Abstract
Background
Alpha-tocopherol, a well-known antioxidative agent, may have a positive effect on bone formation during the remodeling phase of secondary fracture healing. Fracture healing and osseointegration of implants share common biological pathways; hence, alpha-tocopherol may enhance implant osseointegration.
Questions/purposes
This experimental study in rats assessed the ability of alpha-tocopherol to enhance osseointegration of orthopaedic implants as determined by (1) pull-out strength and removal torque and (2) a histomorphological assessment of bone formation. In addition, we asked, (3) is there a correlation between the administration of alpha-tocopherol and a reduction in postoperative oxidative stress (as determined by malondialdehyde, protein carbonyls, reduced and oxidized glutathione and their ratio, catalase activity and total antioxidant capacity) that develops after implantation of an orthopaedic implant?
Methods
This blinded study was performed in study and control groups, each consisting of 15 young adult male Wistar rats. On Day 0, a custom-designed stainless-steel screw was implanted in the proximal metaphysis of both tibias of all rats. On Day 1, animals were randomized to receive either alpha-tocopherol (40 mg/kg once per day intraperitoneally) or saline (controls). Animals were treated according to identical perioperative and postoperative protocols and were euthanized on Day 29. All animals completed the study and all tibias were suitable for evaluation. Implant pullout strength was assessed in the right tibias, and removal torque and histomorphometric evaluations (that is, volume of newly formed bone surrounding the implant in mm3, percentage of newly formed bone, percentage of bone marrow surrounding the implant per optical field, thickness of newly formed bone in μm, percentage of mineralized bone in newly formed bone, volume of mature newly formed bone surrounding the implant in mm3 and percentage of mineralized newly formed bone per tissue area) were performed in the left tibias. The plasma levels of alpha-tocopherol, malondialdehyde, protein carbonyls, glutathione, glutathione disulfide, catalase, and the total antioxidant capacity were evaluated, and the ratio of glutathione to oxidized glutathione was calculated.
Results
All parameters were different between the alpha-tocopherol-treated and control rats, favoring those in the alpha-tocopherol group. The pullout strength for the alpha-tocopherol group (mean ± SD) was 124.9 ± 20.7 newtons (N) versus 88.1 ± 12.7 N in the control group (mean difference -36.7 [95% CI -49.6 to -23.9]; p < 0.001). The torque median value was 7 (range 5.4 to 8.3) versus 5.2 (range 3.6 to 6 ) N/cm (p < 0.001). The newly formed bone volume was 29.8 ± 5.7 X 10-3 versus 25.2 ± 7.8 X 10-3 mm3 (mean difference -4.6 [95% CI -8.3 to -0.8]; p = 0.018), the percentage of mineralized bone in newly formed bone was 74.6% ± 8.7% versus 62.1% ± 9.8% (mean difference -12.5 [95% CI -20.2 to -4.8]; p = 0.003), the percentage of mineralized newly formed bone per tissue area was 40.3 ± 8.6% versus 34.8 ± 9% (mean difference -5.5 [95% CI -10.4 to -0.6]; p = 0.028), the glutathione level was 2 ± 0.4 versus 1.3 ± 0.3 μmol/g of hemoglobin (mean difference -0.6 [95% CI -0.9 to -0.4]; p < 0.001), the median glutathione/oxidized glutathione ratio was 438.8 (range 298 to 553) versus 340.1 (range 212 to 454; p = 0.002), the catalase activity was 155.6 ± 44.6 versus 87.3 ± 25.2 U/mg Hb (mean difference -68.3 [95% CI -95.4 to -41.2]; p < 0.001), the malondialdehyde level was 0.07 ± 0.02 versus 0.14 ± 0.03 μmol/g protein (mean difference 0.07 [95% CI 0.05 to 0.09]; p < 0.001), the protein carbonyl level was 0.16 ± 0.04 versus 0.27 ± 0.08 nmol/mg of protein (mean difference -0.1 [95% CI 0.05 to 0.15]; p = 0.002), the alpha-tocopherol level was 3.9 ± 4.1 versus 0.9 ± 0.2 mg/dL (mean difference -3 [95% CI -5.2 to -0.7]; p = 0.011), and the total antioxidant capacity was 15.9 ± 3.2 versus 13.7 ± 1.7 nmol 2,2-diphenyl-1-picrylhydrazyl radical/g of protein (mean difference -2.1 [95% CI -4.1 to -0.18]; p = 0.008).
Conclusions
These results using an in vivo rat model support that postoperatively administered alpha-tocopherol can enhance the osseointegration of an orthopaedic implant, although a cause and effect relationship between the administration of alpha-tocopherol and a reduction in postoperative stress cannot be securely established.
Clinical Relevance
These findings suggest that postoperative administration of alpha-tocopherol is a promising approach to enhance osseointegration of orthopaedic implants in patients. Further studies with different animal models and/or different implants and those evaluating the alpha-tocopherol dose response are needed before performing clinical trials that will examine whether these promising, preliminary results can be extrapolated to the clinical setting as well.
Introduction
Oxygen free radicals are considered the major common and final pathway of tissue injury in different organ systems [43]. Oxygen free radicals contribute to the development of oxidative stress, which is a chain of oxidative events that leads to increased production of reactive oxygen species that cause tissue injury [51]. After a fracture, oxidative stress injury occurs and may be caused by an ischemia-reperfusion mechanism [26, 38, 45]. Activated polymorphonuclear neutrophils produce oxygen free radicals during the inflammatory phase of fracture healing [4, 49]. This process is further enhanced by impairment of the blood supply to the bone ends [4, 41]. These free radicals also inhibit fracture healing [4, 20] by initiating a chain reaction that will cause lipid peroxidation, which in turn leads to cell membrane damage and eventually cell lysis [4, 49]. Implantation of an orthopaedic device leads to the same ischemia-reperfusion pattern seen after a fracture, at least regarding the initial host response [33, 46, 55, 56]. As a result, oxidative stress is likely to occur after implantation of an orthopaedic device.
Vitamin E is a lipid-soluble molecule with well-known antioxidant properties [4, 7], which protects the cellular membranes by reacting with lipid radicals produced during lipid peroxidation chain reactions [4, 68]. Among vitamin E isomers, alpha-tocopherol has the highest bioavailability [1, 50] because it is absorbed by the body [5, 50, 59]. Furthermore, alpha-tocopherol may exert a substantial (potentially positive) effect on bone formation during the normal bone remodeling phase of secondary fracture healing, thus enhancing fracture healing [17, 34]. Because fracture healing and implant osseointegration share common biological pathways (such as osteoinduction and osteoconduction) [2], alpha-tocopherol may enhance implant osseointegration as well.
This experimental study in rats aimed to assess the ability of alpha-tocopherol to enhance osseointegration of orthopaedic implants as determined by (1) pull-out strength and removal torque and (2) a histomorphological assessment of bone formation. In addition, we asked, (3) is there a correlation between the administration of alpha-tocopherol and reduction in postoperative oxidative stress (as determined by malondialdehyde, protein carbonyls, reduced and oxidized glutathione and their ratio, catalase activity and total antioxidant capacity) that develops after implantation of an orthopaedic implant.
Materials and Methods
This blinded, in vivo animal study was approved by the Department of Animal Health and Welfare, Veterinarian Drugs, and Applications of our institution (Ref. 400864/4401; December 30, 2014) and it was performed in alpha-tocopherol-treated and control groups, each consisting of 15 young adult 12-week-old male Wistar rats. This study was performed in male rats only, since gender is a factor that affects the response to oxidative stress and female rats often suffer less oxidative damage than males [8]. We used the same pharmaceutical perioperative protocol for all animals, which had unrestricted access to commercial food and water, lived in an environment with a controlled temperature (22°C) and humidity (50%), and were exposed to the same period of light daily (12 hours).
