Abstract
Background
Hypoxic‐ischaemic encephalopathy (HIE) is a leading cause of mortality and long‐term neurological sequelae, affecting thousands of children worldwide. Current therapies to treat HIE are limited to cooling. Stem cell‐based therapies offer a potential therapeutic approach to repair or regenerate injured brain tissue. These preclinical findings have now culminated in ongoing human neonatal trials.
Objectives
To determine the efficacy and safety of stem cell‐based interventions for the treatment of hypoxic‐ischaemic encephalopathy (HIE) in newborn infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 5), MEDLINE via PubMed (1966 to 8 June 2020), Embase (1980 to 8 June 2020), and CINAHL (1982 to 8 June 2020). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised controlled trials, quasi‐randomised controlled trials and cluster trials comparing 1) stem cell‐based interventions (any type) compared to control (placebo or no treatment); 2) use of mesenchymal stem/stromal cells (MSCs) of type (e.g. number of doses or passages) or source (e.g. autologous versus allogeneic, or bone marrow versus cord) versus MSCs of other type or source; 3) use of stem cell‐based interventions other than MSCs of type (e.g. mononuclear cells, oligodendrocyte progenitor cells, neural stem cells, hematopoietic stem cells, and inducible pluripotent stem cells) or source (e.g. autologous versus allogeneic, or bone marrow versus cord) versus stem cell‐based interventions other than MSCs of other type or source; or 4) MSCs versus stem cell‐based interventions other than MSCs.
Data collection and analysis
For each of the included trials, two authors independently planned to extract data (e.g. number of participants, birth weight, gestational age, type and source of MSCs or other stem cell‐based interventions) and assess the risk of bias (e.g. adequacy of randomisation, blinding, completeness of follow‐up). The primary outcomes considered in this review are all‐cause neonatal mortality, major neurodevelopmental disability, death or major neurodevelopmental disability assessed at 18 to 24 months of age. We planned to use the GRADE approach to assess the quality of evidence.
Main results
Our search strategy yielded 616 references. Two review authors independently assessed all references for inclusion. We did not find any completed studies for inclusion. Fifteen RCTs are currently registered and ongoing. We describe the three studies we excluded.
Authors' conclusions
There is currently no evidence from randomised trials that assesses the benefit or harms of stem cell‐based interventions for the prevention of morbidity and mortality following hypoxic‐ischaemic encephalopathy in newborn infants.
Plain language summary
Stem cell‐based therapies following poor brain oxygenation at birth (hypoxic‐ischaemic encephalopathy) in newborns
Review question
Do stem cell‐based therapies save the lives, or improve the long‐term development, of newborns who have poor brain oxygenation at birth ('hypoxic‐ischaemic encephalopathy')?
Background
Lack of oxygen at birth may damage the brain of the newborn. Babies with less severe brain damage may make a full recovery or only have mild problems. For other babies with more serious damage, this may lead to death or to problems later in life. For instance, some of these babies develop cerebral palsy, intellectual disabilities, or other problems. We currently have only cooling as an approach to treat this condition. The aim of this review was to assess if stem cell‐based therapies could reduce death and improve the long‐term development of newborns with poor brain oxygenation at birth. During stem cell‐based therapy, stem cells are given to the baby, for instance through injections. These stem cells may have come from humans or animals and may have been taken from cord blood, bone marrow or other parts of the body. These cells then repair the brain cells that the lack of oxygen has damaged.
Key results
We were not able to include any studies in our review. We identified three potential studies, but we excluded them due to the way they were designed which meant that their results could not answer our review question (phase 1 studies). Fifteen studies are ongoing.
How up to date is this review?
We searched for studies that were available up to June 2020.
Background
Description of the condition
Hypoxic‐ischaemic encephalopathy (HIE) is a leading cause of mortality and long‐term neurological sequelae, affecting thousands of children in both developed and developing countries (Lee 2013). Every year 1.15 million infants develop HIE, with 96% born in low‐ and middle‐income countries. Up to 25% of neonates with HIE die and around 35% have long‐term neurodevelopmental sequelae (Lee 2013). Severity of HIE is scored in three stages: Stage I (Sarnat 1) – mild; Stage II (Sarnat 2) – moderate; Stage III (Sarnat 3) – severe (Sarnat 1976). Standard management in neonatal intensive care units consists of providing supportive care to maintain cerebral perfusion and metabolic balance. The only effective intervention is therapeutic hypothermia, which reduces mortality and improves neurocognitive outcome of newborns at 18 months of age (Edwards 2010; Jacobs 2013). The lower brain temperature induced by therapeutic hypothermia may decrease brain baseline metabolism and energy demand (Owji 2017; Pfister 2010; Polderman 2008). The mechanisms include reduction of apoptosis, mitochondrial dysfunction, and free radical production; decreased permeability of blood‐brain barrier; and mitigation of reperfusion injury and neuro‐inflammation (Goss 1995; Gunn 1997; Gunn 1998; Haaland 1997; Iwata 2007; Rothwell 1995; Silverstein 1997; Thoresen 1995). However, treatment should be started during the latent phase of brain injury, which extends up to six hours from the primary brain insult (Gluckman 1992), to avoid reperfusion injury (Gunn 1997; Zhao 1996). Furthermore, the duration of treatment should be long enough to mitigate a secondary phase of neural injury with cytotoxic cell oedema, apoptosis, accumulation of extracellular excitotoxins, and delayed seizures due to reperfusion (Sirimanne 1996; Tan 1996; Williams 1991). Thus, hypothermia should cover the whole reperfusion phase of brain injury and last 72 hours (Wyllie 2015).
Additional therapies for HIE are under investigation and include erythropoietin, allopurinol, xenon, topiramate, and magnesium sulphate. Erythropoietin has anti‐inflammatory, antioxidative, antiapoptotic, and antiexcitotoxic properties (Villa 2003). In addition, it may promote neurogenesis and angiogenesis (Wang 2004). In animal models of HIE, erythropoietin attenuates brain damage, and improves learning memory (Gonzalez 2009; Kumral 2004; Wu 2012). These data seem to be confirmed in small clinical trials (Elmahdy 2010; Rogers 2014; Zhu 2009). Allopurinol may be neuroprotective through direct scavenging of hydroxyl radicals and neutralizing non‐protein‐bound iron (Peeters‐Scholte 2003). Animal studies showed that allopurinol might be neuroprotective (Palmer 1993). Furthermore, its administration to term infants affected by HIE might improve neurocognitive outcome at four to eight years of age (Kaandorp 2012). Xenon has been reported to decrease brain injury in animal studies (David 2008; Ma 2005), with additional efficacy when combined with hypothermia (Hobbs 2008; Thoresen 2009). In one phase 2 clinical trial, xenon seemed to be safe (Dingley 2014). Topiramate may reduce brain damage, decrease the rate of apoptosis, diminish the infarcted area, and improved neurological outcome in animal models of HIE (Noh 2006; Ozyener 2012; Sfaello 2005). One ongoing trial aims to investigate the effects of topiramate on full‐term neonates with HIE undergoing hypothermia (NCT01765218). There are controversies regarding the neuroprotective role of magnesium sulphate in brain damage in term and late preterm infants (Galinsky 2014; Tagin 2013). The data from animal studies show lack of beneficial neuroprotective effect following HIE (de Haan 1997; Greenwood 2000; Penrice 1997). There is an ongoing clinical trial on magnesium sulphate during therapeutic hypothermia in term neonates with HIE (NCT01646619).
Description of the intervention
Mesenchymal stem/stromal cells (MSCs) have emerged as exciting and new therapeutic agents that could potentially ameliorate HIE (Nabetani 2018). MSCs are the most commonly used regenerative cells in clinical trials due to their relatively safe profile, ease of isolation/propagation, ability to reduce inflammation and oxidative stress, restore energy failure, and decrease cell apoptosis (Trounson 2015). The International Society for Cellular Therapy defines cells as MSCs by the following criteria: adhere to plastic in standard culture conditions; express specific surface antigen markers; and have the capacity for multipotent differentiation (Dominici 2006). Although MSCs are ubiquitously used in regenerative studies, additional stem cell‐based therapies (built on what are collectively referred to as 'regenerative cells') are also under consideration. For instance, mononuclear cells, oligodendrocyte progenitor cells, neural stem cells, hematopoietic stem cells, endothelial cells, and inducible pluripotent stem cells have demonstrated efficacy in animal models of brain injury (Pimentel‐Coelho 2012). Furthermore, one ongoing clinical trial is evaluating the efficacy of autologous umbilical cord blood (which contains a combination of MSCs, haematopoietic stem cells, and other mononuclear cells) as a treatment for HIE (NCT02612155). While inducible pluripotent stem cells are typically obtained from skin cells, they can also be retrieved from blood or MSCs to become a specialized cell, such as a neural stem cell or oligodendrocyte (Cai 2010).
Several factors must be taken into account when optimising regenerative cells. For example, the tissue source, laboratory processing, passage number, dose, frequency, timing, and route of administration are all variables that may affect efficacy (Möbius 2015). The perinatal period offers an opportune time to collect umbilical cord tissue/blood, amniotic fluid or placental tissue (Garcia 2014; Parolini 2014; Sanberg 2014; Taghizadeh 2014). These sources, once considered medical waste, offer a vast supply of regenerative cells that are obtained non‐invasively and recognized for their high proliferative rates/differentiation potential, paracrine release of biological factors and low likelihood of mounting an immune response after transplantation (Batsali 2013; Möbius 2015; Moreira 2019; Parolini 2008). The therapeutic potential of regenerative cells may also be influenced by different laboratory cell‐processing techniques (e.g. oxygen tension, cell expansion media, passage number, fresh versus cryopreserved cells) (Frey 2006; Kaplan 2017; Parody 2013). Fewer passages is preferred, as multiple passages may impair cell function (Bellayr 2014; Wagner 2008). Interestingly, larger amounts of immature haematopoietic progenitors and MSCs with high proliferative potential have been detected in the cord blood of preterm as compared with term infants (Podesta 2015). In one systematic review of animal models of HIE, neuroprotective dosages of MSCs ranged between 200,000 to 3,500,000 cells, with most of the studies administering a one‐time intracerebral/intranasal dose of cells 72 hours or less after injury (Archambault 2017).
Though regenerative cells are characterized by low immunogenicity (Gebler 2012), autologous transplantation is likely to be associated with lower risks for infections and immune rejection. Allogeneic transplantation may offer significant practical advantages, however, such as rapid availability of disease‐free products that are generated in a cost‐effective manner (Hare 2017).
How the intervention might work
It is currently unknown which regenerative cell(s) promotes the therapeutic effects in HIE; however, we will present a brief overview of the potential role(s) each cell has after neurological injury (see also Table 1).
1. Types of regenerative cells.
| Cell type | Source | Rationale | Mechanism of action | Preclinical/clinical results | References |
| MSC | Human umbilical cord tissue/blood; rodent/human bone marrow | Safe and feasible in phase 1 RCT for bronchopulmonary dysplasia
|
|
|
Ahn 2016; Boshuizen 2018; Chopp 2002; Hsu 2016; Islam 2012; Liu 2010; Murphy 2013; Park 2016 |
| MNC | Human umbilical cord blood |
|
|
|
Aridas 2016; Cotton 2014; Fan 2005; McDonald 2018; Pimentel‐Coelho 2012; Rowe 2010; Wang 2013 |
| OPC | Rodent/human embryonic stem cell; human NSC derivation |
|
|
|
Chen 2015; Gopagondanahalli 2016; Kim 2018; Manley 2017; Niimi 2018; Xu 2015 |
| NSC | Human fetal striatum; human ESC; human iPSC |
|
|
|
Daadi 2016; Huang 2018; Ji 2015; Mine 2013 |
| HSC | Umbilical cord blood |
|
|
|
Schwarting 2008; Tsuji 2014; Verina 2013 |
| EPC | Human umbilical vein; human umbilical cord blood; human adipose stem cell; human iPSC |
|
|
|
Grandvuillemin 2017; Kidani 2016; McDonald 2018; Nabetani 2018; Wang 2016; Wu 2013 |
| iPSC | Skin fibroblasts, umbilical cord tissue, amniotic tissue |
|
|
|
Cai 2010; Hsu 2016; Oki 2012; Pluchino 2013; Qin 2015; Tornero 2013 |
BDNF: brain‐derived neurotrophic factor; CNS: central nervous system; ESC: embryonic stem cell; EGF: epidermal growth factor; GDNF: glial cell‐line derived neurotrophic factor; HGF: hepatocyte growth factor; HLA: human leukocyte antigen; HSC: haematopoietic stem cells; IGF‐1: insulin‐like growth factor 1; iPSC: inducible pluripotent stem cells; IL: interleukin; MHC: major histocompatibility complex; MSC: mesenchymal stem cell; MNC: mononuclear cells; NF‐κB: nuclear factor kappa beta; NGF: nerve growth factor; NSC: neural stem cells; OPC: oligodendrocyte progenitor cells; RCT: randomised controlled trial; TNF: tumour necrosis factor; VEGF: vascular endothelial growth factor.
Mesenchymal stem cells
The beneficial effects of MSCs occur at the cellular and functional level. The proposed mechanism of action is through the paracrine release of factors known to improve neurogenesis, such as basic fibroblast growth factor, insulin‐like growth factor‐1, and anti‐inflammatory cytokines (Li 2002; Murphy 2013; Qu 2007; van Velthoven 2009; van Velthoven 2011; van Velthoven 2012). MSCs can also modulate the local immune response by regulating the function of immune cells, such as T‐cells and B‐cells, macrophages, and dendritic cells (Iyer 2008). Furthermore, in injured brain tissue MSCs upregulate the expression of genes associated with cell proliferation (e.g. Spp1 and interleukin (IL) 17 (IL‐17)), neurogenesis (neural cell adhesion molecule and nerve growth factor), migration (CXCR4), neuronal survival (glial‐derived neurotrophic factor), and down regulation of genes involved in inflammation (i.e. IL‐1β) (van Velthoven 2011). In addition, MSCs may inhibit apoptosis by transporting mitochondria through tunnelling nanotubules in efforts to rescue aerobic respiration (Liu 2014).
