Abstract
Background
The benefits of surgical treatment of a metastasis of the extremities may be offset by drawbacks such as potential postoperative complications. For this group of patients, the primary goal of surgery is to improve quality of life in a palliative setting. A better comprehension of factors associated with complications and the impact of postoperative complications on mortality may prevent negative outcomes and help surgeons in surgical decision-making.
Questions/purposes
(1) What is the risk of 30-day postoperative complications after surgical treatment of osseous metastatic disease of the extremities? (2) What predisposing factors are associated with a higher risk of 30-day complications? (3) Are minor and major 30-day complications associated with higher mortality at 1 year?
Methods
Between 1999 and 2016, 1090 patients with osseous metastatic disease of the long bones treated surgically at our institution were retrospectively included in the study. Surgery included intramedullary nailing (58%), endoprosthetic reconstruction (22%), plate-screw fixation (14%), dynamic hip screw fixation (2%), and combined approaches (4%). Surgery was performed if patients were deemed healthy enough to proceed to surgery and wished to undergo surgery. All data were retrieved by manually reviewing patients’ records. The overall frequency of complications, which were defined using the Clavien-Dindo classification system, was calculated. We did not include Grade I complications as postoperative complications and complications were divided into minor (Grade II) and major (Grades III-V) complications. A multivariate logistic regression analysis was used to identify factors associated with 30-day postoperative complications. A Cox regression analysis was used to assess the association between postoperative complications and overall survival.
Results
Overall, 31% of the patients (333 of 1090) had a postoperative complication within 30 days. The following factors were independently associated with 30-day postoperative complications: rapidly growing primary tumors classified according to the modified Katagiri classification (odds ratio 1.6; 95% confidence interval, 1.1-2.2; p = 0.011), multiple bone metastases (OR 1.6; 95% CI, 1.1-2.3; p = 0.008), pathologic fracture (OR 1.5; 95% CI, 1.1-2.0; p = 0.010), lower-extremity location (OR 2.2; 95% CI, 1.6-3.2; p < 0.001), hypoalbuminemia (OR 1.7; 95% CI, 1.2-2.4; p = 0.002), hyponatremia (OR 1.5; 95% CI, 1.0-2.2; p = 0.044), and elevated white blood cell count (OR 1.6; 95% CI, 1.1-2.4; p = 0.007). Minor and major postoperative complications within 30 days after surgery were both associated with greater 1-year mortality (hazard ratio 1.6; 95% CI, 1.3-1.8; p < 0.001 and HR 3.4; 95% CI, 2.8-4.2, respectively; p < 0.001).
Conclusion
Patients with metastatic disease in the long bones are vulnerable to postoperative adverse events. When selecting patients for surgery, surgeons should carefully assess a patient’s cancer status, and several preoperative laboratory values should be part of the standard work-up before surgery. Furthermore, 30-day postoperative complications decrease survival within 1 year after surgery. Therefore, patients at a high risk of having postoperative complications are less likely to profit from surgery and should be considered for nonoperative treatment or be monitored closely after surgery.
Level of Evidence
Level III, therapeutic study.
Introduction
Given the prognosis and poor physical condition of most patients with metastatic bone disease, postoperative complications and long rehabilitation periods should be minimized and considered during surgical decision making [19]. Knowledge about postoperative complications may be used to prepare patients for possible adverse events and to monitor high-risk patients closely for early detection and treatment in the event of a postoperative complication. Moreover, for this group of patients, the primary goal of surgery is to improve quality of life in a palliative setting.
A large systematic review has quantified the risk of postoperative complications in this population, reporting complication rates between 5.8% and 25% [19]. The studies included in this review lacked detailed information regarding a definition of complications, and postoperative complications were not the primary outcome. Two other studies primarily focused on postoperative complications and postoperative mortality in a comparable population. One (n = 1497) found a complication rate of 12.1% and identified that older age, type of primary tumor, higher Charlson comorbidity index, and blood transfusion were associated with postoperative morbidity [36]. The other study (n = 1317) comparing prophylactic versus post-fracture stabilization among patients with metastatic long-bone disease found a substantial difference in complication rates between these groups (9.8% versus 16%) [1].
However, none of the previous studies have used the Clavien-Dindo classification to systematically assess complications [6]. Therefore, details about the different kinds of complications that occur and the consequences of these complications remain unclear. A better comprehension of the risk of postoperative complications, predisposing factors, and the impact of complications on survival may help surgeons in their surgical decision-making and postoperative management. Ultimately, for some patients, operative treatment will not be the best option in terms of short-term and long-term complications.
We therefore asked, (1) What is the risk of 30-day postoperative complications after surgical treatment of osseous metastatic disease of the extremities? (2) What predisposing factors are associated with a higher risk of 30-day complications? (3) Are minor and major 30-day complications associated with higher mortality at 1 year?
Patients and Methods
Study Design and Setting
This retrospective study was approved by our institutional review board and a waiver of informed consent was granted. All medical record data were collected at two tertiary care centers. Patients were included if they had an International Classification of Diseases, 9th Revision (ICD-9), code for secondary neoplastic disease or a pathologic bone fracture and a Current Procedural Terminology (CPT) code for fixation of a fracture or an impending fracture or another oncologic orthopaedic procedure.
Participants
After manually assessing the patients’ records, we included those who met the following criteria: age of 18 years and older, receipt of surgical treatment for a pathologic fracture or an impending fracture, and diagnosis of metastatic disease in the treated long bone between January 1999 and December 2016. If a patient had undergone multiple operations, we only included the first surgery per patient to prevent violation of the statistical assumption of independence. Patients who underwent multiple orthopaedic procedures at the same time were included as well. The long bones included the humerus, radius, ulna, femur, tibia, and fibula. We excluded patients who underwent revision procedures and surgical treatments other than endoprosthetic reconstruction, plate-screw fixation, intramedullary nailing, or dynamic hip screw fixation.
Patients with myeloma, multiple myeloma, or lymphoma were also included because the surgical approach to these diseases is similar to the treatment of metastatic long-bone disease.
