 20, 686 10.1186/s12864-019-6046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake L. R., Scamborova P., Hashimoto C. and Steitz J. A. (2002). The divergent U12-type spliceosome is required for pre-mRNA splicing and is essential for development in Drosophila. Mol. Cell 9, 439-446. 10.1016/S1097-2765(02)00441-0 [DOI] [PubMed] [Google Scholar]
- Patel A. A. and Steitz J. A. (2003). Splicing double: insights from the second spliceosome. Nat. Rev. Mol. Cell Biol. 4, 960-970. 10.1038/nrm1259 [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez A. I. and Stanger B. Z. (2015). Organ-size regulation in mammals. Cold Spring Harb. Perspect. Biol. 7, a019240 10.1101/cshperspect.a019240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putoux A., Alqahtani A., Pinson L., Paulussen A. D. C., Michel J., Besson A., Mazoyer S., Borg I., Nampoothiri S., Vasiljevic A. et al. (2016). Refining the phenotypical and mutational spectrum of Taybi-Linder syndrome. Clin. Genet. 90, 550-555. 10.1111/cge.12781 [DOI] [PubMed] [Google Scholar]
- Richardson M. K., Jeffery J. E. and Tabin C. J. (2004). Proximodistal patterning of the limb: insights from evolutionary morphology. Evol. Dev. 6, 1-5. 10.1111/j.1525-142X.2004.04008.x [DOI] [PubMed] [Google Scholar]
- Riddle R. D., Johnson R. L., Laufer E. and Tabin C. (1993). Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401-1416. 10.1016/0092-8674(93)90626-2 [DOI] [PubMed] [Google Scholar]
- Russell A. G., Charette J. M., Spencer D. F. and Gray M. W. (2006). An early evolutionary origin for the minor spliceosome. Nature 443, 863-866. 10.1038/nature05228 [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra M. R., Geiger M. and Schneider R. A. (2016). The taming of the neural crest: a developmental perspective on the origins of morphological covariation in domesticated mammals. R. Soc. Open Sci. 3, 160107 10.1098/rsos.160107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R., Faqeih E., Ansari S., Abdel-Salam G., Al-Hassnan Z. N., Al-Shidi T., Alomar R., Sogaty S. and Alkuraya F. S. (2014). Genomic analysis of primordial dwarfism reveals novel disease genes. Genome Res. 24, 291-299. 10.1101/gr.160572.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshnikova N., Montavon T., Leleu M., Galjart N. and Duboule D. (2010). Functional analysis of CTCF during mammalian limb development. Dev. Cell 19, 819-830. 10.1016/j.devcel.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Szcześniak M. W., Kabza M., Pokrzywa R., Gudyś A. and Makałowska I. (2013). ERISdb: a database of plant splice sites and splicing signals. Plant Cell Physiol. 54, e10 10.1093/pcp/pct001 [DOI] [PubMed] [Google Scholar]
- Tabin C. and Wolpert L. (2007). Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 21, 1433-1442. 10.1101/gad.1547407 [DOI] [PubMed] [Google Scholar]
- Wilkins A. S., Wrangham R. W. and Fitch W. T. (2014). The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795-808. 10.1534/genetics.114.165423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L., Tickle C. and Sampford M. (1979). The effect of cell killing by x-irradiation on pattern formation in the chick limb. J. Embryol. Exp. Morphol. 50, 175-193. [PubMed] [Google Scholar]
- Zuniga A. (2015). Next generation limb development and evolution: old questions, new perspectives. Development 142, 3810-3820. 10.1242/dev.125757 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Total RNA-seq was performed on E10.5 and E11.5 WT and mutant forelimb and hindlimb (n=3 for each sample). RNA extraction was carried out using TRIzol (Thermo Fisher Scientific, 15596018) per the manufacturer's instructions. RNA sample preparation and sequencing were executed by the University of Connecticut's Center for Genome Innovation. Library preparation was carried out using the Illumina TruSeq Stranded Total RNA Library Sample Prep Kit (RS-122-2201) with RiboZero for ribosomal RNA depletion. Sequencing was performed using Illumina NextSeq 500. Reads were mapped to mm10 using Hisat2 (Kim et al., 2015). Gene expression calls were determined through IsoEM2 (Mandric et al., 2017). Differential gene expression was determined using IsoDE2 (Mandric et al., 2017). Minor intron retention and ORF analyses were performed as described previously (Baumgartner et al., 2018). DAVID was employed for functional enrichment analysis of gene sets with significance determined by Benjamini–Hochberg adjusted P-value<0.05. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE146424.