At the functional level, MSC treatment, given within the first 10 days after injury, prevents neuroinflammation and apoptosis (Bonestroo 2015; Donega 2013). The administration of umbilical‐cord‐derived MSCs resulted in nerve fibre remyelination and axonal regeneration, diminished loss of white and grey matter, improved sensorimotor function, and promoted better long‐term neurological recovery (Donega 2013; Donega 2015; Liu 2010; Morán 2017).
Mononuclear cells
In a newborn lamb model of HIE, treatment with mononuclear cells obtained from umbilical cord blood promoted a decrease in neuronal apoptosis and inflammation, along with a trend towards a reduction in seizures (Aridas 2016). Mononuclear cells also reduce motor deficits and cortical brain loss by decreasing CD4+ T‐cell and microglial infiltration to the injury site (McDonald 2018).
Oligodendrocyte progenitor cells
Administration of oligodendrocyte progenitor cells to a small animal model of cervical spinal injury increased myelination and enhanced motor performance (Manley 2017). Differentiation into myelin‐producing cells and expression of brain‐derived neurotrophic factor and bcl‐2 appear to be mechanisms by which oligodendrocyte progenitor cells encourage learning and memory in neonatal asphyxia (Chen 2015).
Neural stem cells
Mine and colleagues showed that intrastriatal transplantation of neural stem cells slows inflammation (microglia/macrophage activation) and promotes axonal connections (Mine 2013). Moreover, it is posited that neural stem cell therapy inhibits IL‐1β expression and improves neural plasticity by upregulating nuclear factor kappa β signalling (Ji 2015).
Haematopoietic stem cells
Human umbilical cord 34+ cells given 48 hours after middle cerebral artery occlusion in mice transiently increased cerebral blood flow and blood vessel diameter in the peri‐infarct area (Tsuji 2014). These findings can be attributed to the release of growth factors (i.e. vascular endothelial growth factor and glial‐derived neurotrophic factor) known to stimulate neurogenesis and angiogenesis (Verina 2013).
Endothelial cells
The neuroprotective effects of endothelial cells are ascribed to their ability in decreasing neuroinflammation and cell apoptosis (Grandvuillemin 2017). Intraperitoneal injection of human umbilical vein endothelial cells preserved microvessels and lessened apoptosis in the cortex of treated animals, via regulation of stromal cell‐derived factor 1 and CXC chemokine receptor 4 (Wu 2013).
Inducible pluripotent stem cells
Using human‐skin‐derived inducible pluripotent stem cells, Tornero and colleagues produced cortical progenitor cells that survived and differentiated into functional neurons that improved performance of impaired limb movement in a rat stroke model (Tornero 2013). Furthermore, inducible pluripotent stem cell treatment reduced the number of inflammatory cells and glial scar formation in a preclinical study of haemorrhagic stroke (Qin 2015).
Taken together, regenerative cells may exert their therapeutic benefit through multiple routes to establish a favourable environment for tissue regeneration, which ultimately leads to better functional outcomes following hypoxic‐ischaemic damage.
Why it is important to do this review
It is important to conduct this review as regenerative cells might be an effective intervention for the prevention and treatment of HIE, which is one of the most severe morbidities in term infants. To date, US clinical trials studying the safety, feasibility, efficacy (or a combination of these) of regenerative cells have been registered for bronchopulmonary dysplasia, HIE, hypoplastic left heart syndrome, and intraventricular haemorrhage. The most widespread tissue used to derive regenerative cells is the umbilical cord (Mitsialis 2016; Yoon 2016). While MSCs dominate as cell type, a few trials are evaluating neural progenitor cells, mononuclear cells, and placenta/cord blood cells (NCT02434965; NCT02854579; NCT02999373).
The efficacy and safety of use of regenerative cell administration has been assessed in several systematic reviews and meta‐analyses. For instance, the safety of MSCs has been evaluated in a meta‐analysis across different disciplines (eight studies including 321 adults): there was no association between acute infusional toxicity, organ system complications, infection, death or malignancy (Lalu 2012). However, the risk of potential tumourigenicity related to MSC‐based interventions needs to be further elucidated (Barkholt 2013). The Cochrane Review 'Stem cell transplantation for ischaemic stroke' included three small trials in adults (Boncoraglio 2010). In newborns, one Cochrane Review has been conducted on MSC for the prevention and treatment of bronchopulmonary dysplasia in preterm infants (Pierro 2017). Early phase trials have been conducted (or are underway) on the use of MSCs or cord blood cells (or both) for bronchopulmonary dysplasia, severe IVH (NCT02274428), and HIE (Chang 2014; Cotten 2014).
Objectives
To determine the efficacy and safety of stem cell‐based interventions for the treatment of hypoxic‐ischaemic encephalopathy (HIE) in newborn infants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs, and cluster trials.
Types of participants
Term infants (37 weeks or greater) and late preterm infants (34+0 to 36+6 weeks' gestation) 10 days of age or less.
-
Evidence of peripartum asphyxia, characterized by evidence of neonatal or foetal distress with each enrolled infant satisfying at least one of the following criteria.
Cord gas or postnatal blood gas (within the first hour of life) with pH 7.0 or less or base deficit 12 mEq/L or greater.
Apgar score 5 or less at five minutes.
Need for mechanical ventilation or resuscitation at 10 minutes of life.
-
With or without evidence of encephalopathy (moderate or severe) according to Sarnat staging (Sarnat 1976):
Stage 1 (mild): hyperalertness, hyper‐reflexia, dilated pupils, tachycardia, absence of seizures;
Stage 2 (moderate): lethargy, hyper‐reflexia, miosis, bradycardia, seizures, hypotonia with weak suck and Moro reflex;
Stage 3 (severe): stupor, flaccidity, small‐to‐mid position pupils that react poorly to light, decreased stretch reflexes, hypothermia, and absent Moro reflex.
No major congenital abnormalities recognizable at birth.
Types of interventions
Comparison 1
Stem cell‐based interventions (any type) compared to control (placebo or no treatment).
Comparison 2
Use of MSCs of type (e.g. number of doses or passages) or source (e.g. autologous versus allogeneic, or bone marrow versus cord) versus MSCs of other type or source.
Comparison 3
Use of stem cell‐based interventions other than MSCs of type (e.g. mononuclear cells, oligodendrocyte progenitor cells, neural stem cells, haematopoietic stem cells, and inducible pluripotent stem cells) or source (e.g. autologous versus allogeneic, or bone marrow versus cord) versus stem cell‐based interventions other than MSCs of other type or source.
Comparison 4
MSCs versus stem cell‐based interventions other than MSCs.
We included all types of transplantation regardless of cell source (bone marrow, cord blood versus Wharton's jelly, placenta, adipose tissue, peripheral blood), type of graft (autologous or allogeneic), and dose. We excluded stem cell‐derived cerebral organoids (Di Lullo 2017).
Characteristic of the interventions and co‐interventions (e.g. cooling) are specified in Subgroup analysis and investigation of heterogeneity.
Types of outcome measures
Primary outcomes
All‐cause neonatal mortality (mortality less than 28 days of age).
Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Mental Developmental Index or Griffiths Mental Development Scale assessment greater than two standard deviations (SD) below the mean) (Bayley 1993; Bayley 2006; Griffiths 1954); intellectual impairment (intelligence quotient (IQ) greater than two SDs below the mean); blindness (vision less than 6/60 in both eyes); or sensorineural deafness requiring amplification (Jacobs 2013). We planned to separately assess data on children aged 18 to 24 months and aged three to five years.
Death or major neurodevelopmental disability assessed at 18 to 24 months of age (defined as cerebral palsy, developmental delay (Bayley or Griffith assessment more than two SD below the mean) or intellectual impairment (IQ more than two SD below mean); blindness (vision less than 6/60 in both eyes); or sensorineural deafness requiring amplification) (Bayley 1993; Bayley 2006; Griffiths 1954; Jacobs 2013).
Secondary outcomes
All‐cause mortality prior to first hospital discharge
-
Each component of major neurodevelopmental disability (we planned to report these components of long‐term outcome for all studies that have evaluated children after 18 months' chronological age; we planned to perform separate analyses for children aged 18 to 24 months and three to five years).
Cerebral palsy
-
Developmental delay
Bayley or Griffith assessment more than two SD below the mean
Neuromotor development (Bayley Scales of Infant Development – Psychomotor Development Index (BSID PDI)) assessed in survivors
Cognitive development (Bayley Scales of Infant Development – Mental Development Index (BSID MDI)) assessed in survivors
Intellectual impairment (IQ more than two SD below mean)
Blindness (vision less than 6/60 in both eyes)
Sensorineural deafness requiring amplification
-
Seizures (suspected clinically or identified by electroencephalogram (EEG) or amplitude‐integrated electroencephalogram (aEEG))
Seizures during neonatal period (after MSC administration)
Seizures or need for anticonvulsants at follow‐up 12 to 14 months of age
Cystic periventricular leukomalacia on brain ultrasound in the first month of life
Brain magnetic resonance imaging (MRI) abnormalities (yes/no), defined as white matter lesions (i.e. cavitations; Rutherford 2010) and punctate lesions (Cornette 2002); germinal matrix‐intraventricular haemorrhage (GM‐IVH) (Parodi 2015); or cerebellar haemorrhage (Limperopoulos 2007)
Duration of hospital stay (days)
Tumour formation, any type, any location, detected by MRI or computed tomography (to assess the risk of tumourigenicity of donor MSCs)
Immune‐rejection or any serious adverse event (certain, probable or possible according to the World Health Organization (WHO) probability scale). We planned to consider post hoc analyses for any unexpected adverse effects reported by the studies.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) and report the date this was done within the review.
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL, 2020 Issue 5) in the Cochrane Library; MEDLINE via PubMed (1996 to 8 June 2020); Embase (1980 to 8 June 2020); and CINAHL (1982 to 8 June 2020). Please see Appendix 1 for the full search strategies for each database. We applied no language restrictions. We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov; the WHO's International Trials Registry and Platform; and the ISRCTN Registry; see Appendix 1).
Searching other resources
We reviewed the reference lists of all identified articles for relevant articles not identified in the primary search.
Data collection and analysis
We used the standard methods of Cochrane Neonatal, as described below.
Selection of studies
Two review authors (MB, OR) independently searched for and identified eligible trials that met the inclusion criteria. We screened the titles and abstracts to identify potentially relevant citations, and retrieved the full texts of all potentially relevant articles; and we independently assessed the eligibility of studies by filling out eligibility forms designed in accordance with the specified inclusion criteria. We excluded studies published only in abstract form unless the final results of the trial were reported and all necessary information can be ascertained from the abstract or authors, or both. We reviewed studies for relevance by assessing study design, types of participants, interventions provided, and outcome measures reported. We resolved disagreements by discussion and, if necessary, by consultation with a third review author (DL). We provide details of excluded studies in the Characteristics of excluded studies table, along with reasons for exclusion. We contacted trial authors if details of primary trials were unclear.
Data extraction and management
Two review authors (MB, OR) independently extracted data using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC Group 2017).
We planned to extract the following characteristics from each included study.
Administrative details: study author(s); published or unpublished; year of publication; year in which study was conducted; presence of vested interest; details of other relevant papers cited.
Details of the study: study design; type, duration, and completeness of follow‐up (e.g. greater than 80%); country and location of study; informed consent; ethics approval.
Details of participants: sex, birth weight, gestational age, number of participants.
Details of interventions: initiation, dose, and duration of MSCs' administration; co‐intervention such as cooling.
Details of outcomes as mentioned above under Types of outcome measures.
We resolved disagreements by discussion. We described ongoing studies identified by our search, when available, detailing the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date.
Should any queries have arisen or we required additional data, we planned to contact study investigators/authors for clarification. Two review authors (MB, OR) used RevMan Web for data entry. We planned to replace any standard error of the mean (SEM) by the corresponding SD.
Assessment of risk of bias in included studies
We planned to independently assess (OR, MB) the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2019).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We planned to resolve any disagreements by discussion or by consultation with a third review author (AM). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We planned to use risk ratios (RRs), risk differences (RDs), numbers needed to treat for an additional beneficial outcome (NNTB) or numbers needed to treat for an additional harmful outcome (NNTH) for categorical variables, and mean differences (MDs) for continuous variables. We planned to replace any within‐group SEM reported in a trial by its corresponding SD using the formula SD = SEM × √n, where n is the number of participants. We planned to report 95% confidence intervals (CIs) for each statistic.
Unit of analysis issues
We planned to include all RCTs and quasi‐RCTs in which the unit of allocation was the individual infant. Should we have found any cluster‐RCTs, we would have adjusted analysis for the designed effect using the method stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Dealing with missing data
We planned to obtain a dropout rate for each study. If we found a significant dropout rate (e.g. greater than 20%), we would have contacted study author(s) to request additional data. We planned to perform a sensitivity analysis to evaluate the overall results with and without inclusion of studies with a significant dropout rate. If a study reported outcomes only for participants completing the trial or only for participants who followed the protocol, we would contact study author(s) to ask them to provide additional information to facilitate an intention‐to‐treat analysis; in instances when this was not possible, we planned to perform a complete‐case analysis.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by comparing the distribution of important participant factors between trials and trial factors (randomisation concealment, blinding of outcome assessment, loss to follow‐up, treatment type, co‐interventions). We planned to assess statistical heterogeneity by examining the I² statistic (Higgins 2019), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than to sampling error.