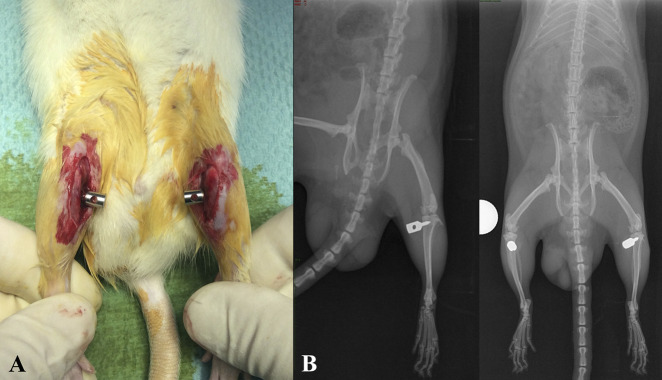
On Day 0, a custom-designed stainless steel 316 L screw (Sanmac®, Sandviken, Sweden) was implanted in the proximal metaphysis of the tibias, with the rats under general anesthesia (Fig. 1A-B). The screw was manufactured to adapt to the length of the transverse axis of the rat tibia (which is < 7 mm) and to penetrate the medial but not the lateral cortex. Anesthesia was induced with intraperitoneal ketamine, 70 mg/kg of body weight (Ketaset®, Pfizer/FDAH, Fort Dodge, IA, USA) and xylazine, 10 mg/kg of body weight (Sedaxylan®, Bimeda, Dublin, Ireland) [10]. Preoperatively, as antibiotic prophylaxis, the animals also received one intraperitoneal dose of 10 mg/kg of body weight of enrofloxacin 0.5% (Baytril®, Bayer Animal Health GmbH, Leverkusen, Germany). A self-drilling and self-tapping screw was implanted 0.5 cm distally to the superior articular surface of the tibia and perpendicular to the long axis of the tibia and was directed from medially to laterally (Fig. 2A-B). The entry point of the screw was made with a 21-gauge needle. Analgesia was administered via four doses of 80 μg/kg body weight of fentanyl (Sublimaze®, Janssen-Cilag, Macquarie Park, Australia). The first dose was given immediately before sedation, the second after the procedure was completed, and the final two every 15 minutes thereafter [10]. The wound was closed with a nonabsorbable 3-0 suture (Prolene®, Ethicon/Johnson & Johnson, Cornelia, GA, USA). The sutures were removed on Day 10.
Fig. 1 A-B.
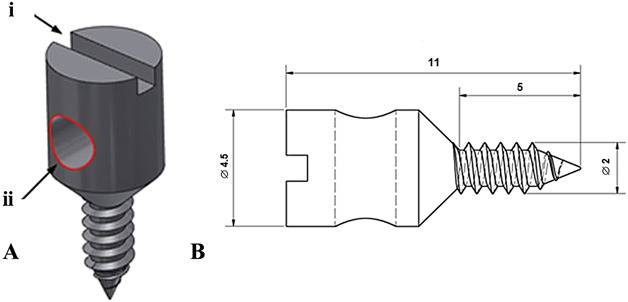
This figure shows the custom-designed 316 L (Sanmac®, Sandviken, Sweden, ASTM: MT 316L) stainless steel screw that was used to evaluate pull-out strength and torque. (A) This illustration of the screw shows an indentation (i) for the screwdriver and a hole (ii) that was used to fix the screw to the test rigs. (B) This image is a graphic diagram of the screw (Scale bar = 2 mm, all dimensions are in mm).
Fig. 2 A-B.
(A) This intraoperative photograph shows the position of the screws in the proximal tibias of the rats. (B) These standard latero-lateral and AP radiographs were taken immediately postoperatively and depict the proper position of the stainless steel screw at the proximal tibia.
On Day 1, animals were randomly assigned [29] to receive either 40 mg/kg body weight of alpha-tocopherol (Nepalm Vitamine E, 100 mg/2ml, Cenexi, France) once per day intraperitoneally (alpha-tocopherol group) or the same volume of saline (control group). The alpha-tocopherol and saline injections were prepared and administered daily. All animals were labeled in a blinded manner to ensure that we were not aware of which animal belonged to which group. Each animal was weighed at the end of each week of the experiment, and the dosage of alpha-tocopherol or saline was adjusted accordingly. On Day 29, all animals were euthanized by exsanguination (cardiac puncture) under general anesthesia, using the same procedure as was used preoperatively [3, 10]. After the study was completed, the results of each animal were unblinded and statistically evaluated by a third-party, non-author statistician (AT).
Osseointegration Evaluation
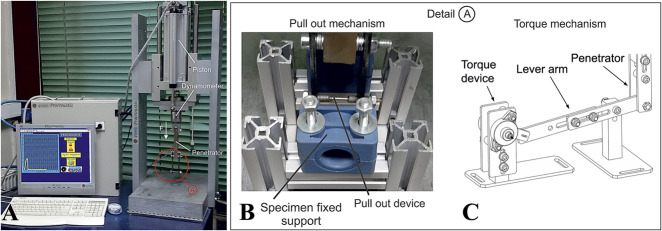
After the rats were euthanized, both tibias were harvested and stripped clear from the surrounding soft tissues. They were placed in a gauze dressing that was soaked in ambient-temperature saline. The implants’ pullout strength and torque were assessed within the first 2 hours after the animals were euthanized, using a custom-designed and manufactured device named “Procroustis” (Laboratory for Machine Tools and Manufacturing Engineering, Department of Mechanical Engineering, Aristotle University of Thessaloniki, Thessaloniki, Greece). This device (Fig. 3A) automatically records the applied force during pulling out (Fig. 3B) or rotating (Fig. 3C) of the screw that is inserted into the bone. The force and the displacement are recorded and displayed as a diagram at the monitor of a computer that is part of the whole device. The force is measured by a Kistler dynamometer (Kistler Group, Winterthur, Switzerland) that is placed under the movement placard of the piston and above the penetrator. The analog signal of the measurement device is turned into a digital signal by an AT converter. The digital control, the data processing, the recording and the results presentation were achieved by a program based on Labview software (National Instruments, Austin, TX, USA). The device can run torsion tests after proper modification (Fig. 3C). Pullout strength was measured in right tibias and maximum removal torque was measured in left tibias.
Fig. 3 A-C.
(A) This figure depicts the experimental device ‘Procroustis’, used for pull-out and torque tests. (B) Detail A of the device with modification for the pull-out tests. (C) Detail A of the device with modification for the torque tests.
Histomorphometric Analysis
After assessing torque in each left tibia, we fixed the bone (with the screw still implanted, since during the torque test, only a slight rotation of the screw was applied until the torque measurement was completed) in a buffered formalin solution (10%) for 1 month in preparation for histomorphometric analysis. Then, tibias were washed in tap water and demineralized in hydrochloric acid (< 7%) for 5 to 8 days. Subsequently, the implants were carefully removed from the bone and tibias were washed in running water for 6 hours, dehydrated in a series of graded alcohol solutions and processed for embedding in paraffin. Semi-serial sections with a thickness of 7 μm were cut along the major axis of the tibia (longitudinal sections) and a plane parallel to the long axis of the screw cavity, using thin-sectioning microtomy (Microm HM315, Rankin Biomedical Corp, Holly, MI, USA). One in 20 consecutive sections (18 to 22 sections) contained in the central part of the implant site were collected and used to estimate each parameter. Sections were placed on superfrost-plus slides (Thermo Scientific, Braunschweig, Germany) for histologic evaluations, rehydrated with decreasing concentrations of ethanol, and treated with two staining techniques: standard hematoxylin and eosin and Masson staining. The Masson trichrome stained mineralized bone in green/turquoise and unmineralized bone (osteoid) in orange/red.