Demographics, Description of Study Population
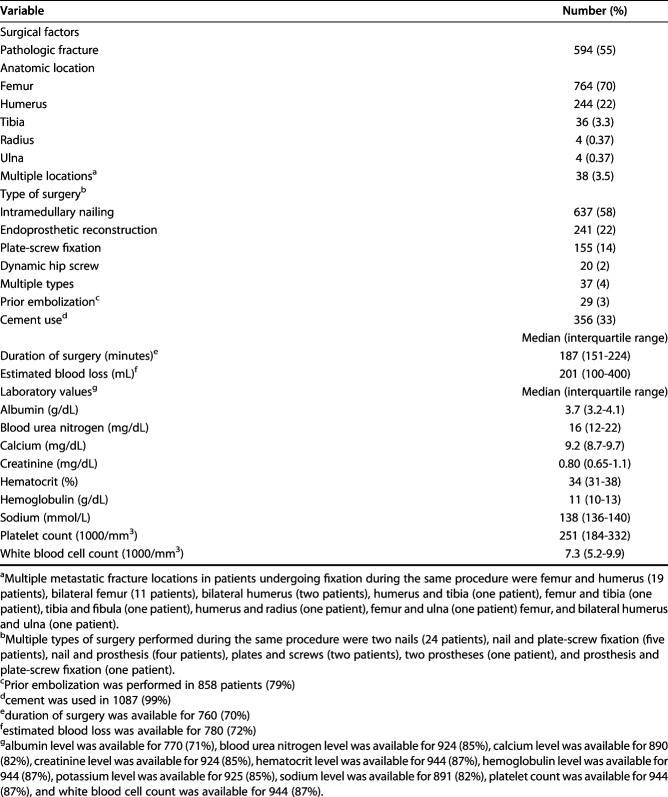
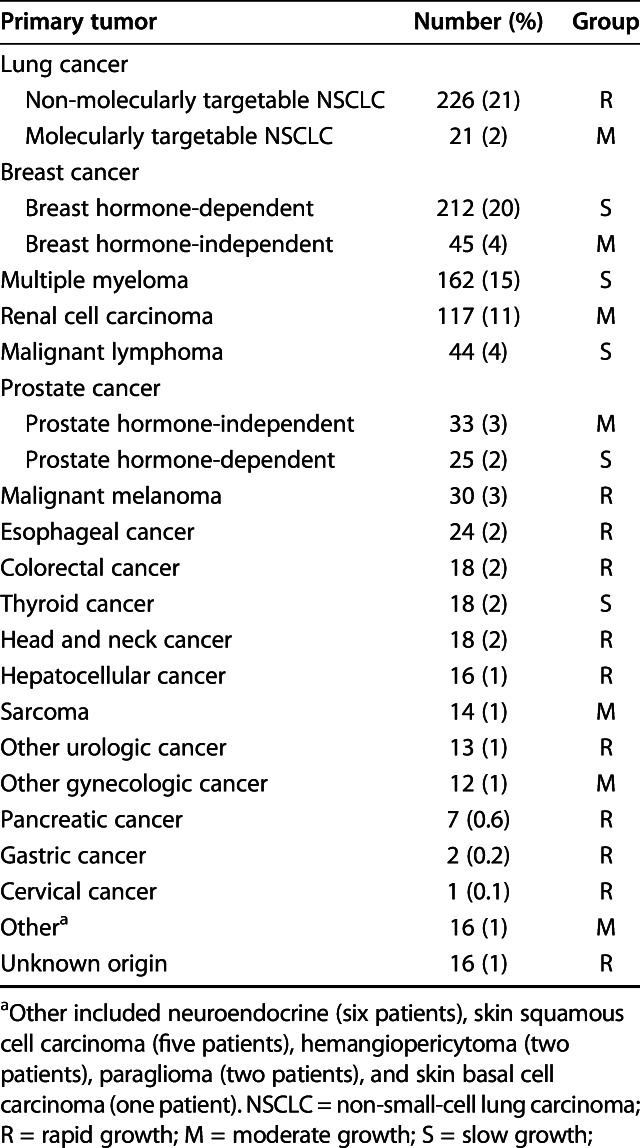
After manually reviewing the patients’ medical records, we included 1090 patients who met the inclusion criteria. The median time of follow-up after surgery was 216 days (IQR 69-651 days) (Table 1). The median age was 63 years (IQR 54-72 years), and 44% (480 of 1090 patients) were men. The median BMI was 26.6 kg/m2 (IQR 23.2-30.3 kg/m2), and the median time from the primary diagnosis to operative treatment of the affected long bone was 611 days (IQR 70-2064 days). In total, 55% (594 patients) had a pathologic fracture and 45% (496 patients) had an impending fracture (Table 2). The femur and humerus were the most operated-on long bones (70% [764 patients] and 22% [244 patients], respectively). Intramedullary nailing was the most commonly used type of surgery (58% [637 patients]), followed by endoprosthetic reconstruction (22% [241 patients]) and plate-screw fixation (14% [155 patients]). The most common primary tumors were breast (24% [257 patients]), lung (23% [247 patients]), multiple myeloma (15% [162 patients]), kidney (11% [117 patients]), and prostate tumors (5% [58 patients]) (Table 3).
Table 1.
Patient demographics (n = 1090)
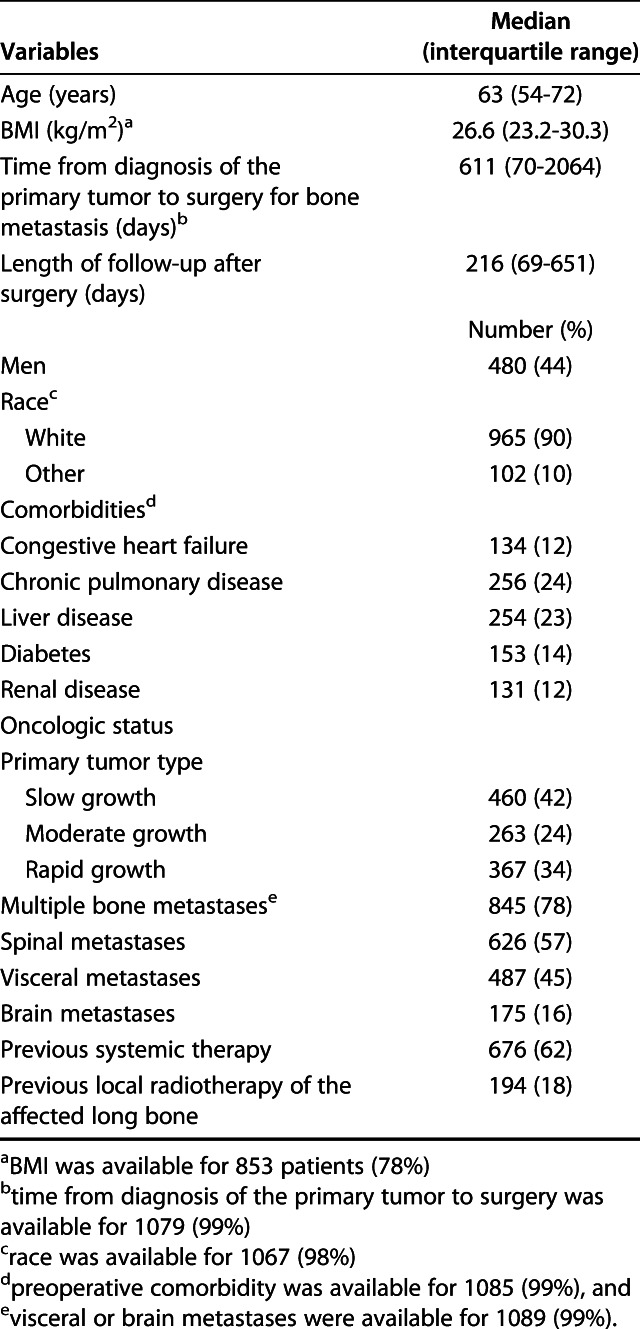
Table 2.