We planned to interpret the I² statistic as follows, as described by Higgins 2003.
Less than 25%: no heterogeneity.
25% to 49%: low heterogeneity.
50% to 74%: moderate heterogeneity.
75% or greater: high heterogeneity.
We planned to consider statistical heterogeneity to be substantial when the I² statistic was 50% or greater. In addition, we planned to employ the Chi² test of homogeneity to determine the strength of evidence that heterogeneity is genuine. We planned to explore clinical variation across studies by comparing the distribution of important participant factors among trials and trial factors (randomisation concealment, blinding of outcome assessment, loss to follow‐up, treatment types, and co‐interventions). We planned to consider a threshold of P value less than 0.1 as an indicator of whether heterogeneity (genuine variation in effect sizes) was present.
Assessment of reporting biases
We planned to examine the possibility of within‐study selective outcome reporting for each study included in the review. We planned to search for trial protocols of included trials on electronic sources such as PubMed, ClinicalTrials.gov, and the WHO ICTRP to assess whether outcome reporting seemed to be sufficiently complete and transparent. We planned to investigate publication bias by using funnel plots if we included 10 or more clinical trials in the systematic review (Egger 1997; Higgins 2019).
Data synthesis
We planned to perform statistical analyses according to the recommendations of Cochrane Neonatal (neonatal.cochrane.org/en/index.html) using RevMan Web. We planned to analyse all infants randomised on an intention‐to‐treat basis. We planned to analyse treatment effects in the individual trials. We planned to use a fixed‐effect model to combine the data. For any meta‐analyses, we would have synthesized data using RR, RD, NNTB, NNTH, MD, and 95% CI. We planned to analyse and interpret individual trials separately when we judged meta‐analysis to be inappropriate.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
For MSCs trials:
gestational age: term infants (≥ 37 weeks' gestation), late preterm infants (34 to 36+6 weeks' gestation);
HIE severity stage: mild, moderate, and severe (Sarnat);
chronological age: less than three days, three days or greater;
co‐intervention: with/without cooling;
MSCs source: bone marrow, cord blood versus Wharton's jelly, placenta, adipose tissue, peripheral blood;
type of graft: autologous or allogeneic;
preconditioned (yes, no);
fresh or frozen and thawed;
MSCs dose: less than 2 × 10⁷/kg; 2 × 10⁷/kg or greater;
number of doses: multiple or single administration;
passage number (i.e. removing cells from a culture flask and plating them into more culture flasks; see Description of the intervention): less than three; three to six; greater than six.
For other cell‐based interventions:
gestational age: term infants (≥ 37 weeks' gestation), late preterm infants (34 to 36+6 weeks' gestation);
HIE severity stage: mild, moderate and severe (Sarnat);
chronological age: less than three days, three days or greater;
co‐intervention: with/without cooling;
cell source: bone marrow, cord blood, peripheral blood, placenta;
type of graft: autologous or allogeneic;
fresh or frozen and thawed;
number of doses: multiple or single administration.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the effect of the methodological quality of trials, checking to ascertain whether studies with a high risk of bias would overestimate the effect of treatment. Differences in study design of included trials might affect the results of the systematic review. We planned to perform a sensitivity analysis to compare the effects of MSCs in truly randomised trials as opposed to quasi‐randomised trials.
Summary of findings and assessment of the certainty of the evidence
We planned to use the GRADE approach to assess the quality of evidence for the following (clinically relevant) outcomes (Schünemann 2013): 1) All‐cause neonatal mortality (mortality less than 28 days of age); 2) Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Mental Developmental Index or Griffiths Mental Development Scale assessment greater than two standard deviations (SD) below the mean), intellectual impairment (intelligence quotient (IQ) greater than two SDs below the mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification; 3) Death or major neurodevelopmental disability assessed at 18 to 24 months of age (defined as cerebral palsy, developmental delay (Bayley or Griffith assessment more than two SD below the mean) or intellectual impairment (IQ more than two SD below mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification; 4) All‐cause mortality prior to first hospital discharge; 5) cognitive development (Bayley Scales of Infant Development – Mental Development Index (BSID MDI)) assessed in survivors; 6) seizures or need for anticonvulsants at follow‐up 12 to 14 months of age; 7) Immune‐rejection or any serious adverse event (certain, probable or possible according to the WHO probability scale).
We planned to independently assess the quality of the evidence for each of the seven outcomes above. We planned to consider evidence from RCTs as high quality but downgrade the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias); consistency across studies; directness of the evidence; precision of estimates; and presence of publication bias. We planned to use the GRADEpro GDT Guideline Development Tool to create a 'Summary of findings' table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
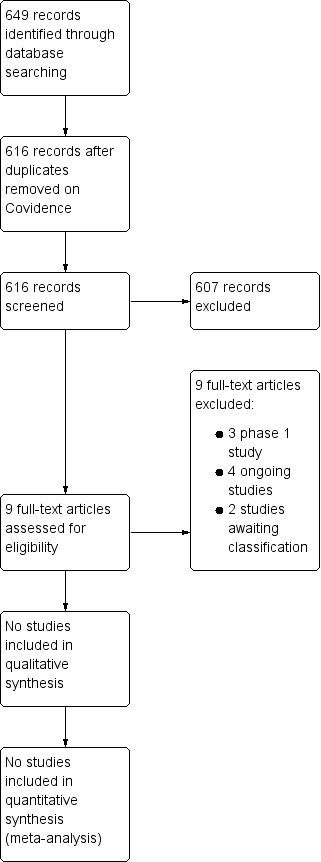
We have provided results of the search for this review in the study flow diagram (Figure 1).
1.
See Characteristics of excluded studies, Characteristics of ongoing studies and Characteristics of awaiting studies.
Results of the search
The literature search run in June 2020 identified 616 references. Upon screening, we considered no trials as potentially eligible.
Three phase 1 studies are currently registered on our targeted patient group and reported in the Excluded studies and Characteristics of excluded studies sections (Cotten 2014; Cotten 2020; Tsuji 2020).
Our search of ClinicalTrials.gov, ICTRP/WHO and ISRCTN registry identified 29, 16 and 12 registered studies, respectively. Fifteen ongoing trials were eligible (ACTRN12610000421033; ChiCTR‐TNRC‐11001591; ChiCTR‐TRC‐10000922; NCT00593242; NCT01506258; NCT01649648; NCT02256618; NCT02434965; NCT02455830; NCT02612155; NCT02854579; NCT02881970; NCT03352310; NCT03635450; NCT01284673). See Characteristics of ongoing studies.
We classified two studies as awaiting classification: in Cotten 2011 the abstract and the full text were not available; in Nabetani 2016 outcomes were not reported in the abstract and the full text was not available (see Characteristics of studies awaiting classification).
Included studies
We identified no trials that matched our inclusion criteria.
Excluded studies
We excluded three studies (Cotten 2014; Cotten 2020; Tsuji 2020). See Characteristics of excluded studies).
Cotten 2014 was a phase 1 open‐label study in 23 infants (greater than or equal to 35 weeks of gestation) fulfilling the criteria for HIE and needing hypothermia treatment. Hypothermia criteria were met if infants had cord or first postnatal hour blood gas results with pH of 7.0 or less, or base deficit equal to or more than −16. Cord blood was collected aseptically via in utero or ex utero techniques by trained staff. Infants were pretreated with 1 mg/kg hydrocortisone, intravenously, and then received up to four infusions of 1 to 5 × 10⁷ cells/kg, with the first dose as soon as possible after birth, and at 24, 48, and 72 postnatal hours. Cells were infused over 15 to 20 minutes, followed by a 1 mL to 2 mL saline flush to clear the intravascular line. Twenty‐three infants were treated with both hypothermia and autologous injection of UCB cells. Eleven infants received four infusions; three infants three infusions; six infants two infusions; and three infants one infusion. Three infants required extracorporeal membrane oxygenation and four infants required seizure medications at discharge.
Cotten 2020 was a phase 1 open‐label study of umbilical cord tissue‐derived MSCs in infants treated with hypothermia for HIE. Infants of more than 35 weeks' gestation treated with hypothermia for HIE based on NICHD hypothermia trial criteria were eligible. Allogeneic umbilical cord tissue‐derived mesenchymal stromal cells (hCT‐MSC) were manufactured from a single cord tissue. Cells were expanded to passage 2 and cryopreserved. The first three infants (cohort 1) received 2 × 106 cells/kg intravenously in the first 48 postnatal hours; the second three (cohort 2) received a second dose at two months. Primary safety outcomes were occurrence of infusion reactions or infections within two weeks of infusion. Six infants (birthweight 2.74 kg to 4.4 kg) were enrolled. Apgar scores ranged from 2 to 6 and 3 to 8 at 5 and 10 minutes, respectively. One of the infants had a positive panel reactive antibody (PRA) screen at six months. Broth culture from thawed hCT‐MSC from an infant grew coagulase‐negative staphylococci; the infant remained asymptomatic after re‐warming and the infant's blood cultures were sterile. Length of stay was between 9 and 10 days for all infants; none was re‐hospitalized.
Tsuji 2020 was a phase 1 open‐label study of intravenous infusion of autologous umbilical cord blood cells to newborns with HIE. When a neonate was born with severe asphyxia following caesarean section, the UCB was collected, volume‐reduced, and divided into three doses. The concentration of CD34+ cells/microliter ranged between 17 and 539 per dose, which were infused at 12 to 24, 36 to 48, and 60 to 72 hours after the birth to each infant. At 30 days of age, the six infants survived without circulatory or respiratory support; at 18 months of age, neurofunctional development according to the Kyoto Scale of Psychological Development was normal in four infants and delayed with cerebral palsy in two infants.
Ongoing studies
We found 15 relevant studies on the clinical trials registries for ongoing or recently completed trials (ChiCTR‐TNRC‐11001591; ChiCTR‐TRC‐10000922; NCT02256618; NCT02612155; NCT02854579; NCT02434965; NCT00593242; NCT01506258; NCT03635450; NCT02455830; NCT02881970; NCT01649648; NCT01284673; NCT03352310; ACTRN12610000421033; see Characteristics of ongoing studies). Nine studies consist of single group assignment studies, whereas we will pool the remaining six studies in comparison 1 of this review, i.e. stem cell‐based interventions versus placebo or no treatment (ChiCTR‐TNRC‐11001591; ChiCTR‐TRC‐10000922; NCT02612155; NCT01506258; NCT03352310; NCT02854579). The latter will have four study arms, with the following interventions given intrathecally: neural progenitor cells; paracrine factors of human MSCs; concentrated paracrine factor plus neural progenitor cell; routine therapy.
Description of the 15 ongoing studies
ChiCTR‐TNRC‐11001591 is a phase 1 non‐randomised control interventional study, aiming to compare routine care plus stem cells versus routine care plus rehabilitation. Both groups will include 10 full‐term infants with an Apgar score at 5 minutes of less than 6 and at 10 minutes of less than 5, neonatal behavioural neurological assessment (NBNA) score less than 28. Outcome measures (all indicated as primary indicators) will be: infant development test of Child Development Centre of China (CDCC), Gross Motor Function Measure (GMFM), movement Assessment Battery for Children (ABC), visual evoke potentials, brainstem auditory evoked potentials, somatosensory evoked potentials, NBNA, fine motor function measure (FMFM), EEG.
ChiCTR‐TRC‐10000922 was registered retrospectively. It is a parallel interventional study with two arms: conventional treatment plus autologous transplantation of cord blood versus conventional treatment. Each arm includes 20 neonates within 24 hours of birth, with cord pH during birth less than 7.0, Apgar's score less than 7 (not specified at which minute) and showing HIE presentation (criteria not specified). Primary indicator outcome measurements are: EEG, intellectual and behavioural development (scales are not specified), neuron specific enolase (NSE), diffusion‐weighted imaging (DWI). Secondary indicators for outcomes are: clinical presentations and blood routine, urine routine, liver function and kidney function.
NCT02256618 is an open‐label interventional study with one group assignment. Six newborns of 36 weeks' or more gestational age (GA) with either Apgar score of 5 or less at 10 minutes or severe acidosis at umbilical or any blood during the first hour after birth, defined as pH less than 7.0 or base deficit of 16 mmol/L or more, moderate or severe HIE (Sarnat II to III), a moderately or severely abnormal background amplitude‐integrated EEG (aEEG) voltage, or seizures identified by aEEG, if monitored will be included. All infants should be less than 24 hours of age. Primary outcome of this study is the rate of adverse events (combined rate of death, continuous respiratory support, and continuous use of vasopressor), which will be compared with historical controls at 30 days of age. Secondary outcomes are neuroimaging at 12 months of age and neurodevelopmental function at 18 months of age (comparison with historical controls).
NCT02612155 is a phase 2 interventional randomised trial, aiming to include 160 newborns with GA greater than or equal to 36 weeks and having less than 6 hours of life with following criteria fulfilled: a cord blood or any blood within the first hour of life with a pH less than or equal to 7.0 or a base deficit (BE) greater than or equal to 16 mmol/L. If, during this time period, a pH was between 7.01 to 7.15 and BE between 10 to 15.9 mmol/L, or non‐available blood gas, additional criteria were: an acute perinatal event (e.g. late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, haemorrhage, or cardiorespiratory arrest) and either Apgar score less than or equal to 5 at 10 minutes or assisted ventilation initiated at birth and continued for at least 10 minutes. Newborns will be randomised in two groups: hypothermia plus two infusions of autologous nucleated cord blood cells versus hypothermia plus placebo (a mix of autologous cord blood red blood cells and plasma) infusions. Primary outcome measures are: survival at one year; percentage of subjects with Bayley III scores in all three domains greater than or equal to 85. Secondary outcome measurements at one year of life are currently: mortality rate, percentage of subjects with seizures, percentage of subjects who require iNO use, percentage of subjects who require ECMO, percentage of subjects who require G‐tube feeding, percentage of subjects who discharged with anticonvulsant treatment.