Histologic evaluations were performed using a light microscope (Eclipse TS 100; Nikon, Tokyo, Japan, with X2 and X4 magnification) equipped with a digital camera (Nikon Digital Sight Fi1-L2, Tokyo, Japan) connected to a computer. The histomorphometric data were analyzed using ImageJ software for microscopic images (version 1.51f, National Institutes of Health, Bethesda, MD, USA). Hematoxylin and eosin and Masson trichrome-stained sections were photomicrographed, and 18 to 22 bone areas for each animal were analyzed.
Bone histomorphometry analysis was performed according to the method of Parfitt et al. [40], as updated by Dempster et al. [15]. The following parameters were estimated: the volume of newly formed bone (mm3) surrounding the implant, the percentage of newly formed bone and the percentage of bone marrow surrounding the implant per optical field, the thickness of newly formed bone (μm), the percentage of mineralized bone in newly formed bone, the volume of mineralized newly formed bone (mm3) surrounding the implant, and the percentage of mineralized newly formed bone per tissue area.
Quantitative data were pooled to obtain the mean ± SD for each group and reported according to the guidelines of the American Society of Bone and Mineral Research [15]. Specifically, 18 to 22 equidistant stained sections from the central part of the implant site on each tibia were used for histomorphometric analysis. In each section, the surrounding peri-implant area was demarcated at low-power magnification (X2) with the aid of the Nikon digital camera and then the appropriate software, already mentioned, and photomicrographs were captured. In each photo a 13.653mm2 (or 1,228,800 pixels) square was drawn around the empty cavity left by the screw and the peri-implant area, which corresponded to the optical field. The area of compact bone, trabecular bone, and bone marrow in the same square represented the tissue area and were calculated also. First, in this square, we measured the area of compact (or lamellar) bone that was in direct contact with the threads of the screw before its removal, and this area represented the newly formed bone. Its volume was calculated with the following formula: [(area of the peri implant compact bone in each section) X 0.007 mm] and expressed as the volume of newly formed bone (in mm3) per optical field. The ratio of the area of newly formed bone divided by the area of the square corresponds to the percentage of newly formed bone surrounding the implant per optical field. Peri-implant tissue area apart from newly formed bone consisted of bone marrow and trabecular bone. The area covered by bone marrow adjacent to the newly formed bone and divided by the area of the above mention square, represented the percentage of bone marrow in an optical field. The thickness of newly formed bone (μm) was the width of compact bone, which was in direct contact with the threads of the screw.
In Masson trichrome-stained sections the green/turquoise pixels in the area of the newly formed bone were translated into the mineralized newly formed bone, whereas the red pixels represented the osteoid or unmineralized newly formed bone. Photomicrographs were captured again under x 4 magnification focusing on areas of the newly formed bone and analyzed with ImageJ software. The ratio of the green/turquoise area to the corresponding area of newly formed bone is the percentage of mineralized bone in newly formed bone. This percentage multiplied by the volume of newly formed bone, already calculated above for each section, represented the volume of mineralized newly formed bone (in mm3). Similarly, the ratio of the same green/turquoise area to the tissue area represented the percentage of mineralized newly formed bone in tissue area.
Biomarkers Assessment Protocols
Blood samples were obtained by cardiac puncture during the euthanasia procedure [3, 10]. The following oxidative stress biomarkers were evaluated: catalase activity and the concentrations of glutathione and oxidized glutathione were measured in red blood cell lysate, whereas protein carbonyls, malondialdehyde, and the total antioxidant capacity were measured in plasma [32, 61-63]. The hemoglobin concentration of the red blood cell lysate samples was measured using a commercially available kit (HUMAN Diagnostics Worldwide, Wiesbaden, Germany). The total protein concentration of the plasma samples was determined using the Bradford reagent. Each assay was performed in triplicate, and the blood samples were stored in multiple aliquots at -80° C and thawed once before analysis. All reagents were manufactured by Sigma-Aldrich (St. Louis, MO, USA).
In an EDTA tube, 500 μl of whole blood was added, the samples were centrifuged (1370 g for 10 minutes at 4° C), and the blood plasma was collected and used to determine protein carbonyls, malondialdehyde in the form of thiobarbituric acid reactive substances, the total antioxidant capacity, and the total protein concentration. Then, an equal volume of distilled H2O was added to the packed erythrocytes, the samples were centrifuged (4000 g for 15 minutes at 4 °C), and the supernatant, which was the red blood cell lysate, was collected and used to determine catalase activity and the hemoglobin concentration. Next, 100 μl of 15% trichloroacetic acid was added to 100 μl of erythrocyte lysate, and the samples were centrifuged (14,000 g for 3 minutes at room temperature). The clear supernatant was collected and used to measure the reduced glutathione concentration. Immediately after blood collection, 60 μl of N-ethylamaleimide (310 mM in ethanol with a pH of 7.4) was added to 200 μl of whole blood, the samples were centrifuged (1370 g for 10 minutes at 4° C), and the supernatant (plasma) was discarded. Then, an equal volume of distilled H2O was added to the packed erythrocytes, the samples were centrifuged (4000 g for 15 minutes at 4° C), and the supernatant red blood cell lysate was collected. Subsequently, 100 μl of 15% trichloroacetic acid was added to 100 μl of the red blood cell lysate samples and centrifuged (14,000 g for 3 minutes at room temperature). The supernatant was collected and treated with three volumes of dichloromethane (typically 250 μl of supernatant for 750 μl of dichloromethane), shaken in a vortex shaker for 5 minutes at room temperature, and centrifuged (14,000 g for 2 minutes at room temperature). The supernatant was collected and used to evaluate the oxidized glutathione concentration.
Protein Carbonyls
Protein oxidation is a common damaging process after oxidative stress. The plasma protein carbonyls is a relatively stable marker of overall protein oxidation, representing an irreversible form of protein modification [67]. Due to this stability, it is one of the most valuable biomarkers assessing oxidative stress.
A volume of 50 μL of 20% trichloroacetic acid was added to 50 μL of plasma, and this mixture was incubated in an ice bath for 15 minutes and centrifuged (15,000 g for 5 minutes at 4° C). The supernatant was discarded and 500 μL of 10 mM 2,4-dinitrophenylhydrazine (in 2.5 N of hydrogen chloride) for the sample, or 500 μL of 2.5 N of hydrogen chloride for the blank, was added to the pellet. The samples were incubated in the dark at room temperature for 1 hour with intermittent vortexing every 15 minutes, and then they were centrifuged (15,000 g for 5 minutes at 4° C). The supernatant was discarded, 1 mL of 10% trichloroacetic acid was added, and the samples were vortexed and centrifuged (15,000 g for 5 minutes at 4° C). The supernatant was discarded, 1 mL of ethanol-ethyl acetate (1:1 v/v) mixture was added, and the samples were vortexed and centrifuged (15,000 g for 5 minutes at 4° C). This washing step was repeated twice. The supernatant was discarded and 1 mL of 5 M urea (pH = 2.3) was added, vortexed, and incubated at 37° C for 15 minutes. The samples were centrifuged (15,000 g for 5 minutes at 4° C) and the absorbance was monitored at 375 nm. The concentration of 2,4-dinitrophenylhydrazine was calculated based on the millimolar extinction coefficient of malondialdehyde (22 L/mmol/cm) and is presented as nmol/mg of protein [13, 61-63].
Malondialdehyde
Malondialdehyde (MDA) is an endogenous genotoxic product of enzymatic and oxygen radical-induced lipid peroxidation and a potentially important contributor to protein damage and mutation [36]. It is one of the most frequently used indicators of lipid peroxidation [37].