Surgical factors and preoperative laboratory values (n = 1090 patients)
Table 3.
Tumor distribution (n = 1090 patients)
Description of Experiment, Treatment, or Surgery
Because of the retrospective nature of this study, there were no predefined criteria regarding operative treatment or surgical strategy, and preferences varied among surgeons. In general, we operated on patients with a bony metastasis when a patient was deemed healthy enough to proceed to surgery and wished to undergo surgery. Among those who had surgery, we generally performed arthroplasty in those with more destructive (and therefore fractured) lesions and more proximally located lesions. In patients with higher estimated survival and smaller or more diaphyseally located lesions, intramedullary nailing or plate-screw fixation was performed. In patients with a shorter life expectancy and femoral neck lesions, dynamic hip screw implementation was performed. Only patients who were surgically treated were included.
Variables, Outcome Measures, Data Sources, and Bias
Our primary outcome was the occurrence of one or more complication within 30 days of surgery. All data were retrieved by manually reviewing patients’ records. We classified complications according to the Clavien-Dindo system [6, 8]. Complications were graded between Grade II and Grade V. Grade II complications were defined as any deviation from the postoperative course resulting in pharmacologic intervention. Grade III complications were defined as any postoperative complications resulting in a surgical, endoscopic, or radiologic intervention. Grade IV complications were defined as any life-threatening postoperative deviation resulting in intensive care unit management. Grade V was defined as any postoperative complication leading to the death of a patient. We did not differentiate between Grade IIIa and IIIb complications or between Grade IVa and IVb complications.
Grade I complications, defined as any deviation from the postoperative course without the need for pharmacologic treatment or surgical, endoscopic, or radiologic interventions, were not graded and were included in the “no complication” group. This type of complication is less serious and was considered clinically irrelevant for this study. Furthermore, it is hard to consequently grade this kind of complication retrospectively.
Our secondary outcome was survival, defined as the time from surgery to death. The date of death was obtained by manually reviewing medical records and by searching the Social Security Death Index, a database of death records from the United States Social Security Administration, which was last updated on February 28, 2014 [15]. Death records of patients who were still alive after this date were manually obtained through the hospital’s medical records and administrative databases.
The following explanatory variables were collected by record review: age, sex, race (self-reported), BMI, preoperative comorbidities, primary tumor type, other bone metastases, spinal involvement, visceral metastases, any previous chemotherapy, previous local radiotherapy of the affected long bone, type of fracture, location of fracture, type of surgery, duration of surgery, estimated blood loss, cement use, and prior embolization of the tumor. We also requested preoperative laboratory values, including the blood urea nitrogen level (mg/dL), calcium level (mg/dL), creatinine level (mg/dL), hematocrit level (%), hemoglobin level (g/dL), sodium level (mmol/L), platelet count (u * 103/mm3), white blood cell count (u * 103/mm3), and albumin level (g/dL).
BMI was categorized as underweight (less than 18.5 kg/m2), normal weight (between 18.5 kg/m2 and 30 kg/m2), and obese (greater than 30 kg/m2).
Preoperative comorbidity data (congestive heart failure, diabetes, chronic obstructive pulmonary disease, renal disease, and liver disease) were obtained by ICD-9 and ICD-10 codes given on the day before surgery [30].
The primary tumor type was categorized according to the method of Katagiri et al. [18] as slow-growth (myeloma, hormone-dependent breast, hormone-dependent prostate, lymphoma, and thyroid), moderate-growth (molecularly targetable lung, hormone-independent breast, hormone-independent prostate, renal cell, sarcoma, gynecologic except cervical, and all other types of cancer), and rapid-growth (non-molecularly targetable lung, melanoma, esophageal, colorectal, head and neck, hepatocellular, urologic, pancreatic, gastric, cervical, and unknown origin).
The fracture type was dichotomized as pathologic or impending. An impending fracture was defined as a bone that did not show a visible fracture line, loss of height, or any other radiographic sign of a fracture that still resulted in surgical treatment.
We assessed radiology reports to evaluate for the presence of metastases in the bones, brain, lung, or liver. Multiple bone metastases were defined as any bone metastasis affecting a bone other than the long bone that resulted in surgery. We dichotomized brain metastases and visceral metastases (lung or liver).
We retrieved the most recent laboratory value within 14 days before surgery for all laboratory values, except for serum albumin, for which the most recent value within 90 days before surgery was used. Most patients underwent preoperative screening within this time period. We assumed a patient’s nutritional status to be reflective of the day of surgery when undergoing preoperative screening. Laboratory values on the day of surgery were excluded because it was unclear whether the values were retrieved preoperatively or postoperatively.
Statistical Analysis
Variables are presented as frequencies and percentages for categorical variables and as medians with interquartile ranges for continuous variables. Bivariate analyses with univariate logistic regression were performed to assess the association between explanatory variables and complications within 30 days.
The aim of this study was to assess whether factors were associated with postoperative complications. Regression models assume that an association between predisposing variables and a dependent variable is uniform. This means that this relation, linear or nonlinear, is constant over the entire range of values [31]. However, in the current study, this did not apply to continuous variables such as laboratory values. We therefore dichotomized all continuous variables before analysis, although dichotomizing continuous variables results in lost information. All laboratory values were dichotomized based on the reference range used in the two tertiary care centers: albumin level < 3.5 g/dL (normal: 3.5-5.5 g/dL), blood urea nitrogen level > 20 mg/dL (normal: 7-20 mg/dL), calcium level < 9 mg/dL (normal: 8.9-10.1 mg/dL), creatinine level > 1.3 mg/dL (normal: 0.4-1.2 mg/dL), sodium level < 135 mmol/L (normal: 135-145 mmol/L), platelet count < 150 or > 450 * 103/mm3 (normal: 150-450 * 103/mm3), and white blood cell count > 11 * 103/mm3 (normal: 4.5-11 * 103/mm3). Hematocrit level < 30% and hemoglobin level < 10 g/dL were dichotomized based on the original threshold for transfusion in other studies [5, 16]. Age was dichotomized as 65 years or older and younger than 65 years. The location of surgery was dichotomized into lower and upper extremity. If patients underwent surgery of multiple locations during the same procedure, they were included in the lower-extremity group if at least one of the locations was a lower extremity [17].