NCT02854579 is a randomised open‐labelled study where newborns with gestational age of 34 weeks or more and body weight of 2 kg or more will be enrolled. The inclusion criteria are 1‐ and 5‐minute Apgar score of 3 or less and 5 or less, respectively, or need for ventilation 5 minutes after birth, or umbilical blood pH less than 7.0 or BE of −12 mmol/L or less at 30 minutes. Infants should have signs of encephalopathy (convulsion, coma, dystonia, abnormal primitive reflex and irregular respiration) within six hours of age or continued abnormal EEG for more than 24 hours. Randomisation in four arms is planned: 1) routine therapy plus neural progenitor cells intrathecally at 48 to 72 hours, 5 days and 10 days after birth; 2) routine therapy plus paracrine factors of human MSCs intrathecally at 12 hours, 24 hours, 48 hours after birth; 3) routine therapy plus concentrated paracrine factor intrathecally at 12 hours, 24 hours, 48 hours after birth plus neural progenitor cell intrathecally at 48 to 72 hours, 5 days and 10 days after birth; 4) routine therapy. Primary outcomes are defined as: Neonatal Behavioral Neurological Assessment at 14 and 28 days of life, number of adverse events (fever, seizures, haemorrhage caused by interventions) at 7 days following cells/factors injection. Secondary outcomes are: Bayley score at 12 and at 18 months after birth, Peabody development measure scale at 12 and at 18 months after birth, death within a year, treatment‐related central nervous tumour as assessed by MRI or CT within 5 years.
NCT02434965 is a phase 2 open‐label interventional study with following inclusion criteria: GA of 36 weeks or more and BW of 1800 grams or more, postnatal age less than 6 hours after birth, autologous cord blood and human placental‐derived stem cells (HPDSCs) available for infusion and one or more of the following criteria: Apgar score at 10 minutes of 5 or less, or need for resuscitation 10 minutes or more after birth, or cord blood pH or arterial blood pH within 60 minutes of birth of 7.0 or less, or BE of −16 or more mEq in cord blood and within 60 minutes of birth, and moderate to severe altered state of consciousness (hypotonia, abnormal reflexes, absent/weak suck). Twenty newborns will be assigned to one arm and treated with autologous HPDSC in combination with autologous cord blood.
NCT00593242 is a phase 1 open‐label non‐randomised interventional study in which newborns are included with GA more than 34 weeks, a history of acute perinatal event or blood pH less than 7.0/BE more than 16 mEq/L, Apgar score at 10 minutes less than 5 or continued need for ventilation. All newborns should present signs of encephalopathy within six hours of age. All infants are recruited in autologous cord blood infusion and they are compared with historical cohort receiving standard treatment. Primary outcome measure is the rate of adverse events within the first 18 postnatal days. Secondary outcomes are neurodevelopmental outcome at 4 to 6 months and 9 to 12 months of age (scales not specified) plus neuroimaging results (not specified).
NCT01506258 is an open‐label non‐randomised study with parallel assignment of full‐term (GA 37 to 42 weeks) with neurological manifestations compatible with HIE, 5‐minute Apgar score less than 5, pH less than 7.0 at cord blood gas and any degree of organic/systemic affectation (cardiovascular, gastrointestinal, hematologic and/or respiratory) either in intervention group (intravascular infusion of autologous stem cells within the first 48 hours after birth) or in comparison group (control group of patients that meet the inclusion criteria but that do not wish to have the intervention). Primary outcome is the effects of stem cell infusion at one week and one year after discharge (clinical assessment, including the Amiel‐Tison Neurological Assessment). No secondary outcomes are listed.
NCT03635450 is a phase 1 open‐label, non‐randomised, parallel assignment study. A total of six newborns with GA of 36+0 or more with signs of encephalopathy within six hours of age will be included. The first cohort newborns (N = 3) will receive a single infusion of human umbilical cord tissue (hCT)‐derived MSC within 48 hours after birth. If no safety concerns appear, the second cohort infants will receive two infusions of umbilical‐cord‐derived MSCs within the first 48 hours and 2 months later, respectively. Primary outcomes of the study are the allergic reactions within 24 hours following hCT‐MSC infusion and the rate of infections within two weeks' post‐infusion. Secondary outcomes are the survival within six months and neurodevelopmental assessment at one year of age (Bayley Scales of Infant and Toddler Development, Third Edition (Bayley III)), assessments in cognitive, language and motor development.
NCT02455830 is a prospective, case‐control, observational study which will include 18 neonates with GA greater than or equal to 36 weeks up to 24 hours of age who will present with moderate to severe encephalopathy (Sarnat II to III) and either an Apgar score of 5 or less at 10 minutes, continued need for resuscitation for at least 10 minutes, or severe acidosis in cord blood or any blood gas during the first postnatal hour (pH less than 7.0 or BE greater than or equal to ‐16 mmol/L). The primary outcome of the study is changes in serum levels of cytokines and trophic factors within the first 10 days of life. Secondary outcomes for the study are neuroimaging at 12 months of age (it is not specified what kind of neuroimaging will be used and what kind of analysis will be done) and neurobehavioural assessment at 18 months of age (scales not specified).
NCT02881970 is a phase 1/2 open‐label, one group assignment study, where neonates who meet the following criteria will be included: GA greater than or equal to 36 weeks with a blood pH less than 7 and BE more than −12 mmol/l at birth or within the first postnatal hour or a blood pH between 7.01 and 7.15 and a history of acute perinatal event and Apgar score at 5 minutes of 5 or less, or a continued need for resuscitation at 5 minutes after birth. Included neonates should have signs of HIE within 12 hours of age (Sarnat and Sarnat classification score greater than or equal to 2) with or without abnormal EEG or aEEG within 12 hours of age and require therapeutic hypothermia. All included infants will receive treatment with autologous cord blood stem cells. The primary outcome of the study is the rate of adverse events due to intervention with a time frame of two years. Secondary outcomes are the neuroimaging (currently not specified what kind and which analysis will be performed) at 12 months of age and neurobehavioral assessment at 18 months of age (scales are not specified).
NCT01649648 is a phase 1, open‐label, single group assignment study. Neonates with GA of more than 36 weeks and brain injury satisfying criteria for therapeutic hypothermia (not specified) will be included in the study. All included infants will receive autologous cord blood infusion. The primary outcome of the study is the rate of adverse events during the first three days of life. The secondary outcomes are neurobehavioural assessment (Peabody tests, Bayley Scales of Infant Development) at one month to two years of age and brain MRI at one to two weeks and four to six months of age.
NCT01284673 is an open‐label, single group study aiming to characterise the cord blood stem cells of neonates with neonatal asphyxia (N = 5) and to compare them with those from healthy newborns (N = 5) within two years of life. Ten full‐term infants (GA > 37 weeks) with normal pregnancy and delivery will be included. No secondary outcomes are reported.
NCT03352310 is a phase 1 non‐randomised study with parallel assignment. Forty infants either with signs of HIE or anaemia will be recruited into the study. The inclusion criteria are: 5‐minute Apgar score of 5 or less; evidence of HIE, defined by UCB pH less than 7.15 or BE of 10 mM or less; subjects with HIE confirmed by clinical features and initial investigations; subjects with evidence of anaemia, defined by haematocrit less than 40% or haemoglobin of 13 g/dL or less within the first 96 hours of life. The comparisons will be standard care plus autologous UCB versus standard care. Primary outcome of the study is mortality rate in HIE subjects and baseline haematocrit changes in anaemia subjects. Secondary outcomes are listed in Characteristics of ongoing studies.
ACTRN12610000421033 is an interventional, single group assignment study. Forty newborns with GA of more than 30 weeks with signs of encephalopathy within the first six hours will be included into the study. Included infants will receive autologous mononuclear cell layer (MNCL) transfusion over 30 minutes. The primary outcome of the study is the rate of adverse events compared to historical controls. Occurrence of blood pressure fluctuation, changes in heart rate, and reaction to blood transfusion will be measured. Secondary outcomes of the study are: MRI score (not specified at which time point) and neurodevelopmental outcome (Bayley II Scales of Infant Development) at four to six and nine to 12 months of age will be compared to historical controls.
Studies in other neonatal populations
The use of stem cell‐based interventions for intraventricular haemorrhage and bronchopulmonary dysplasia is reported in other Cochrane Reviews (Romantsik 2019; Pierro 2017).
Risk of bias in included studies
No study met the eligibility criteria of this review.
Effects of interventions
No study met the eligibility criteria of this review.
Discussion
Summary of main results
Although this review did not identify studies meeting the inclusion criteria, there were 15 early‐phase clinical trials examining the use of stem cell‐based interventions for HIE. Over the next several years these studies will evaluate the safety of MSCs and their impact on neurodevelopment, survival, and brain neuroimaging. The cumulative number of participants will exceed 200 and one of the studies will evaluate brain tumour formation via brain MRI/computed tomography at five years of age (NCT02854579). The number of registered clinical trials suggests that investigators are interested in assessing the therapeutic potential of regenerative therapies for neonatal brain injury.
Most trials will be administering autologous umbilical cord blood cells; one study, however, will deliver several doses of neural progenitor cells (NCT02854579). Intravenous and intrathecal routes will be modes of delivery. Details of the trials were at times sparse, but some studies will include up to four separate cell administrations with a dose as high as 50 million cells/kg birthweight. The clinical trials will be conducted in seven different countries.
We excluded two phase 1 clinical trials on autologous umbilical cord blood cells (Cotten 2014; Tsuji 2020); and one phase 1 clinical trial on allogeneic umbilical cord tissue‐derived mesenchymal stromal cells (Cotten 2020). In Cotten 2014 23 infants, with an average gestational age of 38 weeks and birthweight of 3.1 kg, received a dose of 1 to 5 × 10⁷ cells/kg for each infusion; no adverse events were reported, and vital signs were stable during infusions. In Cotten 2020 six infants received 2 × 10⁶ cells/kg intravascularly in the first 48 postnatal hours, and three of them a second dose at two months; length of stay was between nine and 10 days for all infants and no adverse events were reported. In Tsuji 2020 six infants were infused daily for three days; at 18 months of age, neurofunctional development was normal in four infants and delayed with cerebral palsy in two infants.
At present, no studies have been completed to evaluate the efficacy of stem cell therapies for HIE. Prior to performing phase 3 trials, additional studies evaluating safety and measures of efficacy are required.
Overall completeness and applicability of evidence
We identified no eligible studies for inclusion.
Quality of the evidence
We identified no eligible studies for inclusion.
Potential biases in the review process
We used standard methods of Cochrane Neonatal in conducting this systematic review. It is unlikely that the literature search applied to this review may have missed relevant trials, thus we are confident that this systematic review summarises all the presently available randomised trial evidence on stem cell‐based interventions for HIE. We applied no language restriction.
Agreements and disagreements with other studies or reviews
The use of stem cell‐based interventions for intraventricular haemorrhage and bronchopulmonary dysplasia is reported in other Cochrane Reviews (Romantsik 2019; Pierro 2017).
To our knowledge, this is the first systematic review of clinical studies on stem cell‐based interventions for HIE.
Authors' conclusions
Implications for practice.
There is currently no evidence from randomised trials that assesses the benefit or harms of stem cell‐based interventions for the prevention of morbidity and mortality following hypoxic‐ischaemic encephalopathy in newborn infants.
Implications for research.
Findings from a meta‐analysis of preclinical studies on hypoxic‐ischaemic encephalopathy indicate that treatment with mesenchymal stromal cells might improve neurologic function (Archambault 2017). Although this review was limited by high heterogeneity, results can be useful for future design of clinical trials. For instance, a recent review on the use of mesenchymal stem cells in the treatment of ischaemic heart disease highlighted some potential reasons for a lacklustre response in clinical trials: timing of cell delivery, delivery method, and cell processing (Ward 2018). Thus, clinical trials should focus on standardising some of these variables to optimise the therapeutic potential of stem cell‐based interventions. In doing so, the scientific community would be able to improve the impact of the results (by increased power) which would allow for a more unified interpretation of findings from clinical trials.
History
Protocol first published: Issue 11, 2018 Review first published: Issue 8, 2020
Acknowledgements
We thank Colleen Ovelman and Roger Soll for the editorial support.
We thank Matthias Bank (Library and ICT services, Lund University) for designing and running the search strategy.
We thank Gene Dempsey and Jeffrey Horbar for editorial feedback.