A volume of 100 μL of plasma was mixed with 500 μL of 35% trichloroacetic acid and 500 μL of tris-hydrogen chloride (200 mM, pH = 7.4) and incubated for 10 minutes at room temperature. One milliliter of 2 M Na2SO4 and 55 mM of thiobarbituric acid solution were added and the samples were incubated at 95 °C for 45 minutes. The samples were cooled on ice for 5 minutes and mixed after 1 mL of 70% trichloroacetic acid was added. The samples were centrifuged (15,000 g for 3 minutes) and the absorbance of the supernatant was monitored at 530 nm. The concentration of malondialdehyde was calculated based on the millimolar extinction coefficient of malondialdehyde (156 L/mmol/cm) and is presented as μmol of malondialdehyde/g of protein [14, 61-63].
Reduced Glutathione and Oxidized Glutathione
Glutathione is a water-soluble tripeptide composed of the amino acids glutamine, cysteine, and glycine. Glutathione is an important antioxidant that protects cells from damage due to excessive amounts of reactive O2 species [58]. Under oxidative stress conditions, glutathione is transformed to oxidized glutathione, and the ratio of glutathione/oxidized gluatathione is altered. The latter is used as an important marker of oxidative stress [69].
A volume of 20 μL of the trichloroacetic acid-treated red blood cell lysate for the samples or 20 μL of dH2O for the blank was mixed with 660 μL of phosphate buffer (67 mM, pH = 7.95, containing KH2PO4 and Na2HPO4) and 330 μL of 1 mM 5,5-dithiobis (2-nitrobenzoic acid). The mixture was incubated for 45 minutes in the dark at room temperature and the absorbance was monitored at 412 nm. The concentration of glutathione was calculated based on the millimolar extinction coefficient of 5,5-dithiobis (2-nitrobenzoic acid) (13.6 L/mmol/cm) and is presented as μmol of glutathione/g of hemoglobin [22, 61-63].
A volume of 925 μL of phosphate buffer (200 mM, pH = 7.4, containing KH2PO4 and Na2HPO4), 5 μL of 20 mM of 5,5-dithiobis (2-nitrobenzoic acid) in phosphate buffer, 20 μL of the N-ethylamaleimide-treated red blood cell lysate, and 20 μL of 4.8 mM nicotinamide adenine dinucleotide phosphate in 0.5% NaHCO3 solution for the samples were added in a cuvette. The blank cuvette contained 20 μL of 15% trichloroacetic acid instead of the red blood cell lysate, and the standard cuvette contained 935 μL of phosphate buffer and 10 μL of 10 mM oxidized glutathione solution (dissolved in fourfold diluted phosphate buffer) instead of the red blood cell lysate. The mixtures were incubated for 15 minutes at room temperature; then, a 20 mU/mL solution of glutathione reductase (dissolved in fourfold diluted phosphate buffer) was rapidly added and the absorbance was monitored at 412 nm for 1 minute. The oxidized glutathione concentration was calculated by subtracting the absorbance change of the blank from the absorbance change of the sample in 1 minute, dividing by the absorbance change of the standard. This value is expressed as nmol of oxidized glutathione/g of hemoglobin [22, 61-63]. The glutathione/oxidized glutathione ratio was also calculated.
Catalase
Catalase is a potent antioxidant enzyme detoxifying H2O2. It can be found between blood cells and plasma. Its action renders it a valuable marker of oxidative stress activity [57].
A volume of 4 μL of red blood cell lysate diluted 1/10 in distilled H2O was added to 2991 μL of phosphate buffer (67 mM, pH = 7.4), and the mixture was incubated for 10 minutes at room temperature. Next, 5 μL of 30% H2O2 was added and the absorbance was monitored at 240 nm for 2 minutes. We determined that the catalase activity was based on the molar extinction coefficient of H2O2 (40 L/mol/cm), which is expressed as U/mg of hemoglobin. One unit is 1 mmol/min [23, 61-63].
Total Antioxidant Capacity
Total antioxidant capacity evaluates the cumulative action of all the antioxidants present in plasma and body fluids, thus providing an integrated parameter rather than the simple sum of measurable antioxidants. As a result, total antioxidant capacity assesses the synergistic interaction between known and unknown antioxidants, thus providing a valuable insight into the in vivo balance between oxidants and antioxidants [21, 51].
A volume of 20 μL of plasma was added to 480 μL of 10 mM sodium potassium phosphate buffer (pH = 7.4) and 500 μL of 0.1 mM 2,2-diphenyl-1-picrylhydrazyl radical, and the samples were incubated in the dark for 30 minutes at room temperature. Then, the samples were centrifuged (20,000 g for 3 minutes) and the absorbance was monitored at 520 nm. The total antioxidant capacity is presented as nmol of 2,2-diphenyl-1-picrylhydrazyl radical reduced/g of protein [51-52, 61-63].
Alpha-tocopherol
A volume of 200 μL of ethanol was added to 100 μL of blood plasma to precipitate proteins, and then 500 μL hexan was added as an extraction solvent. After vortex-mixing and centrifugation (12,000 rpm for 15 minutes), 700 μL of supernatant was dried under nitrogen stream. The dry residues were resuspended in 100 μL of solvent (methanol-water, 95-5 [v/v]), followed by filtration and an injection [9]. All samples were analyzed using the Thermo HPLC Spectra System P4000 (Thermo Scientific, Waltham, MA, USA). Detection and identification were performed using a UV-Visible detector (UV2000, Thermo Scientific). The reversed-phase analytical column, Zorbax XDB-C 18 (2.1 x 50 mm, Agilent, Santa Clara, CA, USA), was operated at ambient temperature. Isocratic elution and methanol-water 95-5 (v/v) as the mobile phase were applied. The wavelength absorption was 292 nm. The sample injection volume and flow rate were 20 μL and 0.6 mL/min, respectively.
Animal Follow-up
All rats completed the experimental period successfully and uneventfully. There were no tissue healing problems or fractures at the operative sites. The blood samples taken during the euthanasia procedure were adequate for the biochemical evaluations, and all harvested tibias were suitable for biomechanical and histomorphometric evaluations.
The body weights of the alpha-tocopherol-treated animals (326.00 ± 22.30 g) and control animals (332.33 ± 19.81 g) did not differ on Day 0 (95% CI -27.18 to 14.49; p = 0.739). The body weights of animals in both groups increased by the end of the experiment (alpha-tocopherol group: 370.67 ± 17.10 g; controls: 366.00 ± 19.57 g); p < 0.001 in both comparisons. The weight mean difference in Day 0 was -44.67 ± 12.17 g (95% CI -51.41 to -37.93), while at the end of the experiment (Day 29) it was -33.67 ± 10.77 g (95% CI -39.63 to -27.70). No difference was indicated between the weights of the alpha-tocopherol treated rats and those of the control animals on Day 29, with a mean difference of 4.67 g (95% CI -14.23 to 23.64; p = 0.820).
Statistical Analysis
Parametric and non-parametric statistical methods were applied to statistically evaluate the experimental results. Assumptions of normality and homogeneity of variance were tested using the Shapiro-Wilk and Levene’s tests, respectively. Sometimes, to evaluate assumptions of normality and stability of variance, we transformed data to common logarithms or square roots. When there was normality and variance in homogeneity, we performed a one-way ANOVA to evaluate the possible major effects of treatment. Differences between the mean values of specific treatments were evaluated using post-hoc multiple comparison tests, namely, Tukey’s test and the Duncan multiple range test. Where assumptions about either variability or the form of the population’s distribution were seriously violated, with or without transformed data, we evaluated treatment-dependent differences with the Kruskal-Wallis non-parametric test. Differences between the mean values of specific treatments were evaluated using the Wilcoxon rank-sum test (Mann-Whitney U test). To statistically evaluate the weight of the animals, we applied a repeated-measures ANOVA, and we analyzed the interaction between different levels of treatment and the two timepoints. For the histomorphometric evaluation, the statistical analysis included the mean and SD. All values were proven to have homogeneity of variance (Levene’s test) before two-tailed t tests were performed in the independent groups. All analyses were performed using the statistical software program SPSS version 20.0 (IBM Corp, Armonk, NY, USA). Significance was determined by a p value ≤ 0.05.