All variables with a p value < 0.10 in bivariate testing were tested in a multivariable logistic regression analysis to determine whether these variables were independently associated with complications within 30 days (Table 4). We assumed missing values were random, and multiple imputations (40 imputations) were used to replace missing values if less than 30% were missing [11, 23]. This resulted in the imputation of missing values for albumin (29%), blood urea nitrogen (15%), hematocrit (13%), hemoglobin (13%), sodium (18%), white blood cell count (13%), creatinine (15%), platelet count (13%), visceral metastases (1%), and comorbidities (1%).
Table 4.
Bivariate analysis
To evaluate the correlation between 30-day postoperative complications and our secondary outcome of survival, a Cox regression analysis was performed and visualized with a Kaplan-Meier plot. The patients’ complications (none, minor, or major postoperative complications) were analyzed.
Only a two-sided p value less than 0.05 was considered significant. We used STATA® version 13.0 (StataCorp LP, College Station, TX, USA) for all statistical analyses.
Results
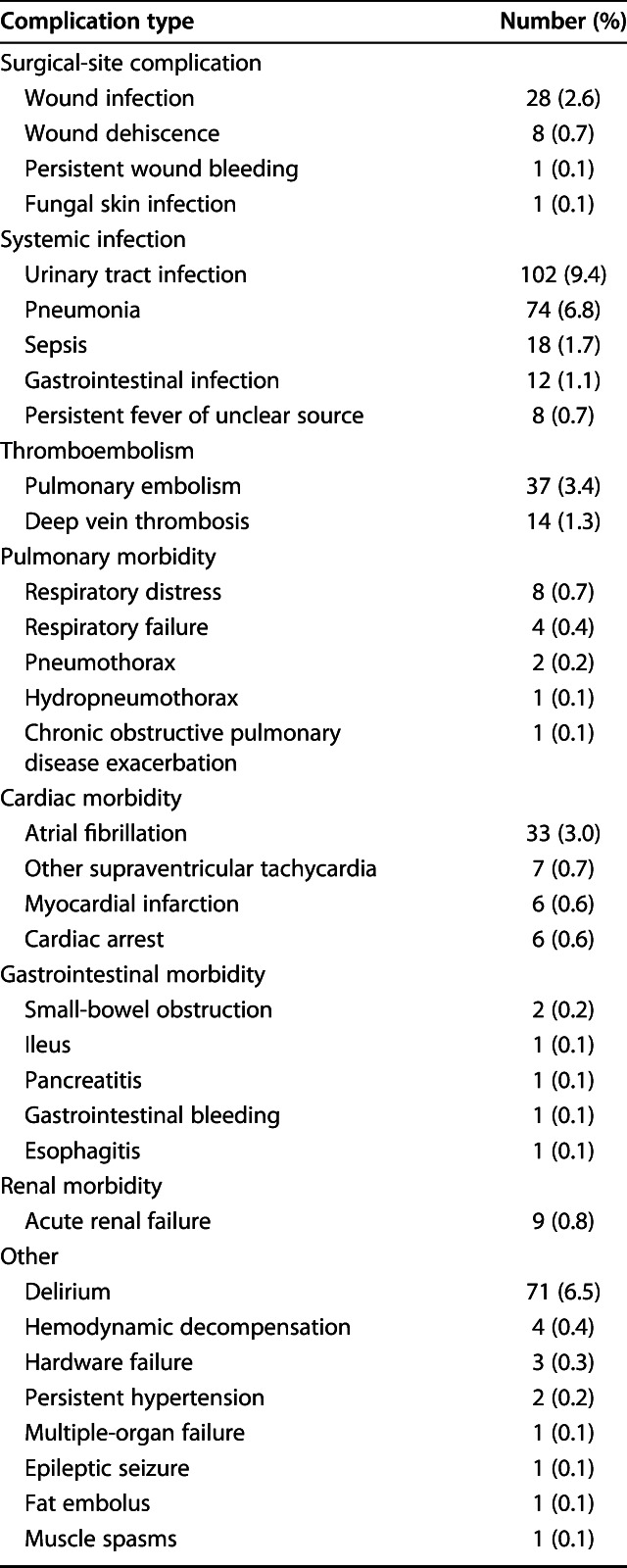
Within 30 days, 31% of the patients (333 of 1090) had a postoperative complication (Table 5). Of those who had at least one complication, 65% (215 of 333 patients) had minor complications (Clavien-Dindo Grade II), and 35% (118 patients) had major complications (Clavien-Dindo Grades III-V). Of the 333 patients who had at least one complication, 69% (231 patients) had only one complication, 23% (77 patients) had two complications, and 7.5% (25 patients) had more than two complications. In general, urinary tract infection (9.4% [102 of 1090 patients]), pneumonia (6.8% [74 patients]), delirium (6.5% [71 patients]), pulmonary embolism (3.4% [37 patients]), and atrial fibrillation (3.0% [33 patients]) were the most common postoperative complications (Table 6). Sixty patients were lost to follow-up within 1 year. Of these, 16 patients were lost to follow-up within 30 days; two still had a registered complication during the follow-up period.
Table 5.
Outcomes (n = 1090 patients)
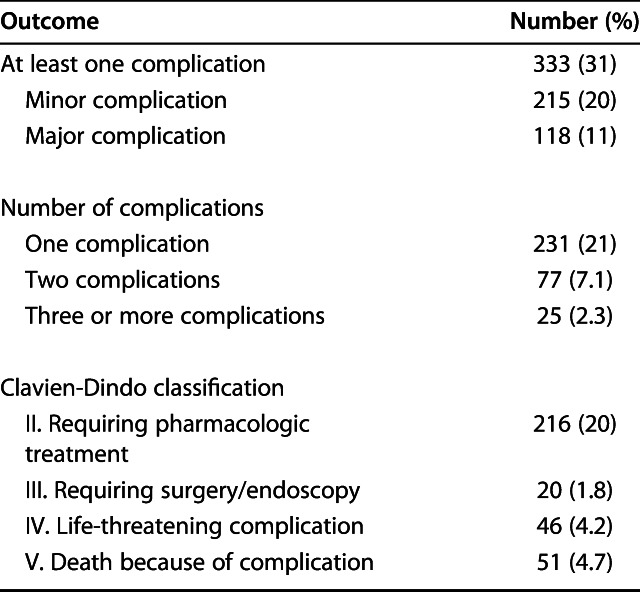
Table 6.