The Methods section is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Search strategy
PubMed, 23 April 2019
#1 (infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies*[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infan*[TIAB] OR neonat*[TIAB]) 1,111,331
#2 ((randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) 3,885,300
#3 (("Stem Cells"[Mesh] OR "Stem Cell Transplantation"[Mesh] OR "Stromal Cells"[Mesh] OR “stem cell”[tiab] OR “stem cells”[tiab] OR “mesenchymal cell” OR “mesenchymal cells” OR “mononuclear cell” OR “mononuclear cells” OR “progenitor cell” OR “progenitor cells” OR “cord blood cell” OR “cord blood cells” OR “regenerative cell” OR “regenerative cells” OR "stromal cell" OR "stromal cells") AND (brain injury OR neuro‐protect* OR neuro‐restorative OR neuroprotect* OR "Asphyxia Neonatorum"[MeSH] OR "Hypoxia‐Ischemia, Brain"[MeSH] OR Asphyxia* OR Hypoxia OR Hypoxic OR Hypoxemia OR Hypoxaemia OR Ischemia OR Ischaemia OR ischaemic OR Ischaemic OR anoxia)) 21,679
#4 #1 AND #2 AND #3 138
Embase, 23 April 2019
#1. 'prematurity'/exp OR 'infant'/exp 1,110,109
#2. newborn*:ti,ab OR 'new born':ti,ab OR 'new borns':ti,ab OR 'newly born':ti,ab OR baby*:ti,ab OR babies:ti,ab OR premature:ti,ab OR prematurity:ti,ab OR preterm:ti,ab OR 'pre term':ti,ab OR 'low birth weight':ti,ab OR 'low birthweight':ti,ab OR vlbw:ti,ab OR lbw:ti,ab OR infant:ti,ab OR infants:ti,ab OR infantile:ti,ab OR infancy:ti,ab OR neonat*:ti,ab 1,012,212
#3. #1 OR #2 1,559,973
#4. 'randomised controlled trial'/exp OR 'randomised controlled trial' OR 'controlled clinical trial'/exp OR 'controlled clinical trial' OR 'randomised':ab,ti OR 'placebo':ab,ti OR 'randomly':ab,ti OR 'trial':ab,ti OR 'clinical study'/exp OR 'clinical trial' 9,939,984
#5. 'stem cell'/exp OR 'stem cell transplantation'/exp OR 'stroma cell'/exp OR 'stem cell':ti,ab OR 'stem cells':ti,ab OR 'mesenchymal cell':ti,ab OR 'mesenchymal cells':ti,ab OR 'mononuclear cell':ti,ab OR 'mononuclear cells':ti,ab OR 'progenitor cell':ti,ab OR 'progenitor cells':ti,ab OR 'cord blood cell':ti,ab OR 'cord blood cells':ti,ab OR 'regenerative cell':ti,ab OR 'regenerative cells':ti,ab OR 'stromal cell':ti,ab OR 'stromal cells':ti,ab 650,828
#6. 'newborn hypoxia'/exp OR 'asphyxia neonatorum':ti,ab OR 'hypoxic ischaemic encephalopathy'/exp OR 'brain hypoxia‐ischemia':ti,ab OR 'brain injury':ti,ab OR 'neuro‐protect*':ti,ab OR 'neuro‐restorative':ti,ab OR neuroprotect*:ti,ab OR asphyxia*:ti,ab OR hypoxia:ti,ab OR hypoxic:ti,ab OR hypoxemia:ti,ab OR
hypoxaemia:ti,ab OR ischemia:ti,ab OR ischaemia:ti,ab OR ischaemic:ti,ab OR ischaemic:ti,ab OR anoxia:ti,ab 857,061
#7. #5 AND #6 30,355
#8. #3 AND #4 AND #7 400
CINAHL, 23 April 2019
| S1 | (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) | 426,815 |
| S2 | ( (MH "Stem Cells+") OR stem cell transplantation OR "stroma cells" OR "stroma cell" OR "stem cell" OR "stem cells" ) OR ( “mesenchymal cell” OR “mesenchymal cells” OR “mononuclear cell” OR “mononuclear cells” OR “progenitor cell” OR “progenitor cells” OR “cord blood cell” OR “cord blood cells” OR “regenerative cell” OR “regenerative cells” OR "stromal cell" OR "stromal cells" ) | 41,467 |
| S3 | ( (MH "Asphyxia Neonatorum") OR (MH "Hypoxia‐Ischemia, Brain+") ) OR ( brain injury OR neuro‐protect* OR neuro‐restorative OR neuroprotect* OR Asphyxia* OR Hypoxia OR Hypoxic OR Hypoxemia OR Hypoxaemia OR Ischemia OR Ischaemia OR ischaemic OR Ischaemic OR anoxia ) | 116,145 |
| S4 | randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR randomised OR randomly OR trial | 462,048 |
| S5 | (MH "Clinical Trials+") OR PT clinical trial | 259,541 |
| S6 | S4 OR S5 | 472,952 |
| S7 | S1 AND S2 AND S3 AND S6 | 35 |
Cochrane Library, 24 April 2019
#1 (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU):ti,ab,kw (Word variations have been searched) 79,485
#2 MeSH descriptor: [Infant, Newborn] explode all trees 14,968
#3 #1 OR #2 79,485
#4 MeSH descriptor: [Stem Cells] explode all trees 763
#5 MeSH descriptor: [Stem Cell Transplantation] explode all trees 1,852
#6 MeSH descriptor: [Stromal Cells] explode all trees 139
#7 (“stem cell” OR “stem cells” OR “mesenchymal cell” OR “mesenchymal cells” OR “mononuclear cell” OR “mononuclear cells” OR “progenitor cell” OR “progenitor cells”):ti,ab,kw OR (“cord blood cell” OR “cord blood cells” OR “regenerative cell” OR “regenerative cells” OR "stromal cell" OR "stromal cells"):ti,ab,kw (Word variations have been searched) 16,535
#8 #4 OR #5 OR #6 OR #7 16,614
#9 MeSH descriptor: [Asphyxia Neonatorum] explode all trees 180
#10 MeSH descriptor: [Hypoxia‐Ischemia, Brain] explode all trees 180
#11 (brain injury OR neuro‐protect* OR neuro‐restorative OR neuroprotect* OR Asphyxia* OR Hypoxia OR Hypoxic OR Hypoxemia OR Hypoxaemia OR Ischemia OR Ischaemia OR ischaemic OR Ischaemic OR anoxia):ti,ab,kw (Word variations have been searched) 55,968
#12 #9 OR #10 OR #11 55,968
#13 #3 AND #8 AND #12 37
Total number of hits in all databases 610
Searches in clinical trial registries, 10 June 2019
ClinicalTrials.gov
Advanced search
Condition or disease: "brain injury" OR neuro‐protective OR neuro‐restorative OR "brain injuries" OR "Asphyxia Neonatorum" OR Asphyxia* OR Hypoxia OR Hypoxic OR Hypoxemia OR Hypoxaemia OR Ischemia OR Ischaemia OR ischaemic OR anoxia
Intervention/treatment: "stem cell" OR "stem cells" OR "stromal cell" OR "stromal cells" OR "mesenchymal cell" OR "mesenchymal cells" OR "mononuclear cell" OR "mononuclear cells" OR "progenitor cell" OR "progenitor cells" OR "cord blood cell" OR "cord blood cells"
Other terms: premature OR prematurity OR preterms OR preterm OR “very low birth” OR “low birth weight” OR newborn OR newborns OR neonate OR neonates OR infant OR infants
No further limits applied
29 results
ICTRP / WHO
Advanced search
Condition: brain injury OR neuro‐protective OR neuro‐restorative OR brain injuries OR Asphyxia Neonatorum OR Asphyxia OR Hypoxia OR Hypoxic OR Hypoxemia OR Hypoxaemia OR Ischemia OR Ischaemia OR ischaemic OR anoxia
Intervention: stem cell OR stromal cell OR mesenchymal cell OR mononuclear cell OR progenitor cell OR cord blood cell
Ticked box: search for clinical trials in children
Recruitment status: ALL, Phases: ALL
16 trials
ISRCTN registry
Condition: encephalopathy
12 results, 4 trials in newborn infants, 0 on stem cell‐based interventions www.isrctn.com/search?q=&filters=condition%3Aencephalopathy%2CconditionCategory%3ANeonatal+Diseases&searchType=advanced-search
Appendix 2. Risk of bias tool
We will use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we will seek information regarding the method of randomisation, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We will assess each criterion as being at a low, high, or unclear risk of bias. Two review authors will separately assess each study. We will resolve any disagreements by discussion. We will add this information to the 'Characteristics of included studies' table. We will evaluate the following issues and enter the findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we will categorise the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we will categorise the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we will categorise the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding will be assessed separately for different outcomes or class of outcomes. We will categorise the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we will categorise the methods used to blind outcome assessment. Blinding will be assessed separately for different outcomes or class of outcomes. We will categorise the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data including attrition and exclusions from the analysis. We will note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported or supplied by the trial authors, we will re‐include missing data in the analyses. We will categorise the methods used to deal with missing data as:
low risk (less than 20% missing data);
high risk (20% or greater missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found. We will search study protocols of the included trials in ClinicalTrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry. For studies in which study protocols were published in advance, we will compare prespecified outcomes versus outcomes eventually reported in the published results. If the study protocols were not published in advance, we will contact study authors to gain access to the study protocol. We will assess the likelihood of selective reporting bias as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we will describe any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We will assess whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk
If needed, we will explore the impact of the level of bias through undertaking sensitivity analyses.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cotten 2014 | Phase 1 study |
| Cotten 2020 | Phase 1 study |
| Tsuji 2020 | Phase 1 study |
Characteristics of studies awaiting classification [ordered by study ID]
Cotten 2011.
| Methods | Abstract and full text not available |
| Participants | Infants with HIE |
| Interventions | Autologous cord blood cells |
| Outcomes | Abstract and full text not available |
| Notes | Abstract and full text not available |
Nabetani 2016.
| Methods | Phase 1 clinical trial study of autologous cord blood cell therapy (ACBCT) in Japan. |
| Participants | Infants admitted to the NICU in the period December 2014 to March 2016, GA > 36 weeks, birth weight > 1800 g with HIE and meet the cooling criteria (JSPNM & MHLW Japan Working Group Practice Guidelines Consensus Statement). |
| Interventions | Umbilical cord blood (UCB) is collected aseptically and prepared by using Sepax (a cell separation system). |
| Outcomes | No significant adverse effects (not defined in the abstract) were reported in the 3 included infants. No other outcomes were reported |
| Notes | Full text not available; outcomes not reported in the abstract. |
GA: gestational age; HIE: hypoxic‐ischaemic encephalopathy; NICU: neonatal intensive care unit; UCB: umbilical cord blood
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000421033.
| Study name | Autologous umbilical cord blood mononuclear layer transfusion for neonates with encephalopathy: a pilot feasibility study |
| Methods | Interventional non‐randomised trial with a single group assignment |
| Participants | Estimated sample size = 40 Inclusion criteria
|
| Interventions | Autologous cord blood collection after birth of the baby (10 min), immediately followed by Mononuclear cell layer (MNCL) separation by density gradient centrifugation (30 min), immediately followed by MNCL transfusion over 30 min to preterm or full‐term neonate with signs of encephalopathy. |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | June 2010 |
| Contact information | Prof. Dr. Sahar MA Hassanein, Faculty of Medicine/Ain Shams University/Abassia Square/Cairo. 11588, Egypt |
| Notes | Study sponsor: University Ain Shams University, Faculty of Medicine |
ChiCTR‐TNRC‐11001591.
| Study name | Early intervention of stem cell transplantation for neonates with hypoxic‐ischemic encephalopathy‐‐‐‐a preliminary clinical study |
| Methods | Phase 1 study |
| Participants | Full‐term infants with Apgar at 1 min ≤ 6' and 5 min < 10' |
| Interventions | Stem cell versus rehabilitation |
| Outcomes | Visual evoked potentials, brainstem auditory evoked potential, somatosensory evoked potentials |
| Starting date | Date of registration: 17 August 2011 |
| Contact information | Xiaodong Wang, xdwang2000@163.com, General Hospital of Chinese People's Armed Police Forces, Beijing, China |
| Notes | Some of the outcomes reported in the register were not clear, e.g. CDCC and GMFM. Study sponsor: General Hospital of the Chinese People’s Armed Police Force |
ChiCTR‐TRC‐10000922.
| Study name | Autologous transplantation of cord blood derived stem cells for neonatal hypoxic‐ischaemic encephalopathy |
| Methods | Randomised parallel controlled trial |
| Participants |
|
| Interventions | Autologous transplantation of cord blood versus standard care |
| Outcomes |
|
| Starting date | Date of registration: 27 June 2010. |
| Contact information | Xiao‐zhuang Zhang, zxz3861@163.com, Guangzhou, China |
| Notes | Study sponsor: Guagndong Maternal and Children's Hospital |
NCT00593242.
| Study name | Cord blood for neonatal hypoxic‐ischemic encephalopathy |
| Methods | Phase 1 open‐label non‐randomised interventional study |
| Participants | Inclusion criteria
|
| Interventions |
|
| Outcomes | Primary: adverse event rates occurring in the pilot study population will be compared between the cord blood cell recipients and historical controls. [Time frame: first 18 postnatal days] Secondary outcomes
|
| Starting date | January 2008 |
| Contact information | Principal Investigator: Charles M Cotton, MD MHS, Duke University |
| Notes | Study sponsor: Michael Cotton |
NCT01506258.
| Study name | Autologous stem cells in newborns with oxygen deprivation |
| Methods | a open‐labelled non‐randomised interventional study with parallel assignment |
| Participants | Infants between 37 to 42 weeks of GA. Inclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcomes Effects of stem cell infusion at 1 week and 1 year after discharge: clinical assessment, including the Amiel‐Tison Neurological Assessment Secondary outcomes: not nominated. |
| Starting date | January 2012 |
| Contact information | Principal Investigator: Consuelo Mancias‐Guerra, MD; Hospital Universitario Dr. Jose E. Gonzalez Study Director: Alma R Marroquin‐Escamilla, MD; Hospital Universitario Dr. Jose E. Gonzalez Study Chair: David Gómez‐Almaguer, MD; Hospital Universitario Dr. Jose E. Gonzalez |
| Notes | Study sponsor: Hospital Universitario Dr. Jose E. Gonzalez |
NCT01649648.