We conducted a post-hoc power analysis for the one-way ANOVA using the software package G*Power (Heinrich Heine Univesität, Düsseldorf, Germany) [18, 19] to confirm that the sample sizes would offer adequate statistical power to detect the effect of a specific treatment on the primary endpoints of pullout strength and removal torque of the implants.
Results
Biomechanical Evaluation
The mean value of pullout strength for the alpha-tocopherol group and its SD was 124.9 ± 20.7 newtons (N) versus 88.1 ± 12.7 N in the control group with a mean difference of -36.7 (95% CI bounds vary from -49.6 to -23.9; p < 0.001) (Fig. 4A). The torque median value was 7 (range 5.4 to 8.3) N/cm versus 5.2 (range 3.6 to 6) N/cm (p < 0.001) (Fig. 4B). This suggested better osseointegration of the implants in animals in the alpha-tocopherol group that postoperatively received alpha-tocopherol (Fig. 5).
Fig. 4 A-B.
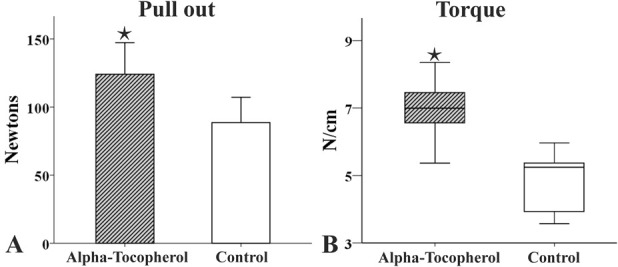
(A) This bar graph illustrates the pullout strength, in Newtons, in control and alpha-tocopherol-treated animals. The bars represent the mean value and the error bars represent the SD. (B) This graph illustrates the torque, in N/cm, in control and alpha-tocopherol treated animals. The boxplots represent the median value (50th percentile) and the range of torque (* represents p < 0.05).
Fig. 5 A-B.
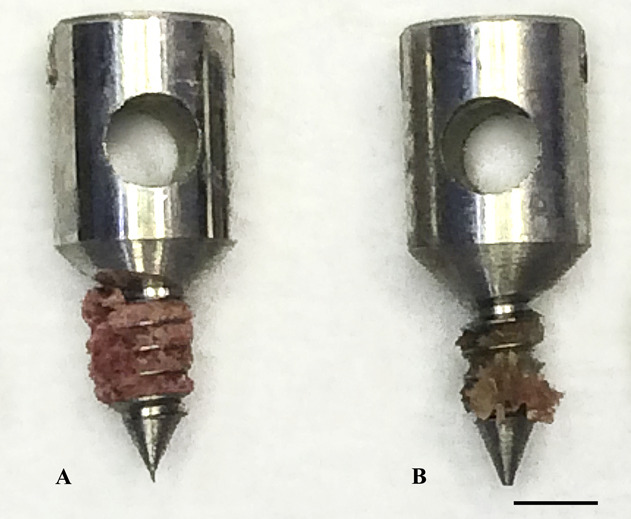
These photographs show pull-out screws in (A) specimens treated with alpha-tocopherol and (B) control specimens after implantation and mechanical testing (pull-out testing). There is more bone in the threads of the screw from the treated specimen than in the threads of the screw from the control specimen.
Histomorphometric Evaluation of Implant Osseointegration
All implants in both groups directly contacted the surrounding bone, with no signs of inflammation at the bone-implant interface. However, better osseointegration was achieved in rats in the alpha-tocopherol group than in the control group (6A-B). The mean volume of newly formed bone surrounding the implant in the alpha-tocopherol group was 29.8 ± 5.7 X 10-3 mm3 versus 25.3 ± 7.8 X 10-3 mm3 in the control group (with a mean difference of -4.6, 95% CI bounds vary from -8.3 to -0.8, p = 0.018). Animals receiving alpha-tocopherol had approximately 4.6 X 10-3 mm3 more newly formed surrounding bone than control rats. The mean percentage of the area of newly formed bone surrounding the implant (alpha-tocopherol group: 31.2% ± 6. 1% versus controls: 26.4% ± 8.2%, with a mean difference -4.8 [95% CI -8.7 to -0.9]; p = 0.018) and the mean percentage of the area of adjacent bone marrow per optical field (alpha-tocopherol group: 27.5% ± 8.6% versus controls: 20.4% ± 6%, with a mean difference -7.1 [95% CI -11.3 to -2.8]; p = 0.002) were higher in the alpha-tocopherol group than in the control group (Fig. 7A). The mean thickness of the newly formed bone was higher in the alpha-tocopherol group than in the control group (51.9 ± 25.5 μm and 30.7 ± 14.9 μm, respectively, with a mean difference -21.2 [95% CI -25.3 to -17.1]; p < 0.001) (Fig. 7B). The mean percentage of mineralized bone in newly formed bone (alpha-tocopherol group: 74.6% ± 8.7% versus controls: 62.1% ± 9.8%, with a mean difference -12.5 [95% CI -20.2 to -4.8]; p = 0.003) (Fig. 7C) and the mean volume of the mineralized newly formed bone surrounding the implant (alpha-tocopherol group: 21.6 ± 5.9 X 10-3 mm3 versus controls: 15.7 ± 4.8 X 10-3 mm3, with a mean difference -6.7 [95% CI -9.2 to -4.2]; p ˂ 0.001) were also higher in the alpha-tocopherol group than in the control group. Finally, in the alpha-tocopherol group, the mean percentage of mineralized newly formed bone per tissue area was elevated compared with the control (40.3 ± 8.6% versus 34.8% ± 9%, with a mean difference -5.5 [95% CI -10.4 to -0.6]; p = 0.028) (Fig. 7D).
Fig. 6 A-D.
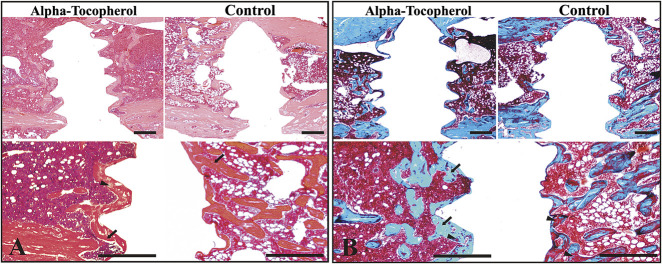
Photomicrographs show histologic sections of rats in alpha-tocopherol and control groups. The sections were stained with either (A) hematoxylin and eosin or (B) Masson trichrome stains. (A) The rats in the alpha-tocopherol group show more continuous direct bone apposition surrounding the implant and thicker newly formed bone than the control rats. At a higher magnification, the mature bone is presented as a compact structure with a reddish orange color (arrows). The immature bone is displayed as light pink (arrowheads). The nuclei are stained blue. (B) Masson trichrome staining allows for identification of mineralized bone tissue by different coloring. Bone structures of mineralized bone are shown in turquoise (arrows), whereas the connective tissue in the newly formed bone (osteoid) is displayed in red (arrowheads). The rats in the alpha-tocopherol group had more mineralized newly formed bone surrounding the implant (turquoise area) and increased cellular density of the bone marrow compared to rats in the control group. (Scale bar = 0.5 mm).
Fig. 7 A-B.