Complication types (n = 1090 patients)
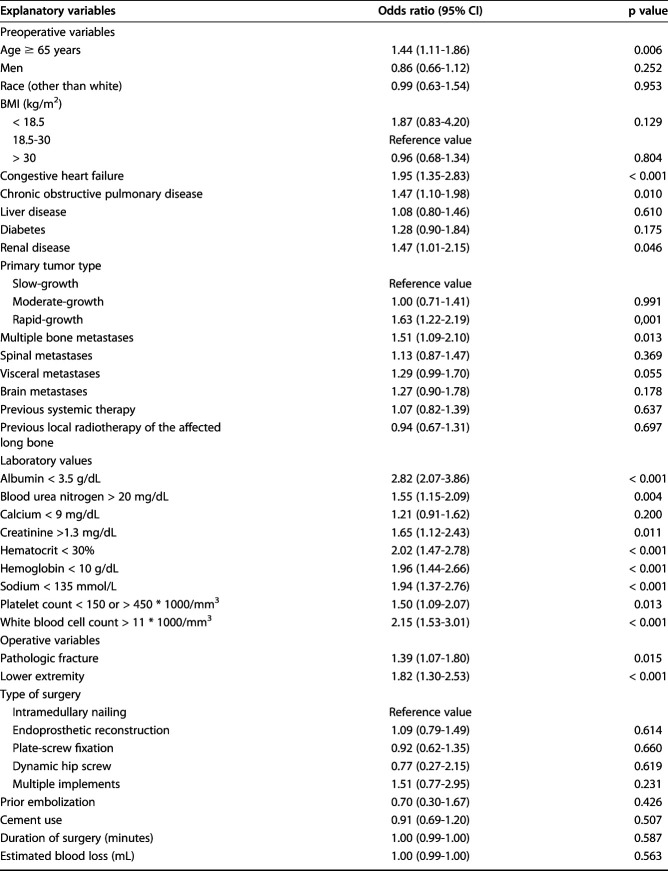
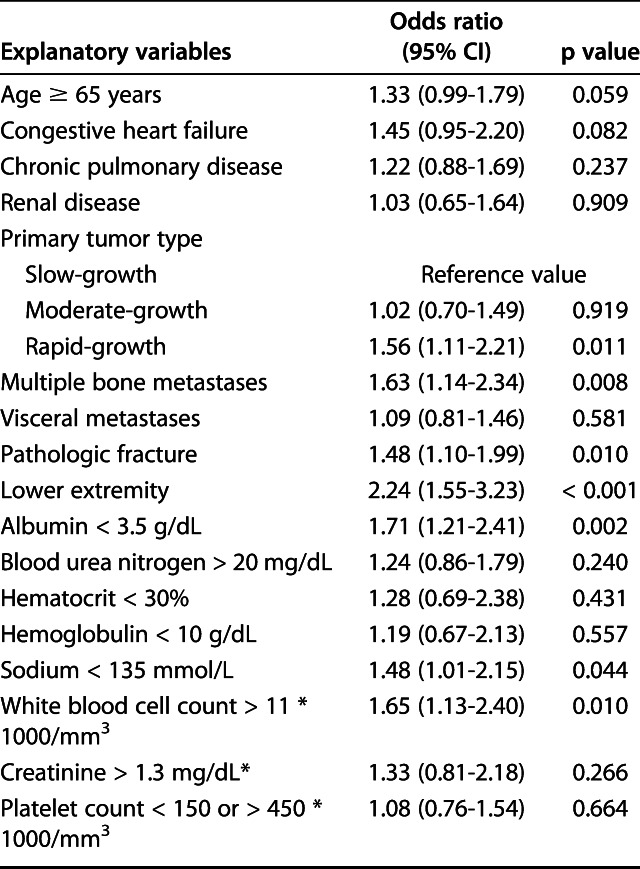
After imputation of the missing variables and controlling for potentially confounding variables such as age, hemoglobin level, and platelet count, we identified the following factors as independently associated with 30-day postoperative complications: rapidly growing primary tumors according to the modified Katagiri classification [18] (OR 1.6; 95% CI, 1.1-2.2; p = 0.011), multiple bone metastases (OR 1.6; 95% CI, 1.1-2.3; p = 0.008), pathologic fracture (OR 1.5; 95% CI, 1.1-2.0; p = 0.010), lower-extremity location (OR 2.2; 95% CI, 1.6-3.2; p < 0.001), albumin level < 3.5 g/dL (OR 1.7; 95% CI, 1.2-2.4; p = 0.002), sodium level < 135 mmol/L (OR 1.5; 95% CI, 1.0-2.2; p = 0.044), and white blood cell count > 11 * 103/mm3 (OR 1.6; 95% CI 1.1-2.4; p = 0.007) (Table 7).
Table 7.
Multivariate analysis
Minor and major complications within 30 days after operative treatment were both associated with an increased risk of mortality (hazard ratio 1.6; 95% CI, 1.3-1.8; p < 0.001 and HR 3.4; 95% CI, 2.8-4.2, respectively; p < 0.001). The survival probability was the lowest at all time points for patients who had major complications and the highest for patients with no complications (Fig. 1).
Fig. 1.
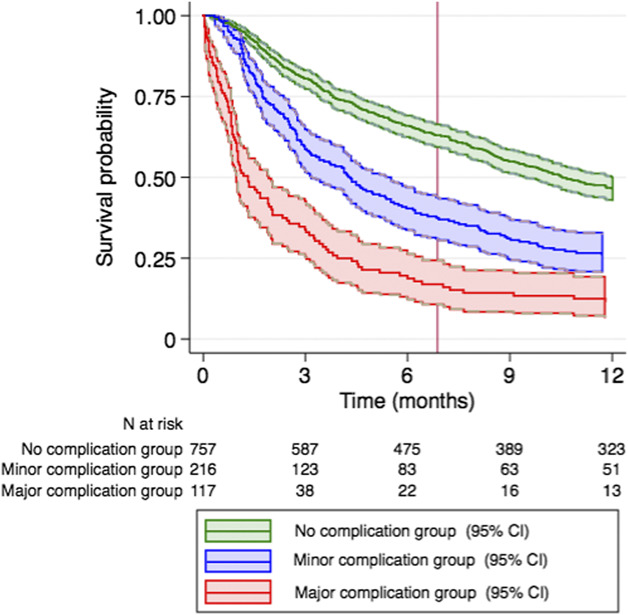
This Kaplan Meier plot shows survival curves by complication grade. Minor complications (HR 1.6; 95% CI, 1.3-1.8; p < 0.001) and major complications (HR 3.4; 95% CI, 2.8-4.2; p < 0.001) were associated with a decreased probability of survival within the first year after surgery.