| Study name | Autologous cord blood cells for brain injury in term newborns |
| Methods | Phase 1, open‐label, single group assignment study |
| Participants | Estimated sample size = 10 Inclusion criteria
|
| Interventions | Autologous cord blood infusion |
| Outcomes | Primary outcome: adverse event rates occurring in the recipients within the 3 first days of life. Secondary outcomes
|
| Starting date | September 2011 |
| Contact information | Principal Investigator: Jiun Lee, MBBS, National University Hospital, Singapore Principal Investigator: Samuel Rajadurai, MBBS, KK Women's and Children's Hospital Principal Investigator: Cheo Lian Yeo, MBBS, Singapore General Hospital |
| Notes | Study sponsor: National University Hospital, Singapore |
NCT02256618.
| Study name | A pilot feasibility and safety study of autologous umbilical cord blood cell therapy in infants with neonatal encephalopathy. |
| Methods | Multicentre pilot study; comparison with historical controls. |
| Participants | Inclusion criteria: infants are eligible if they meet all the following inclusion criteria except 4):
Exclusion criteria
|
| Interventions | Intravenous infusions (up to 3 infusions) of autologous volume reduced cord blood cells in the first 72 hours after birth. The neonate can receive their own non‐cryopreserved volume‐ and red blood cell‐reduced cord blood cells. The cord blood cells are divided into 3 doses and infused at 12 to 24, 36 to 48, and 60 to 72 hours after the birth. |
| Outcomes |
|
| Starting date | 1 August 2014 |
| Contact information | Makoto Nabetani, a103111@ych.or.jp, Yodogawa Christian Hospital, Osaka, Japan |
| Notes | Study sponsor: Neonatal Encephalopathy Consortium, Japan |
NCT02434965.
| Study name | Autologous cord blood and human placental derived stem cells in neonates with severe hypoxic‐ischemic encephalopathy (HPDSC+HIE) |
| Methods | Phase 2 open‐label interventional study |
| Participants | Sample population: 20 Inclusion criteria
|
| Interventions |
|
| Outcomes | Primary: number of subjects with infusion reaction as a measure of safety and tolerability within the first 30 days Secondary: improvement in neurological condition 2 years post HPDSC infusion as shown on head MRI, DTI and neurological development by Sarnat testing. |
| Starting date | June 2019 |
| Contact information | Principal Investigator: Mitchell S Cairo, MD; New York Medical College |
| Notes | Study sponsor: New York Medical College |
NCT02455830.
| Study name | clinicaltrials.gov/ct2/show/record/NCT02455830?term=NCT02455830&rank=1 |
| Methods | Prospective, case‐control, observational study |
| Participants | Sample size = 18 Inclusion criteria: Infants are eligible if they meet all the following inclusion criteria except 4.
|
| Interventions | Autologous cord blood cell therapy The neonates receive their own non‐cryopreserved volume‐ and red blood cell‐reduced cord blood cells. The cord blood cells are divided into 3 doses and intravenously infused at 12 to 24, 36 to 48, and 60 to 72 hours after the birth. |
| Outcomes | Primary outcome: changes in serum levels of cytokines and trophic factors within 10 days after birth. Secondary outcomes: association with neuroimaging (not specified) at 12 months and neurodevelopmental functional outcome (not specified) at 18 months |
| Starting date | April 2015 |
| Contact information | Study Director: Haruo Shintaku, MD, PhD; Osaka City University Graduate School of Medicine |
| Notes | Study sponsor: Neonatal Encephalopathy Consortium, Japan |
NCT02612155.
| Study name | A multi‐site study of autologous cord blood cells for hypoxic ischaemic encephalopathy |
| Methods | Multicenter phase 2 RCT |
| Participants | Estimated sample size: 160 newborns Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcomes: survival at 1 year; percentage of subjects with Bayley III scores in all 3 domains ≥ to 85 Secondary outcomes: percentage of infants with seizures, iNO use; need for ECMO; G‐tube feeding; anti‐epileptic medications |
| Starting date | 20 March 2017 |
| Contact information | Kimberley A Fisher, kimberley.fisher@duke.edu, Duke University Medical Center, Durham, North Carolina, USA |
| Notes | Study sponsor: Michael Cotton |
NCT02854579.
| Study name | Neural progenitor cell and paracrine factors to treat hypoxic ischaemic encephalopathy |
| Methods | Open‐label randomised interventional study |
| Participants | Inclusion criteria
|
| Interventions |
|
| Outcomes | Sample size 120 neonates, up to 14 days of life Primary outcomes
Secondary outcomes
|
| Starting date | January 2013 |
| Contact information | Study Chair: Zuo Luan, MD, Navy General Hospital |
| Notes | Study sponsor: Navy General Hospital, Beijing |
NCT02881970.
| Study name | Neonatal hypoxic ischaemic encephalopathy: safety and feasibility study of a curative treatment with autologous cord blood stem cells (NEOSTEM) |
| Methods | Phase 1, 2, open label study with single group assignment |
| Participants | Sample size = 20 Inclusion criteria Term ≥ 36 weeks of gestation and:
|
| Interventions | Autologous cord blood stem cell infusion |
| Outcomes | Primary outcome: adverse clinical event rates due to stem cell preparation within 2 years of life |
| Starting date | April 2017 |
| Contact information | Study director: Catherine Geindre AP, HM |
| Notes | Study sponsor: Assistance Publique Hopitaux De Marseille |
NCT03352310.
| Study name | Feasibility and safety of umbilical cord blood transfusion in the treatment of neonatal cerebral ischemia and anaemia |
| Methods | Phase 1, non‐randomised trial with parallel assignment |
| Participants | Expected sample size = 40 Inclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
Anaemia
|
| Starting date | April 2018 |
| Contact information | Principal Investigator: Simon Lam, MD; Chinese University of Hong Kong |
| Notes | Study sponsor: Mononuclear Therapeutics Ltd. |
NCT03635450.
| Study name | Study of hCT‐MSCs in newborn infants With moderate or severe HIE |
| Methods | Phase 1, open‐label, non‐randomised, parallel assignment |
| Participants | Sample size = 6 Inclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
Death prior to discharge from initial hospitalisation
1 year (12 to 16 postnatal months) Bayley Scales of Infant and Toddler Development, Third Edition (Bayley III) assessments in cognitive, language and motor development. Moderate to Severe CP will be assigned with cognitive score, 70, motor score, 70 and with Gross Motor Function Classification System ≥ 2. |
| Starting date | December 2018 |
| Contact information | Principal Investigator: Michael Cotton, MD; Duke University |
| Notes | Study sponsor: Joanne Kurtzberg, MD |
NCT01284673.
| Study name | Characterization of the cord blood stem cell in situation of neonatal asphyxia (NEOCORD) |
| Methods | Open‐label, single group assignment study |
| Participants | Estimated sample size = 10 Inclusion criteria:
|
| Interventions | in vitro characterization of the cord blood stem cell |
| Outcomes | The primary outcome is the characterisation of cord blood stem cells of neonates with neonatal asphyxia and to compare them with those from healthy newborn. Time frame: 2 years. |
| Starting date | April 2010 |
| Contact information | Principal investigator: Umberto SIMEONI, MD, PhD AP‐HM |
| Notes | Study sponsor: Assistance Publique Hopitaux De Marseille |
Differences between protocol and review
The search strategy has been updated following the publication of the protocol and is reported in Appendix 1.
Contributions of authors
MB, OR, and AM reviewed the literature and wrote the review.
DL and BT commented on and oversaw the review.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
MB, OR and DL are employed by this organisation
-
The University of Texas Health San Antonio School of Medicine Clinical Investigator Kickstart Pilot Grant, USA
to AM
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute for Health Research, UK
: This report is independent research funded by a UK NIHR Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
Declarations of interest
MB has received research funding from ALF grant (non‐profit ‐ Lund University) and Crafoord Foundation (non‐profit) for research projects not related to Cochrane.
OR: no known conflicts of interest.
AM: is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR001118. AM's institution has received a grant from the Francis Family Foundation (Parker B. Francis Fellowship Award). AM also has submitted a provisional patent to the US Patent and Trademark Office that involves by‐products of stem cells ("cell‐free therapies"). This patent does not conflict with this planned systematic review, as the review is only considering different types of cell‐based interventions.
DL owns shares in Premacure AB, and has received consultancy fees from Shire Inc. The collaboration with Premacure AB and Shire Inc. is specifically related to treatment of extremely preterm infants with a protein complex aiming to decrease prevalence of severe morbidity associated with extremely preterm birth.
BT's work on stem cells is supported by the Canadian Institute for Health Research, the Canadian Stem Cell Network, the Canadian Thoracic Society, the Ottawa Hospital Research Institute and the Children's Hospital of Eastern Ontario Research Institute, and the Ontario Institute of Regenerative Medicine.
New
References
References to studies excluded from this review
Cotten 2014 {published data only}
- Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. Journal of Pediatrics 2014;164(5):973-9. [DOI: 10.1016/j.jpeds.2013.11.036] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotten 2020 {published data only}
- Cotten CM, Fisher K, Kurtzberg J, Simmons R. Phase I trial of allogeneic umbilical cord tissue-derived mesenchymal stromal cells in neonates with hypoxic-ischemic encephalopathy. Cytotherapy 2020;22(5):S192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuji 2020 {published data only}
- Tsuji M, Sawada M, Watabe S, Sano H, Kanai M, Tanaka E, et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Scientific reports 20202;10(1):4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cotten 2011 {published data only}
- Cotten CM, Murtha A, Goldberg R, Smith PB, Grotegut C, Goldstein RF, et al. Autologous cord blood cells for infants with hypoxic-ischemic encephalopathy (HIE): a feasibility study. In: Pediatric Academic Societies (PAS) Annual Meeting; 2011 April 30 to May 3; Denver (CO). 2011.
Nabetani 2016 {published data only}
- Nabetani M, Shintaku H, Tsuji M, Tamura M, Kusuda S, Hayakawa M, et al. Clinical trial study of autologous cord blood cell therapy for newborn with hypoxic ischemic encephalopathy in Japan. In: European Journal of Pediatrics. 6th Congress of the European Academy of Paediatric Societies; 2016 Oct 21-24; Geneva (Switzerland). 2016.
References to ongoing studies
ACTRN12610000421033 {published data only}
- ACTRN12610000421033. Autologous umbilical cord blood mononuclear layer transfusion for neonates with encephalopathy: a pilot feasibility study [Efficacy of autologous umbilical cord blood mononuclear layer transfusion to prevent brain injury after neonatal encephalopathy]. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12610000421033 (first received 26 May 2010).
ChiCTR‐TNRC‐11001591 {published data only}
- ChiCTR-TNRC-11001591. Early intervention of stem cell transplantation for neonates with hypoxic-ischemic encephalpathy----a preliminary clinical study. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TNRC-11001591 (first received 17 August 2011).
- ChiCTR-TNRC-11001591. Early intervention of stem cell transplantation for neonates with hypoxic-ischemic encephalpathy----a preliminary clinical study. www.chictr.org.cn/showprojen.aspx?proj=7950 (first received 17 August 2011).
ChiCTR‐TRC‐10000922 {published data only}
- ChiCTR-TRC-10000922. Autologous transplantation of cord blood derived stem cells for neonatal hypoxic - ischemic encephalopathy. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-10000922 (first received 26 June 2010).
NCT00593242 {published data only}
- NCT00593242. Cord blood for neonatal hypoxic-ischemic encephalopathy. clinicaltrials.gov/ct2/show/record/NCT00593242?term=NCT00593242&rank=1 (first received 14 January 2008).
NCT01284673 {published data only}
- NCT01284673. Characterization of the cord blood stem cell in situation of neonatal asphyxia (NEOCORD). clinicaltrials.gov/ct2/show/record/NCT01284673?term=NCT01284673&rank=1 (first received 27 January 2011).
NCT01506258 {published data only}
- NCT01506258. Autologous stem cells in newborns with oxygen deprivation [Effects of the infusion of autologous non-cryopreserved CD34+ cells in newborns with asphyxia]. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01506258 (first received 1 May 2012).
- NCT01506258. Autologous stem cells in newborns with oxygen deprivation. clinicaltrials.gov/ct2/show/record/NCT01506258?term=NCT01506258&rank=1 (first received 9 January 2012).
NCT01649648 {published data only}
- NCT01649648. Autologous cord blood cells for brain injury in term newborns. clinicaltrials.gov/ct2/show/NCT01649648?term=NCT01649648&rank=1 (first received 9 January 2012).
NCT02256618 {published data only}
- JPRN-UMIN000014903. A pilot feasibility and safety study of autologous umbilical cord blood cell therapy in infants with neonatal encephalopathy. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000014903 (first received 26 August 2014).
- NCT02256618. A pilot feasibility and safety study of autologous umbilical cord blood cell therapy in infants with neonatal encephalopathy. ichgcp.net/clinical-trials-registry/NCT02256618 (first received 1 August 2014).
NCT02434965 {published data only}
- NCT02434965. Autologous cord blood and human placental derived stem cells in neonates with severe hypoxic-ischemic encephalopathy (HPDSC+HIE). clinicaltrials.gov/ct2/show/record/NCT02434965?term=NCT02434965&rank=1 (first received 6 May 2015).
NCT02455830 {published data only}
- NCT02455830. Cytokines associated with cord blood cell therapy for neonatal encephalopathy. clinicaltrials.gov/ct2/show/record/NCT02455830?term=NCT02455830&rank=1 (first received 28 May 2015).
NCT02612155 {published data only}
- NCT02612155. A multi-site study of autologous cord blood cells for hypoxic ischemic encephalopathy. clinicaltrials.gov/ct2/show/nct02612155 (first received 23 November 2015).