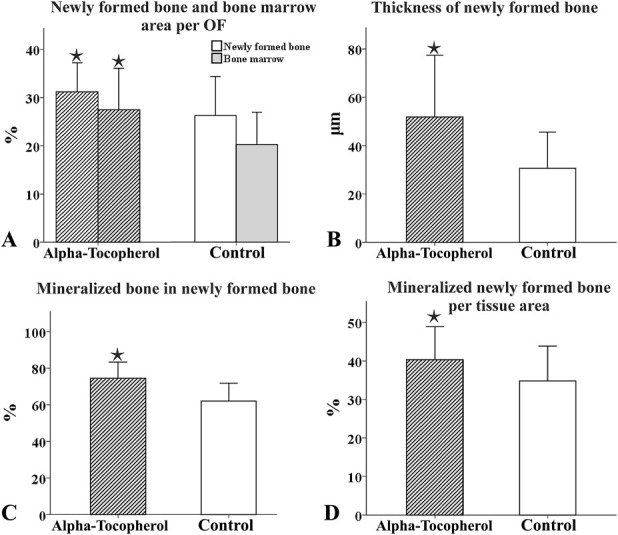
These bar graphs depict differences in the histomorphometric parameters between the alpha-tocopherol and control groups. The graphs illustrate in (A) the percentage area of newly formed bone surrounding the implant and the percentage area of adjacent bone marrow, per optical field (OF), in (B) the thickness of newly formed bone (µm) in (C) the percentage of mineralized bone to newly formed bone in (D) the percentage of mineralized newly formed bone per tissue area in alpha-tocopherol and control groups. The bars represent the mean value and the error bars the SD (*represents p < 0.05).
Biochemical Evaluation of Oxidative Biomarkers and Alpha-tocopherol
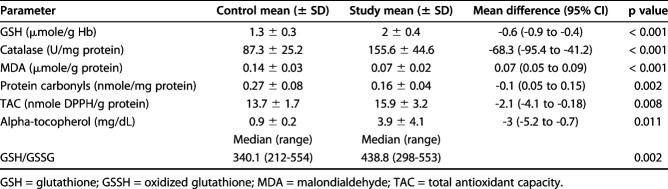
All studied parameters were different in favor of the alpha-tocopherol group, suggesting decreased postoperative oxidative stress in the animals receiving alpha-tocopherol. The glutathione level in the alpha-tocopherol group was 2 ± 0.4 versus 1.3 ± 0.3 μmol/g of hemoglobin in the control group, with a mean difference of -0.6 (95% CI bounds vary from -0.9 to -0.4, p < 0.001), the median glutathione/oxidized glutathione ratio was 438.8 in the alpha-tocopherol group (range 298 to 553) versus 340.1 (range 212 to 454; p = 0.002) in the control, the mean catalase activity in the alpha-tocopherol group was 155.6 ± 44.6 versus 87.3 ± 25.2 U/mg Hb in the control group, with a mean difference of -68.3 (95% CI -95.4 to -41.2; p < 0.001), the mean malondialdehyde level in the alpha-tocopherol group was 0.07 ± 0.02 versus 0.14 ± 0.03 μmol/g protein in the control, with a mean difference of 0.07 (95% CI 0.05 to 0.09; p < 0.001), the mean protein carbonyl level in the alpha-tocopherol group was 0.16 ± 0.04 versus 0.27 ± 0.08 nmol/mg of protein in the control group, with a mean difference of -0.1 (95% CI 0.05 to 0.15, p = 0.002), the mean alpha-tocopherol level was 3.9 ± 4.1 in the alpha-tocopherol group versus 0.9 ± 0.2 mg/dL in the control group, with a mean difference of -3 (95% CI -5.2 to -0.7; p = 0.011), and the mean total antioxidant capacity in the alpha-tocopherol group was 15.9 ± 3.2 versus 13.7 ± 1.7 nmol 2,2-diphenyl-1-picrylhydrazyl radical/g of protein in the control group, with a mean difference of -2.1 (95% CI -4.1 to -0.2; p = 0.008) (Table 1).
Table 1.
Postoperative oxidative status of animals after alpha-tocopherol administration
Discussion
Αlpha-tocopherol, a potent antioxidant agent, seems to exert a positive effect on the fracture healing procedure [17, 34]. Since osseointegration shares common biological pathways with fracture healing [2], this study assessed the ability of alpha-tocopherol to enhance osseointegration of orthopaedic implants as determined by (1) pull-out strength and removal torque and (2) a histomorphological assessment of bone formation. In addition, we examined (3) if there is a correlation between the administration of alpha-tocopherol and the reduction in postoperative oxidative stress that develops after implantation of an orthopaedic implant. Using an in vivo rat model, our results support the research question that postoperatively administered alpha-tocopherol enhances osseointegration of an orthopaedic implant. Although we found differences in all the biochemical parameters examined in favor of the alpha-tocopherol group, a cause and effect relationship between the administration of alpha-tocopherol and a reduction in postoperative stress, cannot be securely established.
As with similar studies, this one has limitations. One limitation is that an animal model was used [4, 53, 55]. However, the model chosen seemed to be useful, given that multiple evaluation procedures were implemented. Evaluating implant osseointegration immediately postoperatively in clinical studies is extremely difficult and has several limitations, and the long-term evaluation of the same procedure in a clinical setting is time-consuming, costly, necessitates a large number of patients, and can only be used to assess the long-term failure of implant-to-bone bonding (which may be the result of several causes). Although rat bone is similar to human bone [66], the results of experimental studies should be interpreted cautiously and should not be extrapolated to humans without further evaluation.
The existence of many oxidative stress markers renders the choice of markers used in a study evaluating postoperative oxidative stress relatively difficult. However, all markers were selected based on previous studies with reliable results [12, 24, 39, 44, 48]. Furthermore, the combination of all the markers chosen increased the reliability of our evaluations.
The dosage of alpha-tocopherol was also a limitation. Studies evaluating alpha-tocopherol’s anti-oxidative action had nonstandard dosages [4, 16, 27, 29, 30, 34, 64]. Avoiding the extreme high and low dosages of these prior studies, we implemented a dosage of 40 mg/kg of body weight per day. However, even this dose seems extremely high; as translated to the human 40 mg/kg would result in 2-3 g of alpha-tocopherol, when the recommended dosage of alpha-tocopherol for human consumption is about 100 international units (IUs) per day [28]. Apart from the fact that a dosage of up to 1500 IUs per day appears to be safe and even a dosage of 3200 IUs per day does not have a toxic effect in humans [28], an extrapolation of the dosage to the human based on body weight only is not correct. Such an extrapolation should be based on allometric scaling, with the introduction of a correction factor reflecting the relationship between the body weight and the body surface [35]. Based on these calculations, a dose to humans of 6.49 mg/kg of alpha-tocopherol would reflect the dosage used in this study. This dose is well above the daily requirement for humans but remains still within the nontoxic therapeutic range [28].
Another limitation of our study was the use of only two experimental groups, alpha-tocopherol-treated and saline-treated (control) rats. Though these two groups were sufficient to determine whether alpha-tocopherol enhances osseointegration of steel implants, the lack of a sham group results in an incomplete analysis of whether alpha-tocopherol suppresses postoperative oxidative stress and eliminates any causation between alterations in oxidative stress and implant osseointegration. Further, there was also not a nonsurvival group that would have helped to determine the primary stability of the implant.
The use of 12-week old rats might be considered as another limitation. As the skeletal growth of a rat does not taper off until 7-8 months, a 12-week old rat may be quite young in the musculoskeletal sense. Although the 12-week adulthood is strongly supported by the evidence [11, 42, 47, 65], it is true that at this age the musculoskeletal system is still developing. The time period of the experiment was also determined based on past studies and was considered to be adequate to evaluate the primary stability of an orthopaedic implant [4, 53, 54].