Discussion
Background and Rationale
The aim of surgical treatment of metastatic disease is to relieve pain and regain biomechanical stability and functional independence [19]. However, surgery also has drawbacks such as postoperative complications [19]. Therefore, the decision to operate on metastases in the long bones must be carefully considered in terms of positive and negative outcomes. A better comprehension of the risk of postoperative complications, predisposing factors, and the impact of complications on survival may help surgeons in surgical decision making and postoperative management. We found several factors were associated with 30-day postoperative complications. In addition, minor and major complications were both associated with increased 1-year mortality. Therefore, patients who are less likely to have postoperative complications might benefit more from surgery and patients with a high risk of postoperative complications should be considered for nonoperative treatment or be monitored closely after surgery.
Limitations
This study has several limitations. The primary limitation of this study is the high percentage of missing data for some explanatory variables, such as albumin (29%). We used multiple imputation to address this issue when less than 30% of the data was missing, assuming the missing data to be random [11, 23]. Future studies may seek to conform our findings. Second, because of the retrospective nature of the study, we were not able to ascertain any predefined criteria for operative treatment. This might have resulted in selection bias. For example, some patients were not considered for surgery because of their very poor overall health status. Our results can therefore only be interpreted for patients with metastatic bone disease who are primarily considered fit enough for surgery. Furthermore, there was no uniform protocol to actively screen for and register complications. We may have missed some complications, although these would probably be minor and all available medical reports were screened. Additionally, there was no standard screening protocol before surgery. Patients with active preexisting infections such as pneumonia did not undergo surgery, but we cannot determine whether there were patients with an asymptomatic infection. However, we believe our results are valid because they reflect the patients’ state before surgery. Third, the use of ICD-9 and CPT codes to find eligible patients might have resulted in missing patients. However, we think the number of patients with missing data was low and therefore did not influence our results. Fourth, because data on postoperative complications were collected by reviewing medical records retrospectively, we may have underestimated the risk of complications. However, we manually reviewed all medical records, including discharge summaries, follow-up visit notes, and medical microbiology, pathology, and radiology reports to minimize the underestimation of postoperative complications. Sixteen patients were lost to follow-up within 30 days; two still had a complication registered. We could have missed complications if these patients have been treated elsewhere; however, we assume this amount was too small to influence our results. Fifth, the time period in which data was collected was quite long (1999 to 2016). Changes in medical guidelines regarding pharmacy or surgery during this time period might have influenced our results. Therefore, validation in a prospective setting would contribute greatly to the strength of our results.
Risk of 30-day Complications After Surgery for Bone Metastases
We found a high incidence (31%) of 30-day postoperative complications. A recently published study found a comparable risk (26%) of postoperative complications among 141 patients with metastatic disease in the long bones [28]. In that study, systemic complications were defined as major adverse events such as pneumonia, deep vein thrombosis, and pulmonary embolism within 30 days postoperatively, and local complications were defined as any complications at the surgical site at any time. Complications such as urinary tract infections, delirium, and respiratory failure treated with pharmacologic treatment were not included. In other studies, the complication rates differ widely, between 9.6% and 25%, but most of these studies lack detailed information about how complications were defined [1, 10, 17, 32, 36, 41, 42, 44]. None of these studies used the Clavien-Dindo classification system to identify postoperative complications, although the Clavien-Dindo system has a broader definition than most studies [6]. This might explain the higher risk we found in our study. A standardized classification grading system makes it easier to compare detailed information on complications between different studies, and we encourage future studies to use the same classification system for this purpose [35].
Factors Associated With a Higher Risk of 30-day Postoperative Complications?
We found the following factors were independently associated with postoperative complications: primary tumor category by growth, multiple bone metastases, pathologic fracture, surgery of the lower extremities, low albumin level, low sodium level, and high white blood cell count.
First, the type of primary tumor was an independent risk factor of 30-day postoperative complications. Rapidly growing tumors, as categorized according to Katagiri et al.’s method [18], increase the risk of complications. Other studies found similar results regarding esophageal and lung cancer [17, 36]. A large Scandinavian study, however, did not find the primary type of tumor to be associated with systemic complications [32]. This may be explained by the fact that the authors did not differentiate between rapid-growth and moderate-growth tumors that were classified according to the method of Katagiri et al. [18]. Second, in our study, the presence of multiple bone metastases was independently associated with postoperative complications, which was not seen by others [43]. However, another study found that an advanced cancer status, including the presence of multiple bone metastases, was associated with the development of postoperative complications [17]. Third, we found that patients with a pathologic fracture had a higher risk of having postoperative complications than patients with an impending fracture. This confirms the finding of previous studies [1, 29, 33], although other studies did not find this association [28, 32].
The type of primary tumor and the presence of multiple bone metastases and a pathologic fracture have been identified as risk factors for decreased survival [3, 16]. Our results again emphasize the importance of assessing a patient’s cancer status before selecting patients for surgery, because an advanced preoperative cancer status is associated with not only worse prognosis, but also postoperative complications.
In the current study, patients who underwent surgery of the lower extremities had a higher complication rate than those with upper-extremity surgery. In general, this correlates with the results of other studies. A large systematic review [19] reported widespread complication rates of the femur between 5.8% and 20% [24, 27, 40, 44, 45], but only two studies mentioned a complication rate lower than 15%. However, these studies reported only surgical [24] or only systemic complications [40]. A study examining humeral metastases found a complication rate of 9% [41]. This difference could be explained by the fact that surgery of the lower extremity is a more complicated and lengthier operation than surgery of the upper extremity. Additionally, postoperatively, patients stay in the hospital longer and require a more intense rehabilitation period. Preoperatively, surgeons should inform patients receiving surgery of the lower extremities about their vulnerability to postoperative complications and monitor these patients more closely after operative treatment.
After controlling for confounding variables, we found that age older than 65 years was not independently associated with complications in this study. Although one study reported similar results [32], more recent studies reported age as a risk factor [17, 36, 43]. This might be explained by the threshold of 65 years; a higher threshold or categorized approach might have been different.