NCT02854579 {published data only}
- NCT02854579. Neural progenitor cell and paracrine factors to treat hypoxic ischemic encephalopathy. clinicaltrials.gov/ct2/show/NCT02854579?term=NCT02854579&rank=1 (first received 3 August 2016).
- Neural progenitor cell and paracrine factors to treat hypoxic ischemic encephalopathy (HIE). apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02854579 (first received 23 November 2015).
NCT02881970 {published data only}
- NCT02881970. Neonatal hypoxic ischemic encephalopathy: safety and feasibility study of a curative treatment with autologous cord blood stem cells (NEOSTEM). clinicaltrials.gov/ct2/show/record/NCT02881970?term=NCT02881970&rank=1 (first received 29 August 2016).
- NCT02881970. Neonatal hypoxic ischemic encephalopathy: safety and feasibility study of a curative treatment with autologous cord blood stem cells NEOSTEM. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02881970 (first received 20 April 2017).
NCT03352310 {published data only}
- NCT03352310. Feasibility and safety of umbilical cord blood transfusion in the treatment of neonatal cerebral ischemia and anemia. clinicaltrials.gov/ct2/show/record/NCT03352310?term=NCT03352310&rank=1 (first received 24 November 2017).
NCT03635450 {published data only}
- NCT03635450. Study of hCT-MSC in newborn infants with moderate or severe HIE. clinicaltrials.gov/ct2/show/record/NCT03635450?term=NCT03635450&rank=1 (first received 17 August 2018).
Additional references
Ahn 2016
- Ahn SY, Chang YS, Park WS. Stem cells for neonatal brain disorders. Neonatology 2016;109(4):377-83. [DOI: 10.1159/000444905] [PMID: ] [DOI] [PubMed] [Google Scholar]
Archambault 2017
- Archambault J, Moreira A, McDaniel D, Winter L, Sun L, Hornsby P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: a systematic review and meta-analysis of preclinical studies. PloS One 2017;12(12):e0189895. [DOI: 10.1371/journal.pone.0189895] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aridas 2016
- Aridas JD, McDonald CA, Paton MC, Yawno T, Sutherland AE, Nitsos I, et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb. Journal of Physiology 2016;594(5):1421-35. [DOI: 10.1113/JP271104] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkholt 2013
- Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies – bridging scientific observations and regulatory viewpoints. Cytotherapy 2013;15(7):753-9. [DOI: 10.1016/j.jcyt.2013.03.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Batsali 2013
- Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications. Current Stem Cell Research & Therapy 2013;8(2):144-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd edition. San Antonio (TX): The Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Bellayr 2014
- Bellayr IH, Catalano JG, Lababidi S, Yang AX, Lo Surdo JL, Bauer SR, et al. Gene markers of cellular aging in human multipotent stromal cells in culture. Stem Cell Research & Therapy 2014;5(2):59. [DOI: 10.1186/scrt448] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boncoraglio 2010
- Boncoraglio GB, Bersano A, Candelise L, Reynolds BA, Parati EA. Stem cell transplantation for ischemic stroke. Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No: CD007231. [DOI: 10.1002/14651858.CD007231.pub2] [DOI] [PubMed] [Google Scholar]
Bonestroo 2015
- Bonestroo HJ, Heijnen CJ, Groenendaal F, Bel F, Nijboer CH. Development of cerebral gray and white matter injury and cerebral inflammation over time after inflammatory perinatal asphyxia. Developmental Neuroscience 2015;37(1):78-94. [DOI: 10.1159/000368770] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boshuizen 2018
- Boshuizen MC, Steinberg GK. Stem cell-based immunomodulation after stroke: effects on brain repair processes. Stroke 2018;49(6):1563-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2010
- Cai J, Li W, Su H, Qin D, Yang J, Zhu F, et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. Journal of Biological Chemistry 2010;285(15):11227-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2014
- Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. Journal of Pediatrics 2014;164(5):966-72.e6. [DOI: 10.1016/j.jpeds.2013.12.011] [ClinicalTrials.gov NCT01297205] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen LX, Ma SM, Zhang P, Fan ZC, Xiong M, Cheng GQ, et al. Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic-ischemic injury. PloS One 2015;10(3):e0115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chopp 2002
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurology 2002;1(2):92-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochrane EPOC Group 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). Screening, data extraction and management. EPOC Resources for Review Authors, 2017. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed prior to 20 September 2018).
Cornette 2002
- Cornette LG, Tanner SF, Ramenghi LA, Miall LS, Childs AM, Arthur RJ, et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(3):F171-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daadi 2016
- Daadi MM, Klausner JQ, Bajar B, Goshen I, Lee-Messer C, Lee SY, et al. Optogenetic stimulation of neural grafts enhances neurotransmission and downregulates the inflammatory response in experimental stroke model. Cell Transplantation 2016;25(7):1371-80. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2008
- David HN, Haelewyn B, Rouillon C, Lecoq M, Chazalviel L, Apiou G, et al. Neuroprotective effects of xenon: a therapeutic window of opportunity in rats subjected to transient cerebral ischemia. FASEB Journal 2008;22(4):1275-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
de Haan 1997
- Haan HH, Gunn AJ, Williams CE, Heymann MA, Gluckman PD. Magnesium sulfate therapy during asphyxia in near-term fetal lambs does not compromise the fetus but does not reduce cerebral injury. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 1):18-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Di Lullo 2017
- Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nature Reviews. Neuroscience 2017;18(10):573-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dingley 2014
- Dingley J, Tooley J, Liu X, Scull-Brown E, Elstad M, Chakkarapani E, et al. Xenon ventilation during therapeutic hypothermia in neonatal encephalopathy: a feasibility study. Pediatrics 2014;133(5):809-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dominici 2006
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315-7. [DOI: 10.1080/14653240600855905] [PMID: ] [DOI] [PubMed] [Google Scholar]
Donega 2013
- Donega V, Velthoven CT, Nijboer CH, Bel F, Kas MJ, Kavelaars A, et al. Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PloS One 2013;8(1):e51253. [DOI: 10.1371/journal.pone.0051253] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donega 2015
- Donega V, Nijboer CH, Velthoven CT, Youssef SA, Bruin A, Bel F, et al. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatric Research 2015;78(5):520-6. [DOI: 10.1038/pr.2015.145] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2010
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ (Clinical Research Ed.) 2010;340:c363. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmahdy 2010
- Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics 2010;125(5):e1135-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fan 2005
- Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han ZC. Human umbilical cord blood cells express neurotrophic factors. Neuroscience letters 2005;380(3):322-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Frey 2006
- Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the 'cold shoulder'? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplantation 2006;38(6):399-405. [PMID: ] [DOI] [PubMed] [Google Scholar]
Galinsky 2014
- Galinsky R, Bennet L, Groenendaal F, Lear CA, Tan S, Bel F, et al. Magnesium is not consistently neuroprotective for perinatal hypoxia-ischemia in term-equivalent models in preclinical studies: a systematic review. Developmental Neuroscience 2014;36(2):73-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia 2014
- Garcia O, Warburton D. Amniotic fluid stem cell therapy for lung disease. In: Atala A, Murphy S, editors(s). Perinatal Stem Cells. New York (NY): Springer, 2014:59-66. [DOI: 10.1007/978-1-4939-1118-9_6] [DOI] [Google Scholar]
Gebler 2012
- Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends in Molecular Medicine 2012;18(2):128-34. [DOI: 10.1016/j.molmed.2011.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gluckman 1992
- Gluckman PD, Williams CE. When and why do brain cells die? Developmental Medicine and Child Neurology 1992;34(11):1010-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gonzalez 2009
- Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Developmental Neuroscience 2009;31(5):403-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gopagondanahalli 2016
- Gopagondanahalli KR, Li J, Fahey MC, Hunt RW, Jenkin G, Miller SL, et al. Preterm hypoxic-ischemic encephalopathy. Frontiers in Pediatrics 2016;4:114. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goss 1995
- Goss JR, Styren SD, Miller PD, Kochanek PM, Palmer AM, Marion DW, et al. Hypothermia attenuates the normal increase in interleukin 1 beta RNA and nerve growth factor following traumatic brain injury in the rat. Journal of Neurotrauma 1995;12(2):159-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 09 January 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grandvuillemin 2017
- Grandvuillemin I, Garrigue P, Ramdani A, Boubred F, Simeoni U, Dignat-George F, et al. Long-term recovery after endothelial colony-forming cells or human umbilical cord blood cells administration in a rat model of neonatal hypoxic-ischemic encephalopathy. Stem Cells Translational Medicine 2017;6(11):1987-96. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenwood 2000
- Greenwood K, Cox P, Mehmet H, Penrice J, Amess PN, Cady EB, et al. Magnesium sulfate treatment after transient hypoxia-ischemia in the newborn piglet does not protect against cerebral damage. Pediatric Research 2000;48(3):346-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R. The Abilities of Babies: a Study in Mental Measurement. New York (NY): McGraw-Hill Book Co. Inc, 1954. [Google Scholar]
Gunn 1997
- Gunn AJ, Gunn TR, Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. Journal of Clinical Investigation 1997;99(2):248-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunn 1998
- Gunn AJ, Gunn TR. The 'pharmacology' of neuronal rescue with cerebral hypothermia. Early Human Development 1998;53(1):19-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haaland 1997
- Haaland K, Loberg EM, Steen PA, Thoresen M. Posthypoxic hypothermia in newborn piglets. Pediatric Research 1997;41(4 Pt 1):505-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hare 2017
- Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. Journal of the American College of Cardiology 2017;69(5):526-37. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook (accessed prior to 20 September 2018).
Hobbs 2008
- Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 2008;39(4):1307-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hsu 2016
- Hsu YC, Wu YT, Yu TH, Wei YH. Mitochondria in mesenchymal stem cell biology and cell therapy: from cellular differentiation to mitochondrial transfer. Seminars in Cell & Developmental Biology 2016;52:119-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huang 2018
- Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Progress in Neurobiology 2018 May 21 [Epub ahead of print]. [DOI: 10.1016/j.pneurobio.2018.05.004] [PMID: ] [DOI] [PMC free article] [PubMed]
Islam 2012
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Medicine 2012;18(5):759-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwata 2007
- Iwata O, Iwata S, Thornton JS, De Vita E, Bainbridge A, Herbert L, et al. "Therapeutic time window" duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Research 2007;1154:173-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Iyer 2008
- Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opinion on Biological Therapy 2008;8(5):569-81. [DOI: 10.1517/14712598.8.5.569] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2015
- Ji G, Liu M, Zhao XF, Liu XY, Guo QL, Guan ZF, et al. NF-kappaB signaling is involved in the effects of intranasally engrafted human neural stem cells on neurofunctional improvements in neonatal rat hypoxic-ischemic encephalopathy. CNS Neuroscience & Therapeutics 2015;21(12):926-35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaandorp 2012
- Kaandorp JJ, van Bel F, Veen S, Derks JB, Groenendaal F, Rijken M, et al. Long-term neuroprotective effects of allopurinol after moderate perinatal asphyxia: follow-up of two randomised controlled trials. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(3):F162-6. [DOI: 10.1136/archdischild-2011-300356] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaplan 2017
- Kaplan A, Sackett K, Sumstad D, Kadidlo D, McKenna DH. Impact of starting material (fresh versus cryopreserved marrow) on mesenchymal stem cell culture. Transfusion 2017;57(9):2216-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kidani 2016
- Kidani Y, Miki Y, Nomimura N, Minakawa S, Tanaka N, Miyoshi H, et al. The therapeutic effect of CD133(+) cells derived from human umbilical cord blood on neonatal mouse hypoxic-ischemic encephalopathy model. Life Sciences 2016;157:108-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2018
- Kim TK, Park D, Ban YH, Cha Y, An ES, Choi J, et al. Improvement by human oligodendrocyte progenitor cells of neurobehavioral disorders in an experimental model of neonatal periventricular leukomalacia. Cell Transplantation 2018;27(7):1168-77. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumral 2004
- Kumral A, Uysal N, Tugyan K, Sonmez A, Yilmaz O, Gokmen N, et al. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behavioural Brain Research 2004;153(1):77-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lalu 2012
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS One 2012;7(10):e47559. [DOI: 10.1371/journal.pone.0047559] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013
- Lee ACC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatric Research 2013;74(Suppl 1):50-72. [DOI: 10.1038/pr.2013.206] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2002
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 2002;59(4):514-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Limperopoulos 2007
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007;120(3):584-93. [DOI: 10.1542/peds.2007-1041] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2010
- Liu AM, Lu G, Tsang KS, Li G, Wu Y, Huang ZS, et al. Umbilical cord-derived mesenchymal stem cells with forced expression of hepatocyte growth factor enhance remyelination and functional recovery in a rat intracerebral hemorrhage model. Neurosurgery 2010;67(2):357-65; discussion 365-6. [DOI: 10.1227/01.NEU.0000371983.06278.B3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvascular Research 2014;92:10-8. [DOI: 10.1016/j.mvr.2014.01.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ma 2005
- Ma D, Hossain M, Chow A, Arshad M, Battson RM, Sanders RD, et al. Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Annals of Neurology 2005;58(2):182-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Manley 2017
- Manley NC, Priest CA, Denham J, Wirth ED 3rd, Lebkowski JS. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Translational Medicine 2017;6(10):1917-29. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2018
- McDonald CA, Penny TR, Paton MC, Sutherland AE, Nekkanti L, Yawno T, et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. Journal of Neuroinflammation 2018;15(1):47. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mine 2013
- Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiology of Disease 2013;52:191-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mitsialis 2016
- Mitsialis SA, Kourembanas S. Stem cell-based therapies for the newborn lung and brain: possibilities and challenges. Seminars in Perinatology 2016;40(3):138-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreira 2019
- Moreira A, Alayli Y, Balgi S, Winter C, Kahlenberg S, Mustafa S, et al. Upcycling umbilical cords: bridging regenerative medicine with neonatology. Journal of Maternal-fetal & Neonatal Medicine 2019;32(8):1378-87. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morán 2017
- Morán J, Stokowska A, Walker FR, Mallard C, Hagberg H, Pekna M. Intranasal C3a treatment ameliorates cognitive impairment in a mouse model of neonatal hypoxic-ischemic brain injury. Experimental Neurology 2017;290:74-84. [DOI: 10.1016/j.expneurol.2017.01.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murphy 2013
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine 2013;45:e54. [DOI: 10.1038/emm.2013.94] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Möbius 2015
- Möbius MA, Thebaud B. Stem cells and their mediators – next generation therapy for bronchopulmonary dysplasia. Frontiers in Medicine 2015;2:50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabetani 2018
- Nabetani M, Shintaku H, Hamazaki T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatric Research 2018;83(1-2):356-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT01646619
- NCT01646619. Efficacy study of hypothermia plus magnesium sulphate (MgSO4) in the management of term and near term babies with hypoxic ischemic encephalopathy (MagCool) [A multicenter randomized controlled trial of therapeutic hypothermia plus magnesium sulphate (MgSO4) versus therapeutic hypothermia plus placebo in the management of term and near term babies with hypoxic ischemic encephalopathy]. clinicaltrials.gov/ct2/show/NCT01646619 (first received 20 July 2012).