Our primary hypothesis—that the administration of alpha-tocopherol immediately postoperatively and for 28 days after implantation of a stainless steel orthopaedic implant in a rat experimental model would enhance osseointegration of the implant to the bone—seems to be confirmed by our results. This is based on biomechanical and histomorphometric data. Any agent that reduces or eliminates postoperative stress should enhance fracture healing and could positively affect implant osseointegration. Vitamin E is considered the most potent, lipid-soluble, chain-breaking antioxidant in nature [4, 7]. Based on existing animal studies, the effect of vitamin E on fracture healing remains inconclusive [4]. Nonetheless, the effect of alpha-tocopherol on bone formation during the normal bone remodeling phase of secondary fracture healing is promising, although it has not been proven so far to be effective for callous formation [4]. Based on our initial background search for studies on the issue, the most popular proposed mechanism of action for the effect of vitamin E on fracture healing is based on the cellular-protective property of the antioxidant. The protective role of vitamin E was seen in past studies in which alpha-tocopherol in the cell membranes acted as an antioxidant that inhibited lipid peroxidation by scavenging free radicals and breaking the chain reaction [6, 60]. Even vitamin E-coated implants (without systemic administration of vitamin E) showed promising, yet preliminary, results for bone-implant osseointegration [31].
A cause-and-effect relationship between the administration of alpha-tocopherol and a reduction in postoperative stress cannot be securely established by the results of this study alone and certainly requires further confirmation. The effect exerted by oxidative stress on fracture healing remains largely unknown, although it may have a negative impact. After a fracture, oxygen free radicals are produced by both the activation of polymorphonuclear neutrophils in the inflammatory phase of bone fracture healing [4, 49] and by the impairment of the blood supply to the bone’s ends [67]. These free radicals inhibit fracture healing [20, 25] by initiating a chain reaction that will cause lipid peroxidation, leading to cell membrane damage and eventually cell lysis [49]. This negative effect exerted by the oxidative stress might also negatively affect osseointegration [33].
To the best of our knowledge, this is the first study examining the ability of alpha-tocopherol to enhance osseointegration of stainless steel orthopaedic implants. By performing a blinded, biomechanical, histomorphometric, and biochemical study, we made every effort to eliminate bias. However, our results should be interpreted with caution and extrapolation of these results to humans should be supported by more research. Further studies with different animal models and/or different implants, studies evaluating the alpha-tocopherol dose response and/or evaluating differences in the sedimentation rate and/or blood coagulation (likely affected by the administration of alpha-tocopherol) should be the next step to securely reach firm conclusions. Large-scale clinical trials confirming the effect exerted by alpha-tocopherol on osseointegration will certainly be needed before moving towards a possible clinical application.
Acknowledgments
We thank Assistant Professor Alexandros Theodoridis (Dr. Vet) for his contribution to the statistical analysis of the data. We also thank Emeritus Professor George A. Kapetanos, Dr Eng Ioannis Mirisidis and Dr Eng Ioannis Zyganitidis, who contributed significantly to the completion of this study. We also thank Dr Eng Ioannis Zyganitidis, for his design of the stainless steel screw (Sanmac) used in this study. We also thank the late Professor John M. Kirkos for his support and for being a valuable source of inspiration.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the School of Veterinary Medicine, Laboratory of Physiology. Aristotle University of Thessaloniki, Thessaloniki, Greece; School of Physical Education and Sport Science at Serres and Laboratory of Exercise Physiology and Biochemistry, Aristotle University of Thessaloniki, Serres, Greece; and School of Mechanical Engineering, Laboratory of Machine Tools and Manufacturing Engineering, Aristotle University of Thessaloniki, Thessaloniki, Greece.
References
- 1.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80:1613-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10:S96-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mousawi AM, Kulp GA, Branski LK, Kraft R, Mecott GA, Williams FN, Herndon DN, Jeschke MG. Impact of anesthesia, analgesia, and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury. Shock. 2010;34:261-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borhanuddin B, Mohd Fozi NF, Naina Mohamed I. Vitamin E and the healing of bone fracture: the current state of evidence. Evid Based Complement Alternat Med. 2012;2012:684510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigelius-Flohé R, Traber M. Vitamin E: function and metabolism. FASEB J. 1999;13:1145-1155. [PubMed] [Google Scholar]
- 6.Callahan BC, Lisecki EJ, Banks RE, Dalton JE, Cook SD, Wolff JD. The effect of warfarin on the attachment of bone to hydroxyapatite-coated and uncoated porous implants. J Bone Joint Surg Am. 1995;77:225-230. [DOI] [PubMed] [Google Scholar]
- 7.Castellini C, Mourvaki E, Dal Bosco A, Galli F. Vitamin E biochemistry and function: a case study in male rabbit. Reprod Domest Anim. 2007;42:248-256. [DOI] [PubMed] [Google Scholar]
- 8.Català-Niell A, Estrany ME, Proenza AM, Gianotti M, Lladó I. Skeletal muscle and liver oxidative metabolism in response to a voluntary isocaloric intake of a high fat diet in male and female rats. Cell Physiol Biochem. 2008;22:327-336. [DOI] [PubMed] [Google Scholar]
- 9.Catignani GL, Bieri JG. Simultaneous determination of retinol and alpha-tocopherol in serum or plasma by liquid chromatography. Clin Chem. 1983;29:708-712. [PubMed] [Google Scholar]
- 10.Célèrier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465-472. [DOI] [PubMed] [Google Scholar]
- 11.Coelho MS, Passadore MD, Gasparetti AL, Bibancos T, Prada PO, Furukawa LL, Furukawa LN, Fukui RT, Casarini DE, Saad MJ, Luz J, Chiavegatto S, Dolnikoff MS, Heimann JC. High- or low-salt diet from weaning to adulthood: effect on body weight, food intake and energy balance in rats. Nutr Metab Cardiovasc Dis. 2006;16:148-155. [DOI] [PubMed] [Google Scholar]
- 12.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23-38. [DOI] [PubMed] [Google Scholar]
- 13.Davies MJ. Protein oxidation and peroxidation. Biochem J. 2016;473:805-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316-328. [DOI] [PubMed] [Google Scholar]
- 15.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieber-Rotheneder M, Puhl H, Waeg G, Striegl G, Esterbauer H. Effect of oral supplementation with D-alpha-tocopherol on the vitamin E content of human low density lipoproteins and resistance to oxidation. J Lipid Res. 1991;32:1325-1332. [PubMed] [Google Scholar]
- 17.Durak K, Sonmez G, Sarisozen B, Ozkan S, Kaya M, Ozturk C. Histological assessment of the effect of alpha-tocopherol on fracture healing in rabbits. J Int Med Res. 2003;31:26-30. [DOI] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149-1160. [DOI] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Lang AG, Buchner A. G.*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. [DOI] [PubMed] [Google Scholar]
- 20.Foschi D, Trabucchi E, Musazzi M, Castoldi L, Di Mattia D, Radaelli E, Marazzi M, Franzini P, Berlusconi A. The effects of oxygen free radicals on wound healing. Int J Tissue React. 1988;10:373-379. [PubMed] [Google Scholar]
- 21.Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29:1106-1114. [DOI] [PubMed] [Google Scholar]