Our study revealed that several preoperative laboratory values were independently associated with the risk of postoperative complications. This is an interesting finding, as these laboratory values are easy to obtain and cost effective. The association of hypoalbuminemia with a poor outcome is in line with the results of previous studies [4, 9, 20, 22, 25, 37]. Low serum albumin level is related to poor nutritional status; therefore, preoperative supplementation 7 to 14 days before surgery might improve postoperative outcomes [14, 37]. However, there are indications that hypoalbuminemia in patients with cancer is also related to an inflammatory response. Thus, only treating malnutrition to resolve hypoalbuminemia might oversimplify the problem. The ongoing inflammatory response results in an increasing loss of protein elements, with death as a consequence [7, 26]. According to the American Society of Parental and Enteral Nutrition Clinical Guidelines, moderately or severely malnourished patients might benefit from nutritional support therapy, but routinely starting supplementation for every patient with hypoalbuminemia is not recommended [13, 14]. Screening of serum albumin should be part of the standard preoperative work-up to help predict complications and outcomes.
A high white blood cell count was associated with a higher risk of postoperative complications in our study. No studies that we know of have assessed the association between preoperative white blood cell count and the risk of postoperative complications among patients with metastatic long-bone disease. Leukocytosis is associated with an infectious or inflammatory process [2, 34]. The association between preoperative leukocytosis and postoperative complications could be explained by the presence of a pre-existing infection or inflammatory process. If patients were not considered healthy enough for operative treatment (for example, they suffered from pneumonia), they did not undergo surgery. Furthermore, all preoperative assessments were manually reviewed and only new-onset problems were graded as a postoperative complication. However, there were no uniform predefined criteria for operative treatment; therefore, not every patient underwent complete screening before surgery. Our results indicate that white blood cell count should be measured before surgery and if it is elevated, surgeons should reconsider these patients for operative treatment and monitor this group more closely after surgery.
Hyponatremia was independently associated with the risk of postoperative complications in our study. Preoperative sodium levels were described as a prognostic factor for general 30-day mortality and morbidity in an American nationwide study of 964,263 adults [21]. Hyponatremia was associated with a higher rate of postoperative major coronary events, wound infections, and pneumonia. Another large study of 11,079 patients found that in-hospital mortality was increased in patients undergoing orthopaedic surgery, but the rates of preoperative and postoperative hyponatremia were not differentiated [39]. A smaller retrospective article also found more adverse events after orthopaedic surgery among 1067 patients with preoperative hyponatremia [12]. However, it is unclear whether preoperative hyponatremia is a marker or mediator of postoperative complications [21, 38]. One study recommends perioperative use of a more physiologic crystalloid solution instead of ringer lactate or 0.9% saline solution for orthopaedic patients to prevent a further drop of sodium levels postoperatively [12]. However, we do not know the actual cause of hyponatremia in this group of patients. As a consequence, it remains unclear whether this would be a modifiable intervention and would result in better postoperative outcomes.
Association Between Complications and Survival at 1 Year
In this study, we found that minor and major complications were both associated with higher mortality within 1 year. This confirms the finding of a previous study on 1497 patients with femoral metastases showing that postoperative complications were associated with 30-day mortality [36]. A study focusing on patients with spine metastases found a comparable association between postoperative complications and survival, with a 1-year mortality rate of 28% for patients with a complications versus 6.8% for those without complications [29]. Thus, when selecting patients for operative treatment, surgeons must assess who is at a high risk of having postoperative complications. Regarding 1-year survival, patients with an increased risk of postoperative complications are less likely to profit from surgery and should be considered for nonoperative treatment.
Future research is needed to validate our findings. The decision to operate or not in patients with a skeletal metastasis is difficult, and the surgeon must weigh the benefits and drawbacks of surgical treatment. Negative consequences of nonoperative treatment such as pain and loss of function and mobility should be considered as well. One must strive to have the best understanding of all these factors. Therefore, it would be interesting to further explore the outcomes of patients regarding their quality of life when the decision is made not to treat the patient surgically.
Conclusions
Patients with metastatic disease in the long bones are vulnerable to postoperative adverse events. When selecting patients for surgery, surgeons should carefully assess a patient’s cancer status. Rapid-growing primary tumors such as lung and esophageal cancer, the presence of multiple bone metastases or a pathologic fracture, and surgery of the lower extremities increase the risk of postoperative complications. In addition, preoperative laboratory values such as low albumin level, hyponatriemia, and elevated white blood cell count were identified as predisposing factors and should be part of the standard preoperative work-up. Furthermore, 30-day postoperative complications decrease survival within 1 year after surgery. Therefore, patients at a high risk of having postoperative complications are less likely to profit from surgery and should be considered for nonoperative treatment or be monitored closely after surgery.
Acknowledgements
None.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Departments of Orthopedics at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, MA, USA.
References
- 1.Abiad El JM, Raad M, Puvanesarajah V, Rao SS, Morris CD, Levin AS. Prophylactic versus postfracture stabilization for metastatic lesions of the long bones: a comparison of 30-day postoperative outcomes. J Am Acad Orthop Surg. 2019;27:e709–e716. [DOI] [PubMed] [Google Scholar]
- 2.Abramson N, Melton B. Leukocytosis: basics of clinical assessment. Am Fam Physician. 2000;62:2053–2060. [PubMed] [Google Scholar]
- 3.Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases: prognostication in 241 patients. Acta Orthop. 1995;66:143–146. [DOI] [PubMed] [Google Scholar]
- 4.Bohl DD, Shen MR, Kayupov E, Della Valle CJ. Hypoalbuminemia independently predicts surgical site infection, pneumonia, length of stay, and readmission after total joint arthroplasty. J Arthroplasty. 2016;31:15–21. [DOI] [PubMed] [Google Scholar]
- 5.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao S V, Roback JD, Shander A, Tobian AAR. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–2035. [DOI] [PubMed] [Google Scholar]