NCT01765218
- NCT01765218. Topiramate in neonates receiving whole body cooling for hypoxic ischemic encephalopathy [Topiramate as an adjuvant to therapeutic hypothermia for infants with hypoxic ischemic encephalopathy]. clinicaltrials.gov/ct2/show/NCT01765218 (first received 10 January 2013).
NCT02274428
- NCT02274428. Phase 1 clinical trial of PNEUMOSTEM® treatment in premature infants with intraventricular hemorrhage. clinicaltrials.gov/ct2/show/NCT02274428 (first received 2 October 2014).
NCT02999373
- NCT02999373. Prevention of preterm infection by autologous umbilical cord blood mononuclear cells therapy [Autologous cord blood mononuclear cells for bronchopulmonary dysplasia in very preterm neonates]. clinicaltrials.gov/ct2/show/NCT02999373 (first received 21 December 2016).
Niimi 2018
- Niimi Y, Levison SW. Pediatric brain repair from endogenous neural stem cells of the subventricular zone. Pediatric Research 2018;83(1-2):385-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Noh 2006
- Noh MR, Kim SK, Sun W, Park SK, Choi HC, Lim JH, et al. Neuroprotective effect of topiramate on hypoxic ischemic brain injury in neonatal rats. Experimental Neurology 2006;201(2):470-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Oki 2012
- Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells (Dayton, Ohio) 2012;30(6):1120-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Owji 2017
- Owji ZP, Gilbert G, Saint-Martin C, Wintermark P. Brain temperature is increased during the first days of life in asphyxiated newborns: developing brain injury despite hypothermia treatment. AJNR. American Journal of Neuroradiology 2017;38(11):2180-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozyener 2012
- Ozyener F, Cetinkaya M, Alkan T, Goren B, Kafa IM, Kurt MA, et al. Neuroprotective effects of melatonin administered alone or in combination with topiramate in neonatal hypoxic-ischemic rat model. Restorative Neurology and Neuroscience 2012;30(5):435-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Palmer 1993
- Palmer C, Towfighi J, Roberts RL, Heitjan DF. Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatric Research 1993;33(4 Pt 1):405-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2016
- Park WS, Sung SI, Ahn SY, Sung DK, Im GH, Yoo HS, et al. Optimal timing of mesenchymal stem cell therapy for neonatal intraventricular hemorrhage. Cell Transplantation 2016;25(6):1131-44. [DOI: 10.3727/096368915X689640] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parodi 2015
- Parodi A, Morana G, Severino MS, Malova M, Natalizia AR, Sannia A, et al. Low-grade intraventricular hemorrhage: is ultrasound good enough? Journal of Maternal-fetal & Neonatal Medicine 2015;28(Suppl 1):2261-4. [DOI: 10.3109/14767058.2013.796162] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parody 2013
- Parody R, Caballero D, Marquez-Malaver FJ, Vazquez L, Saldana R, Madrigal MD, et al. To freeze or not to freeze peripheral blood stem cells prior to allogeneic transplantation from matched related donors. European Journal of Haematology 2013;91(5):448-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parolini 2008
- Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells 2008;26(2):300-11. [DOI] [PubMed] [Google Scholar]
Parolini 2014
- Parolini O, De D, Rodrigues M, Caruso M. Placental stem/progenitor cells: isolation and characterization. In: Perinatal Stem Cells. New York (NY): Springer, 2014:141-57. [DOI: 10.1007/978-1-4939-1118-9_13] [DOI] [Google Scholar]
Peeters‐Scholte 2003
- Peeters-Scholte C, Braun K, Koster J, Kops N, Blomgren K, Buonocore G, et al. Effects of allopurinol and deferoxamine on reperfusion injury of the brain in newborn piglets after neonatal hypoxia-ischemia. Pediatric Research 2003;54(4):516-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Penrice 1997
- Penrice J, Amess PN, Punwani S, Wylezinska M, Tyszczuk L, D'Souza P, et al. Magnesium sulfate after transient hypoxia-ischemia fails to prevent delayed cerebral energy failure in the newborn piglet. Pediatric Research 1997;41(3):443-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pfister 2010
- Pfister RH, Soll RF. Hypothermia for the treatment of infants with hypoxic-ischemic encephalopathy. Journal of Perinatology 2010;30 Suppl:S82-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pierro 2017
- Pierro M, Thebaud B, Soll R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD011932. [DOI: 10.1002/14651858.CD011932.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pimentel‐Coelho 2012
- Pimentel-Coelho PM, Rosado-de-Castro PH, da Fonseca LM, Mendez-Otero R. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatric Research 2012;71(4 Pt 2):464-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pluchino 2013
- Pluchino S, Peruzzotti-Jametti L. Rewiring the ischaemic brain with human-induced pluripotent stem cell-derived cortical neurons. Brain 2013;136(Pt 12):3525-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Podesta 2015
- Podesta M, Bruschettini M, Cossu C, Sabatini F, Dagnino M, Romantsik O, et al. Preterm cord blood contains a higher proportion of immature hematopoietic progenitors compared to term samples. PloS One 2015;10(9):e0138680. [DOI: 10.1371/journal.pone.0138680] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Polderman 2008
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 2008;371(9628):1955-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Qin 2015
- Qin J, Ma X, Qi H, Song B, Wang Y, Wen X, et al. Transplantation of induced pluripotent stem cells alleviates cerebral inflammation and neural damage in hemorrhagic stroke. PloS One 2015;10(6):e0129881. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qu 2007
- Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology 2007;27(4):355-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Rogers 2014
- Rogers EE, Bonifacio SL, Glass HC, Juul SE, Chang T, Mayock DE, et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatric Neurology 2014;51(5):657-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romantsik 2019
- Romantsik O, Bruschettini M, Moreira A, Thébaud B, Ley D. Stem cell-based interventions for the prevention and treatment of germinal matrix-intraventricular haemorrhage in preterm infants. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013201. [DOI: 10.1002/14651858.CD013201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothwell 1995
- Rothwell NJ, Strijbos PJ. Cytokines in neurodegeneration and repair. International Journal of Developmental Neuroscience 1995;13(3-4):179-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rowe 2010
- Rowe DD, Leonardo CC, Hall AA, Shahaduzzaman MD, Collier LA, Willing AE, et al. Cord blood administration induces oligodendrocyte survival through alterations in gene expression. Brain Research 2010;1366:172-88. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutherford 2010
- Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 2010;52(6):505-21. [DOI: 10.1007/s00234-010-0700-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sanberg 2014
- Sanberg PR, Eve DJ, Borlongan CV. Umbilical cord blood cells in the repair of central nervous system diseases. In: Atala A, Murphy S, editors(s). Perinatal Stem Cells. New York (NY): Springer, 2014:269-87. [DOI: 10.1007/978-1-4939-1118-9_25] [DOI] [Google Scholar]
Sarnat 1976
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology 1976;33(10):696-705. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schwarting 2008
- Schwarting S, Litwak S, Hao W, Bahr M, Weise J, Neumann H. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke 2008;39(10):2867-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sfaello 2005
- Sfaello I, Baud O, Arzimanoglou A, Gressens P. Topiramate prevents excitotoxic damage in the newborn rodent brain. Neurobiology of Disease 2005;20(3):837-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Silverstein 1997
- Silverstein FS, Barks JD, Hagan P, Liu XH, Ivacko J, Szaflarski J. Cytokines and perinatal brain injury. Neurochemistry International 1997;30(4-5):375-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sirimanne 1996
- Sirimanne ES, Blumberg RM, Bossano D, Gunning M, Edwards AD, Gluckman PD, et al. The effect of prolonged modification of cerebral temperature on outcome after hypoxic-ischemic brain injury in the infant rat. Pediatric Research 1996;39(4 Pt 1):591-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Taghizadeh 2014
- Taghizadeh RR, Holzer PW, Marino T, Cetrulo KJ, Cetrulo CL, Cetrulo CL. Towards clinical applications of umbilical cord derived mesenchymal stem cells. In: Atala A, Murphy S, editors(s). Perinatal Stem Cells. New York (NY): Springer, 2014:347-59. [DOI: 10.1007/978-1-4939-1118-9_31] [DOI] [Google Scholar]
Tagin 2013
- Tagin M, Shah PS, Lee KS. Magnesium for newborns with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. Journal of Perinatology 2013;33(9):663-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 1996
- Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, et al. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatric Research 1996;39(5):791-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thoresen 1995
- Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatric Research 1995;37(5):667-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thoresen 2009
- Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J. Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. Journal of Cerebral Blood Flow and Metabolism 2009;29(4):707-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tornero 2013
- Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 2013;136(Pt 12):3561-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Trounson 2015
- Trounson A, Mcdonald C. Stem cell therapies in clinical trials: progress and challenges. Stem Cell 2015;17(1):11-22. [DOI] [PubMed] [Google Scholar]
Tsuji 2014
- Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Sato Y, Tsuda H, et al. Effects of intravenous administration of umbilical cord blood CD34(+) cells in a mouse model of neonatal stroke. Neuroscience 2014;263:148-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
van Velthoven 2009
- Velthoven CT, Kavelaars A, Bel F, Heijnen CJ. Regeneration of the ischemic brain by engineered stem cells: fuelling endogenous repair processes. Brain Research Reviews 2009;61(1):1-13. [10.1016/j.brainresrev.2009.03.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Velthoven 2011
- Velthoven CT, Kavelaars A, Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behavioral Immunology 2011;25(7):1342-8. [DOI: 10.1016/j.bbi.2011.03.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Velthoven 2012
- Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatric Research 2012;71(4 Pt 2):474-81. [DOI: 10.1038/pr.2011.64] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verina 2013
- Verina T, Fatemi A, Johnston MV, Comi AM. Pluripotent possibilities: human umbilical cord blood cell treatment after neonatal brain injury. Pediatric Neurology 2013;48(5):346-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Villa 2003
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. Journal of Experimental Medicine 2003;198(6):971-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2008
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PloS One 2008;3(5):e2213. [DOI: 10.1371/journal.pone.0002213] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004;35(7):1732-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang XL, Zhao YS, Hu MY, Sun YQ, Chen YX, Bi XH. Umbilical cord blood cells regulate endogenous neural stem cell proliferation via hedgehog signaling in hypoxic ischemic neonatal rats. Brain Research 2013;1518:26-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2016
- Wang J, Chen Y, Yang Y, Xiao X, Chen S, Zhang C, et al. Endothelial progenitor cells and neural progenitor cells synergistically protect cerebral endothelial cells from Hypoxia/reoxygenation-induced injury via activating the PI3K/Akt pathway. Molecular Brain 2016;9:12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2018
- Ward MR, Abadeh A, Connelly KA. Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease. Stem Cells Translational Medicine 2018;7(7):543-50. [DOI: 10.1002/sctm.17-0210] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1991
- Williams CE, Gunn A, Gluckman PD. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 1991;22(4):516-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2012
- Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics 2012;130(4):683-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2013
- Wu CC, Chen YC, Chang YC, Wang LW, Lin YC, Chiang YL, et al. Human umbilical vein endothelial cells protect against hypoxic-ischemic damage in neonatal brain via stromal cell-derived factor 1/C-X-C chemokine receptor type 4. Stroke 2013;44(5):1402-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wyllie 2015
- Wyllie J, Perlman JM, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2015;95:e169-201. [PMID: ] [DOI] [PubMed] [Google Scholar]
Xu 2015
- Xu L, Ryu J, Hiel H, Menon A, Aggarwal A, Rha E, et al. Transplantation of human oligodendrocyte progenitor cells in an animal model of diffuse traumatic axonal injury: survival and differentiation. Stem Cell Research & Therapy 2015;6(1):93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2016
- Yoon S, Yun A, Chang S, Park WS. Stem cells for neonatal brain disorders. Neonatology 2016;109(4):377-83. [DOI] [PubMed] [Google Scholar]
Zhao 1996
- Zhao W, Richardson JS, Mombourquette MJ, Weil JA, Ijaz S, Shuaib A. Neuroprotective effects of hypothermia and U-78517F in cerebral ischemia are due to reducing oxygen-based free radicals: an electron paramagnetic resonance study with gerbils. Journal of Neuroscience Research 1996;45(3):282-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhu 2009
- Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics 2009;124(2):e218-26. [PMID: ] [DOI] [PubMed] [Google Scholar]