- 22.Giustarini D, Colombo G, Garavaglia ML, Astori E, Portinaro NM, Reggiani F, Badalamenti S, Aloisi AM, Santucci A, Rossi R, Milzani A, Dalle-Donne I. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic Biol Med. 2017;112:360-375. [DOI] [PubMed] [Google Scholar]
- 23.Glorieux C, Calderon PB. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem. 2017;398:1095-1108. [DOI] [PubMed] [Google Scholar]
- 24.Gokturk E, Turgut A, Baycu C, Gunal I, Seber S, Gulbas Z. Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand. 1995;66:473-475. [DOI] [PubMed] [Google Scholar]
- 25.Gupta RK, Padmanabhan TV. Resonance frequency analysis. Indian J Dent Res. 2011;22:567-573. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y, Anderson JH, Long DM. Oxygen free radicals in the genesis of traumatic and peritumoral brain edema. Neurosurgery. 1989;24:679-684. [DOI] [PubMed] [Google Scholar]
- 27.Jialal I, Grundy SM. Effect of dietary supplementation with alpha-tocopherol on the oxidative modification of low density lipoprotein. J Lipid Res. 1992;33:899-906. [PubMed] [Google Scholar]
- 28.Kappus H, Diplock AT. Tolerance and safety of vitamin E: a toxicological position report. Free Radic Biol Med. 1992;13:55-74. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Shin W. How to do random allocation (randomization). Clin Orthop Surg. 2014;6:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewallen EA, Riester SM, Bonin CA, Kremers HM, Dudakovic A, Kakar S, Cohen RC, Westendorf JJ, Lewallen DG, van Wijnen AJ. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng Part B Rev. 2015;21:218-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovati AB, Bottagisio M, Maraldi S, Violatto MB, Bortolin M, De Vecchi E, Bigini P, Drago L, Romanò CL. Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clin Orthop Relat Res. 2018;476:1324-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margaritelis NV, Cobley JN, Paschalis V, Veskoukis AS, Theodorou AA, Kyparos A, Nikolaidis MG. Going retro: oxidative stress biomarkers in modern redox biology. Free Radic Biol Med. 2016;98:2-12. [DOI] [PubMed] [Google Scholar]
- 33.Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. Biology of implant osseointegration. J Musculoskelet Neuronal Interact. 2009;9:61-71. [PubMed] [Google Scholar]
- 34.Mohamad S, Shuid AN, Mokhtar SA, Abdullah S, Soelaiman IN. Tocotrienol supplementation improves late-phase fracture healing compared to alpha-tocopherol in a rat model of postmenopausal osteoporosis: a biomechanical evaluation. Evid Based Complement Alternat Med. 2012;2012:372878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016r;7:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426-31433. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209-1214. [PubMed] [Google Scholar]
- 38.Oda T, Nakai I, Mitou M, Yamagisi H, Oka T, Yoshikawa T. Role of oxygen radicals and synergistic effect of superoxide dismutase and catalase on ischemia reperfusion injury of the rat pancreas. Transplant Proc. 1992;24:797-798. [PubMed] [Google Scholar]
- 39.Pablos AB, Ramalho SA, König B, Jr, Furuse C, deAraújo VC, Cury PR. Effect of meloxicam and diclofenac sodium on peri-implant bone healing in rats. J Periodontol. 2008;79:300-306. [DOI] [PubMed] [Google Scholar]
- 40.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595-610. [DOI] [PubMed] [Google Scholar]
- 41.Paskalev MD, Goranov NV, Krastev SJ, Roydev RT. Antioxidant and bone healing effect of vitamin E in an experimental osteotomy model in dogs. Comparat Clin Pathol. 2011;20:403-408. [Google Scholar]
- 42.Prada P, Okamoto MM, Furukawa LN, Machado UF, Heimann JC, Dolnikoff MS. High-or low-salt diet from weaning to adulthood: effect on insulin sensitivity in Wistar rats. Hypertension. 2000;35:424-249. [DOI] [PubMed] [Google Scholar]
- 43.Prasad G, Dhillon MS, Khullar M, Nagi ON. Evaluation of oxidative stress after fractures. A preliminary study. Acta Orthop Belg. 2003;69:546-551. [PubMed] [Google Scholar]
- 44.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159-3165. [DOI] [PubMed] [Google Scholar]
- 45.Rangan U, Bulkley GB. Prospects for treatment of free radical mediated tissue injury. Br Med Bull. 1993;4:700-718. [DOI] [PubMed] [Google Scholar]
- 46.Rigo EC, Boschi AO, Yoshimoto M, Allegrini S, Jr, Konig B, Jr, Carbonari MJ. Evaluation in vitro and in vivo of biomimetic hydroxyapatite coated on titanium dental implants. Mater Sci Eng C. 2004;24:647-651. [Google Scholar]
- 47.Sharpe RM, Turner KJ, McKinnell C, Groome NP, Atanassova N, Millar MR, Buchanan DL, Cooke PS. Inhibin B levels in plasma of the male rat from birth to adulthood: effect of experimental manipulation of Sertoli cell number. J Androl. 1999;20:94-101. [PubMed] [Google Scholar]
- 48.Shearer DW, Youm J, Bozic KJ. Short-term complications have more effect on cost-effectiveness of THA than implant longevity. Clin Orthop Relat Res. 2015;473:1702-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr Drug Metab. 2007;8:519-525. [DOI] [PubMed] [Google Scholar]
- 50.Shuid AN, Mohamad S, Muhammad N, Fadzilah FM, Mokhtar SA, Mohamed N, Soelaiman IN. Effects of α-tocopherol on the early phase of osteoporotic fracture healing. J Orthop Res. 2001;29:1732-1738. [DOI] [PubMed] [Google Scholar]
- 51.Sies H. Total antioxidant capacity: appraisal of a concept. J Nutr. 2007;137:1493-5. [DOI] [PubMed] [Google Scholar]
- 52.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86;715-748 [DOI] [PubMed] [Google Scholar]
- 53.Skripitz R, Aspenberg P. Early effect of parathyroid hormone (1-34) on implant fixation. Clin Orthop Relat Res. 2001;392:427-432. [DOI] [PubMed] [Google Scholar]
- 54.Skripitz R, Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg Br. 2001;83:437-440. [DOI] [PubMed] [Google Scholar]
- 55.Søballe K, Hansen ES, Brockstedt-Rasmussen H, Bünger C. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J Bone Joint Surg Br. 1993;75:270-278. [DOI] [PubMed] [Google Scholar]
- 56.Søballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand Suppl. 1993;255:1-58. [DOI] [PubMed] [Google Scholar]
- 57.Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Córdova A, Tur JA, Pons A. Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res. 2005;39:1317-1324. [DOI] [PubMed] [Google Scholar]
- 58.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Acker SA, Koymans LM, Bast A. Molecular pharmacology of vitamin E: structural aspects of antioxidant activity. Free Radic Biol Med. 1993;15:311-328. [DOI] [PubMed] [Google Scholar]
- 61.Veskoukis AS, Goutianos G, Paschalis V, Margaritelis NV, Tzioura A, Dipla K, Zafeiridis A, Vrabas IS, Kyparos A, Nikolaidis MG. The rat closely mimics oxidative stress and inflammation in humans after exercise but not after exercise combined with vitamin C administration. Eur J Appl Physiol. 2016;116:791-804. [DOI] [PubMed] [Google Scholar]
- 62.Veskoukis AS, Kyparos A, Paschalis V, Nikolaidis MG. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers. 2016;21:208-217. [DOI] [PubMed] [Google Scholar]
- 63.Veskoukis AS, Nikolaidis MG, Kyparos A, Kokkinos D, Nepka C, Barbanis S, Kouretas D. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl Physiol Nutr Metab. 2008;33:1140-1154. [DOI] [PubMed] [Google Scholar]
- 64.Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, De Greeff A, Poston L, Shennan A. WHO Vitamin C and Vitamin E trial group. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116:780-788. [DOI] [PubMed] [Google Scholar]
- 65.Walker AK, Nakamura T, Hodgson DM. Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats. Stress. 2010;13:506-515. [DOI] [PubMed] [Google Scholar]
- 66.Wancket LM. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet Pathol. 2015;52:842-850. [DOI] [PubMed] [Google Scholar]
- 67.Weber D, Davies MJ, Grune T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015;5:367-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]