- 6.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 7.Crumley ABC, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010;34:2393–2398. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh SL, De Silva RP, Dhital K, Gett RM. Is low serum albumin associated with postoperative complications in patients undergoing oesophagectomy for oesophageal malignancies? Interact Cardiovasc Thorac Surg. 2015;20:107–113. [DOI] [PubMed] [Google Scholar]
- 10.Hansen BH, Keller J, Laitinen M, Berg P, Skjeldal S, Trovik C, Nilsson J, Walloe A, Kalen A, Wedin R. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11–15. [DOI] [PubMed] [Google Scholar]
- 11.Hayati Rezvan P, Lee KJ, Simpson JA. The rise of multiple imputation: a review of the reporting and implementation of the method in medical research. BMC Med Res Methodol. 2015;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennrikus E, Ou G, Kinney B, Lehman E, Grunfeld R, Wieler J, Damluji A, Davis C, 3rd, Mets B. Prevalence, timing, causes, and outcomes of hyponatremia in hospitalized orthopaedic surgery patients. J Bone Joint Surg Am. 2015;97:1824–1832. [DOI] [PubMed] [Google Scholar]
- 13.Huhmann MB, August DA. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) clinical guidelines for nutrition support in cancer patients: nutrition screening and assessment. Nutr Clin Pract. 2008;23:182–188. [DOI] [PubMed] [Google Scholar]
- 14.Huhmann MB, August DA. Nutrition support in surgical oncology. Nutr Clin Pract. 2009;24:520–526. [DOI] [PubMed] [Google Scholar]
- 15.Huntington JT, Butterfield M, Fisher J, Torrent D, Bloomston M. The Social Security Death Index (SSDI) most accurately reflects true survival for older oncology patients. Am J Cancer Res. 2013;3:518–522. [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen SJ, van der Heijden AS, van Dijke M, Ready JE, Raskin KA, Ferrone ML, Hornicek FJ, Schwab JH. 2015. Marshall Urist Young Investigator Award: prognostication in patients with long bone metastases: does a boosting algorithm improve survival estimates? Clin Orthop Relat Res. 2015;473:3112–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen SJ, Kortlever JTP, Ready JE, Raskin KA, Ferrone ML, Hornicek FJ, Lozano-Calderon SA, Schwab JH. Complications after surgical management of proximal femoral metastasis: a retrospective study of 417 patients. J Am Acad Orthop Surg. 2016;24:483–494. [DOI] [PubMed] [Google Scholar]
- 18.Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, Nishimura T, Asakura H, Ogawa H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkinis MN, Lyne CJ, Wilson MD, Choong PFM. Metastatic bone disease: a review of survival, prognostic factors and outcomes following surgical treatment of the appendicular skeleton. Eur J Surg Oncol. 2016;42:1787–1797. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan CK, Han I, Kim H-S. Outcome after surgery for metastases to the pelvic bone: a single institutional experience. Clin Orthop Surg. 2017;9:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung AA, McAlister FA, Rogers SOJ, Pazo V, Wright A, Bates DW. Preoperative hyponatremia and perioperative complications. Arch Intern Med. 2012;172:1474–1481. [DOI] [PubMed] [Google Scholar]
- 22.Lohsiriwat V, Lohsiriwat D, Boonnuch W, Chinswangwatanakul V, Akaraviputh T, Lert-Akayamanee N. Pre-operative hypoalbuminemia is a major risk factor for postoperative complications following rectal cancer surgery. World J Gastroenterol. 2008;14:1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackinnon A. The use and reporting of multiple imputation in medical research - a review. J Intern Med. 2010;268:586–593. [DOI] [PubMed] [Google Scholar]
- 24.Mavrogenis AF, Pala E, Romagnoli C, Romantini M, Calabro T, Ruggieri P. Survival analysis of patients with femoral metastases. J Surg Oncol. 2012;105:135–141. [DOI] [PubMed] [Google Scholar]
- 25.McMillan DC, Crozier JEM, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. [DOI] [PubMed] [Google Scholar]
- 26.McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39:210–213. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson J, Gustafson P. Surgery for metastatic lesions of the femur: good outcome after 245 operations in 216 patients. Injury. 2008;39:404–410. [DOI] [PubMed] [Google Scholar]
- 28.Nooh A, Goulding K, Isler MH, Mottard S, Arteau A, Dion N, Turcotte R. Early improvement in pain and functional outcome but not quality of life after surgery for metastatic long bone disease. Clin Orthop Relat Res. 2018;476:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulino Pereira NR, Ogink PT, Groot OQ, Ferrone ML, Hornicek FJ, van Dijk CN, Bramer JA, Schwab JH. Complications and reoperations after surgery for 647 patients with spine metastatic disease. Spine J. 2019;19:144–156. [DOI] [PubMed] [Google Scholar]
- 30.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 31.Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: logistic regression. Perspect Clin Res. 2017;8:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratasvuori M, Wedin R, Keller J, Nottrott M, Zaikova O, Bergh P, Kalen A, Nilsson J, Jonsson H, Laitinen M. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132–138. [DOI] [PubMed] [Google Scholar]
- 33.Ristevski B, Jenkinson RJ, Stephen DJG, Finkelstein J, Schemitsch EH, McKee MD, Kreder HJ. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302–308. [PMC free article] [PubMed] [Google Scholar]
- 34.Shoenfeld Y, Tal A, Berliner S, Pinkhas J. Leukocytosis in non hematological malignancies--a possible tumor-associated marker. J Cancer Res Clin Oncol. 1986;111:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470:2220–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuda Y, Yasunaga H, Horiguchi H, Fushimi K, Kawano H, Tanaka S. Complications and postoperative mortality rate after surgery for pathological femur fracture related to bone metastasis: analysis of a nationwide database. Ann Surg Oncol. 2016;23:801–810. [DOI] [PubMed] [Google Scholar]
- 37.Uppal S, Al-Niaimi A, Rice LW, Rose SL, Kushner DM, Spencer RJ, Hartenbach E. Preoperative hypoalbuminemia is an independent predictor of poor perioperative outcomes in women undergoing open surgery for gynecologic malignancies. Gynecol Oncol. 2013;131:416–422. [DOI] [PubMed] [Google Scholar]
- 38.Vassalotti JA, DuPree E. Preoperative hyponatremia: an opportunity for intervention? Arch Intern Med. 2012;172:1482–1483. [DOI] [PubMed] [Google Scholar]
- 39.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wedin R, Bauer HCF. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br. 2005;87:1653–1657. [DOI] [PubMed] [Google Scholar]
- 41.Wedin R, Hansen BH, Laitinen M, Trovik C, Zaikova O, Bergh P, Kalén A, Schwarz-Lausten G, Vult von Steyern F, Walloe A, Keller J, Weiss RJ. Complications and survival after surgical treatment of 214 metastatic lesions of the humerus. J Shoulder Elbow Surg. 2012;21:1049–1055. [DOI] [PubMed] [Google Scholar]
- 42.Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss RJ, Tullberg E, Forsberg JA, Bauer HC, Wedin R. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286–290. [DOI] [PubMed] [Google Scholar]
- 44.Xing Z, Moon BS, Satcher RL, Lin PP, Lewis VO. A long femoral stem is not always required in hip arthroplasty for patients with proximal femur metastases. Clin Orthop Relat Res. 2013;471:1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zacherl M, Gruber G, Glehr M, Ofner-Kopeinig P, Radl R, Greitbauer M, Vecsei V, Windhager R. Surgery for pathological proximal femoral fractures, excluding femoral head and neck fractures: resection vs. stabilisation. Int Orthop. 2011;35:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]